To the Editor:
Hereditary hemochromatosis (HH) is an autosomal recessive disorder of iron metabolism wherein the body accumulates excessive iron. The excess iron is deposited in a variety of organs leading to their failure, which results in serious illness including cirrhosis, hepatoma, diabetes, cardiomyopathy, arthritis, and hypogonadotrophic hypogonadism. The locus of the gene responsible for HH has been mapped by linkage to the HLA locus on chromosome 6p, within approximately 1 to 2 centimorgans (cM) of the HLA-A gene.1,2 The identification of different genes in the critical region of the HH locus has been described previously. One of the genes, HLA-H, contains two missense alterations (Cys 282 Tyr and His 63 Asp). Cys 282 Tyr, one of these two alterations, is predicted to inactivate this class of proteins and was found to be homozygous in 83% of 178 patients of white origin, or 85% of all HH chromosomes. In contrast, only 3.2% of the control chromosomes carried the mutation — a carrier frequency of 6.4%.3 Jazwinska et al4 and Jouanolle et al5 reported even higher rates of homozygous Cys 282 Tyr in their HH patients (100% and 91%, respectively). Although the His 63 Asp variant is frequent in Cys 282 Tyr heterozygous patients, its high frequency on control chromosomes (17%) and the relative absence of patients homozygous for this allele raises the possibility that this variant is not directly causative of the disease. The Cys 282 Tyr mutation can be detected by allelic oligonucleotide hybridization method.3
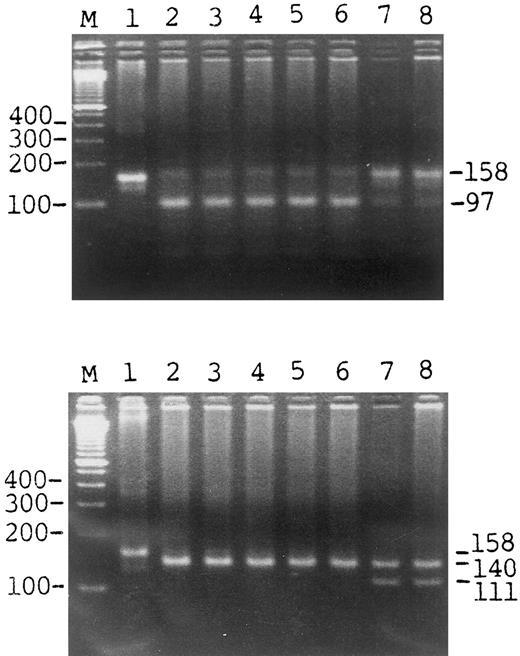
Detection of the HLA-H Cys 282 Tyr mutation by the presence or absence of specific PCR products. The top panel shows the results of the gel pattern digested by Bcg I; the bottom panel was digested by Rsa I. Lane 1 is the uncut control, lanes 2 to 6 are cases of known hemochromatosis, lane 7 is 1 of the 221 β-thalassemia minor cases, lane 8 is 1 of the 280 cases of the general population. M represents the marker.
Detection of the HLA-H Cys 282 Tyr mutation by the presence or absence of specific PCR products. The top panel shows the results of the gel pattern digested by Bcg I; the bottom panel was digested by Rsa I. Lane 1 is the uncut control, lanes 2 to 6 are cases of known hemochromatosis, lane 7 is 1 of the 221 β-thalassemia minor cases, lane 8 is 1 of the 280 cases of the general population. M represents the marker.
We report a simple, rapid, and convenient method in which polymerase chain reaction (PCR) products, digested by restriction enzyme and visualized in a gel under UV light give a clear and specific pattern for the Cys 282 Tyr mutation. PCR products of the Cys 282 Tyr mutation can be readily distinguished because the mutation creates a recognized site for the restriction enzyme Rsa I. There is a second Rsa I site just 29 bp upstream from the mutation site, which can be used as an internal control. For the normal codon 282 Cys, no such difference in the restriction site for any known enzyme exists. Therefore, we developed an oligonucleotide primer to detect the Cys 282 Tyr mutation, and a Bcg I restriction site was created in the normal codon 282 Cys by introducing a mismatch base (can also detect other mutations of codon 282 different from the Cys282Tyr mutation). HLA-H codon 282 region was amplified with the upstream primer: 5′-CTGGATAACCTTGGCTGTACCACCTGGGGAAGAG-CAGCGA-3′ and downstream primer: 5′-CTCAGGCACTCCTCTCAACC-3′. The underlined base is the mismatch base. This mutagenic base in combination with codon 282 Cys “TGC” will create a Bcg I site: 5′-10 (N)CGA(N)6TGC(N)12-3′. The 35 cycles of the PCR were performed at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute using Taq polymerase (Dynazyme; Finnzymes Oy, Espoo, Finland) in a reaction buffer as recommended by the supplier. PCR products were subsequently digested by the restriction enzymes Rsa I and Bcg I, respectively, and then electrophoresed in 3.5% agarose gel. The PCR product showed a 158 bp band. After digestion by Bcg I, there are a 97-bp and a 61-bp fragment in normal allele (the band would not be visualized well if the size were less than 65 bp). The 158-bp band was undigested in mutant. For the Rsa I enzyme, there is a restriction site in the upstream primer as an internal control of enzyme activity. After digestion with Rsa I, the PCR products of normal allele were digested to 140-bp and 18-bp bands, and three fragments (111 bp, 29 bp, and 18 bp) in mutant. The result is shown in Fig 1.
We analyzed 280 cases of general population, 221 cases of β-thalassemia minor, 40 cases of rheumatoid arthritis, 37 cases of ankylosing spondilitis, 18 cases of hepatoma, and 5 cases of HH by the methods mentioned above. Only 2 of the 596 nonhemochromatosis cases showed heterozygosity for the Cys 282 Tyr mutation, whereas the mutation was not present in any of the HH cases. Our results show that there is a very low frequency of the Cys 282 Tyr mutation in the Chinese population (0.33% of carrier rate). Perhaps different ethnic groups have different mutations, or the mutation resulting in HH is heterogenous. The main advantages of our method are that neither radioactive nor nonradioactive hybridization is required and we can obtain clear and unambiguous results within 1 day. In addition, we can detect the other changes in codon 282 Cys which are critical for the function of the HLA-H gene.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal