Abstract
The surface Ig on each B-cell lymphoma has unique portions (idiotypes), which can be recognized by the immune system. In this study, we immunized patients against the Ig expressed by their tumor and observed their clinical outcomes. After standard chemotherapy, 41 patients with non-Hodgkin's B-cell lymphoma received a series of injections with a vaccine consisting of tumor Ig protein coupled to keyhole limpet hemocyanin and emulsified in an immunologic adjuvant. Subjects were observed for toxicity, immune responses, and tumor status. The median duration of follow-up of all patients is 7.3 years from diagnosis and 5.3 years from the last chemotherapy given before vaccine treatment. Twenty patients (49%) generated specific immune responses against the idiotypes of their tumor Ig. Two patients who had residual disease experienced complete tumor regression in association with the development of these immune responses. The median duration of freedom from disease progression and overall survival of all 20 patients mounting an anti-idiotype immune response are significantly prolonged compared to the patients who did not mount an immune response. Thirty-two patients were in their first remission and nine were in subsequent remissions before beginning vaccine treatments. Analysis of the 32 first remission patients also shows an improved clinical outcome for those patients who mounted a specific immune response compared to those who did not (freedom from progression, 7.9 years v 1.3 years P = .0001; median survival from time of last chemotherapy not yet reached v 7 years, P = .04). This study confirms an earlier report that patients with B-cell lymphoma can be induced to make a specific immune response against the Ig expressed by their own tumor. It further shows that the ability to make such an immune response is correlated with a more favorable clinical outcome. Prospective controlled trials will be needed to prove a causal relationship between anti-idiotype immunity and improved clinical outcome.
CHEMOTHERAPY and radiation therapy can both induce remissions in patients with low grade non-Hodgkin's lymphoma (NHL). However, despite this responsiveness to treatment, the majority of patients ultimately relapse and cannot be cured with these standard therapies. Therefore, alternative methods of treatment are needed. An attractive approach would be to evoke the ability of the immune system to recognize and eliminate neoplastic cells while sparing normal cells. B-cell malignancies present such an opportunity because a tumor-specific cell surface antigen exists. The surface immunoglobulin (Ig) of these tumor cells display antigenic determinants within the variable regions of the Ig heavy and light chains.1-4 These determinants (termed “idiotypes” or Id) are unique because they are encoded by genes formed by the combinatorial association of several different genetic elements. Since these malignancies are monoclonal, all the cells of each tumor produce the same Ig protein. Therefore, these tumor-specific idiotypes can distinguish neoplastic from normal cells.
Studies in animals and humans have shown the effectiveness of the immune system to target idiotypes and kill lymphoma cells.5-9 Treatment with monoclonal anti-Id antibodies can cause tumor regression and, in some cases, induce long-term clinical remissions.8-11 However, in a few cases, responding tumors have recurred with cells containing mutations in their Ig protein which altered the unique Id determinants recognized by the monoclonal antibodies.12 Tumors that escaped based on these subtle genetic mutations could have arisen either from pre-existing or ongoing intratumor variation.13 14
An alternative approach might be to induce a broad antitumor immune response within the host. Animal studies have shown that active immunization with tumor-derived Id vaccines can induce host immunity.5-7,15 Vaccination with the tumor Ig protein leads to polyclonal antibody and T-cell responses. Such immune responses are capable of recognizing multiple antigenic determinants and, therefore, may prevent the escape of tumor cells with mutations in their idiotypes. These anti-Id responses can protect animals against tumor challenge and can even cure animals with established lymphomas.16 Based on these preclinical results, we began a clinical trial to evaluate Id vaccines in the treatment of patients with B-cell lymphoma. The early results of this trial showed that patients with B-cell lymphoma could be induced to make specific immune responses against the Id expressed on their own tumors.17 18 Here, we present the long-term clinical outcome and immunologic results of the initial 41 patients completing treatment on this ongoing study.
MATERIALS AND METHODS
Patients.At the time of entry into the study, all patients had B-cell NHL with a peripheral site of disease available to obtain a tumor sample. Patients with second malignancies, debilitating diseases, or Karnofsky performance <70% were not eligible. A lymph node biopsy was obtained from each patient for the purpose of producing a custom Id vaccine. The biopsies were classified according to the Working Formulation19 (see Table 1). All patients had follicular low grade lymphoma except one who had diffuse small cleaved cell histology. All patients then received chemotherapy, the timing and choice of which were determined by their physician. Therapy was continued until the patient achieved a maximal response. Of the 41 patients who completed vaccine treatments, 32 were in their first remission, and 9 were in subsequent remissions. Four of these latter 9 patients were treated with autologous bone marrow transplantation. Of the 32 first remission patients, all had advanced stage (III or IV), follicular small cleaved (FSC) or follicular mixed small cleaved and large cell (FM) lymphomas, and none were treated with a transplant. The average elapsed time between the initial diagnosis and the end of chemotherapy given before vaccine therapy was 2.6 years (median 1.2 years) for all 41 patients or 1.6 years (median 1 year) for the 32 first remission patients.
Patient Characteristics
| . | All (N = 41) . | IR+ (N = 20) . | IR− (N = 21) . |
|---|---|---|---|
| Age at end of last chemotherapy | |||
| Mean (median) | 45 (45) yr | 43 (42) yr | 48 (47) yr |
| Range | 23 to 74 yr | 23 to 70 yr | 32 to 74 yr |
| Gender | 18 M, 23 F | 8 M, 12 F | 10 M, 11 F |
| Histology | |||
| FSC | 27 | 12 | 15 |
| FM | 13 | 7 | 6 |
| DSC | 1 | 1 | |
| Stage | |||
| II | 1 | 1 | |
| III | 10 | 5 | 5 |
| IV | 30 | 14 | 16 |
| Tumor isotypes | |||
| IgM κ | 16 | 8 | 8 |
| IgM λ | 15 | 8 | 7 |
| IgG κ | 4 | 2 | 2 |
| IgG λ | 5 | 1 | 4 |
| IgA λ | 1 | 1 | |
| Treatment | |||
| First remission | 32 | 14 | 18 |
| Subsequent remission | 9 | 6 | 3 Time from Dx to end of last chemotx |
| Mean (median) | 2.6 (1.2) yr | 3.0 (0.9) yr | 2.3 (1.7) yr Time from end of last chemotx to vaccine |
| Mean (median) | 8.4 (6) mo | 9.6 (7) mo | 7.2 (5) mo |
| Cycles of chemotx | |||
| ≤8 | 17 | 7 | 10 |
| >8 | 24 | 13 | 11 |
| Disease status | |||
| Before Tx: | |||
| Clinical remission | 21 | 15 | 6 |
| Residual disease | 20 | 5 | 15 |
| . | All (N = 41) . | IR+ (N = 20) . | IR− (N = 21) . |
|---|---|---|---|
| Age at end of last chemotherapy | |||
| Mean (median) | 45 (45) yr | 43 (42) yr | 48 (47) yr |
| Range | 23 to 74 yr | 23 to 70 yr | 32 to 74 yr |
| Gender | 18 M, 23 F | 8 M, 12 F | 10 M, 11 F |
| Histology | |||
| FSC | 27 | 12 | 15 |
| FM | 13 | 7 | 6 |
| DSC | 1 | 1 | |
| Stage | |||
| II | 1 | 1 | |
| III | 10 | 5 | 5 |
| IV | 30 | 14 | 16 |
| Tumor isotypes | |||
| IgM κ | 16 | 8 | 8 |
| IgM λ | 15 | 8 | 7 |
| IgG κ | 4 | 2 | 2 |
| IgG λ | 5 | 1 | 4 |
| IgA λ | 1 | 1 | |
| Treatment | |||
| First remission | 32 | 14 | 18 |
| Subsequent remission | 9 | 6 | 3 Time from Dx to end of last chemotx |
| Mean (median) | 2.6 (1.2) yr | 3.0 (0.9) yr | 2.3 (1.7) yr Time from end of last chemotx to vaccine |
| Mean (median) | 8.4 (6) mo | 9.6 (7) mo | 7.2 (5) mo |
| Cycles of chemotx | |||
| ≤8 | 17 | 7 | 10 |
| >8 | 24 | 13 | 11 |
| Disease status | |||
| Before Tx: | |||
| Clinical remission | 21 | 15 | 6 |
| Residual disease | 20 | 5 | 15 |
Abbreviation: DSC, diffuse small cleaved cell lymphoma.
All patients were staged with computerized tomographic (CT) scans of the chest, abdomen, and pelvis before vaccine treatments. Immunizations were initiated at least 2 months after the completion of chemotherapy and administered according to the schedule below. Patients were then surveyed for tumor recurrence in a standardized and rigorous manner. They received physical examinations, blood counts and chemistries, chest radiographs, and abdominal films if lymphangiogram dye was present every 3 months. Repeat CT scans of the chest, abdomen, and pelvis were performed once a year or earlier if clinically indicated.
Vaccine production.Single cell suspensions of tumor cells were prepared under sterile conditions and used immediately or stored cryogenically in liquid nitrogen in fetal calf serum supplemented with 10% dimethyl sulfoxide (DMSO). Tumor cells were fused to the cell line K6H6B5 as previously described.20 The resulting hybridomas were initially screened by an enzyme-linked immunosorbent assay for the production of Ig matching the isotype of the tumor. High protein producing cell lines were identified. The Ig was then confirmed to be derived from the original tumor by either immunologic or genetic analysis. In the first method, Ig protein was used to vaccinate animals, and the protein was determined to be derived from the tumor when the hyperimmune serum from the animals was found to bind to the original tumor after absorption against normal human Ig. Alternatively, the Ig heavy chain variable region (VH) gene of each hybridoma was amplified and sequenced as previously described.21 Hybridomas were confirmed to be derived from the tumor when the sequence corresponding to the third complementarity-determining region (CDR3) of the heavy chain gene matched that of the original tumor. In two cases, the tumor Ig was found to have cross-reactive epitopes identified by monoclonal antibodies (shared idiotypes). Previously produced proteins expressing these shared idiotypes were used to vaccinate these two patients. Ig protein was purified from hybridoma culture supernatants by affinity chromatography (Protein A for IgG, anti-IgM antibody columns for IgM, and anti-IgA antibody columns for IgA). Keyhole limpet hemocyanin (KLH; Calbiochem, La Jolla, CA) was depleted of endotoxin and coupled to the tumor Ig protein using glutaraldehyde as previously described.17
Vaccine treatments.Each patient received a series of 5 subcutaneous immunizations each consisting of 0.5 mg of tumor Ig protein conjugated to 0.5 mg of KLH carrier protein and mixed with an immunologic adjuvant. Vaccines were administered on day 0 and then 2, 6, 10, and 20 weeks later. The initial 9 patients received their vaccinations of Id-KLH protein in “incomplete adjuvant” (5% squalane [Aldrich Chemical, Milwaukee, WI], 2.5% pluronic L121 [BASF, Parsippany, NJ], 0.2% Tween 80 [Aldrich] and phosphate-buffered saline), the second 9 patients received the “complete” adjuvant (incomplete adjuvant containing increasing doses [3 patients per dose level] of threonyl-muramyl dipeptide [Thr-MDP; Peninsula Laboratories, Burlingame, CA]) as part of a dose finding study. The remaining 23 patients received the complete adjuvant mixture containing the maximum tolerated dose (0.4 mg/vaccine) of Thr-MDP.
Humoral responses.Tumor Ig protein or isotype matched Igs were captured onto microtiter plates coated with goat anti-human heavy chain antibodies (BioSource International, Camarillo, CA). When the tumor Ig was an IgG, F(ab′ )2 fragments were produced by digestion with immobilized pepsin (Pierce, Rockford, IL) and used to coat microtiter plates directly. Preimmunization and postimmunization patient serum were serially diluted and allowed to bind to the target proteins. The binding of anti-Id antibodies was detected by polyclonal goat antihuman IgG antibodies (BioSource International) coupled to horseradish peroxidase (HRP). A response was interpreted as positive when a fourfold increase in anti-Id antibody titer was found when compared to the prevaccine serum and to the binding to irrelevant isotype matched proteins used as specificity targets. Antibody responses to KLH were measured by directly coating microtiter plates with KLH and allowing patient serum to bind. Anti-KLH antibodies were detected with KLH coupled to HRP. Serum titers of anti-KLH antibodies were determined by comparison to a standardized lot of polyclonal human anti-KLH serum. A titer greater than 0.5 μg/mL was considered positive.
Cellular proliferation assay.Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll Hypaque (Pharmacia, Uppsala, Sweden) density gradient separation of 40 to 60 mL of heparinized blood samples. PBMC were cultured in quadruplicate in media containing either KLH, tumor Ig protein or isotype matched, irrelevant protein at concentrations of 0 to 100 μg/mL as previously described.17 Determination of [3H]-thymidine incorporation was performed over days 5 to 6. A response was interpreted as positive when incorporation of more than two times background was found on two or more occasions.
Statistical methods.Freedom from disease progression and survival data were analyzed using Kaplan-Meier analysis and two-sample log rank tests of significance. Freedom from disease progression was measured from the date of last chemotherapy before vaccine treatment to the date of progression or last follow-up. Survival was measured from the date of last chemotherapy before vaccine treatment to the date of death or last follow-up. The date of last chemotherapy was the reference time point used for these calculations because the impact of vaccine treatments can only be tested when compared to the time of progression from the last treatment proven to cause tumor responses. It is at this point in time in which the relevant baseline clinical status is represented.
Side Effects of Vaccine Adjuvant
| . | Toxicity Grade . | |||
|---|---|---|---|---|
| . | 0 . | 1 . | 2 . | 3 . |
| Skin reaction | ||||
| Adjuvant vehicle | 0 | 5 | 4 | 0 |
| +Thr-MDP | 0 | 7 | 24 | 1 |
| Myalgias/arthralgias | ||||
| Adjuvant vehicle | 8 | 0 | 1 | 0 |
| +Thr-MDP | 1 | 3 | 20 | 8 |
| Fever | ||||
| Adjuvant vehicle | 8 | 0 | 1 | 0 |
| +Thr-MDP | 13 | 14 | 5 | 0 |
| Fatigue/malaise | ||||
| Adjuvant vehicle | 8 | 1 | 0 | 0 |
| +Thr-MDP | 4 | 25 | 3 | 0 |
| . | Toxicity Grade . | |||
|---|---|---|---|---|
| . | 0 . | 1 . | 2 . | 3 . |
| Skin reaction | ||||
| Adjuvant vehicle | 0 | 5 | 4 | 0 |
| +Thr-MDP | 0 | 7 | 24 | 1 |
| Myalgias/arthralgias | ||||
| Adjuvant vehicle | 8 | 0 | 1 | 0 |
| +Thr-MDP | 1 | 3 | 20 | 8 |
| Fever | ||||
| Adjuvant vehicle | 8 | 0 | 1 | 0 |
| +Thr-MDP | 13 | 14 | 5 | 0 |
| Fatigue/malaise | ||||
| Adjuvant vehicle | 8 | 1 | 0 | 0 |
| +Thr-MDP | 4 | 25 | 3 | 0 |
Grading scale: Skin 0 none; 1 mild-erythema, swelling, tenderness; 2 moderate-induration, erythema, swelling; 3 severe-infection or skin breakdown. Myalgia/arthalgia 0 none; 1 mild-no treatment; 2 moderate-required anlagesics; 3 severe-not controlled with analgesics and/or interfered with daily activities. Fever 0, none; 1, 37.1°C to 38°C; 2, 38.1°C to 40°C; 3, >40°C. Fatigue/malaise 0 none; 1 mild-performs activities; 2 moderate-impairment of normal activity or bedrest <50% waking hours; 3 severe-bedrest >50% of waking hours.
RESULTS
Vaccine treatments.A vaccine for a given individual was successfully manufactured in 85% of cases attempted. Protein production required between 2 to 6 months to complete. During the same time period, patients were treated with cytoreductive chemotherapy. Failure to produce vaccines occurred because of poor tumor hybridoma growth or the inadequate secretion of Id protein by these hybridomas. At least three hybridoma fusion attempts were made to manufacture Id protein before an individual case was abandoned.
Forty-one patients have completed all vaccine treatments and have been followed for at least 1 year. All patients received a vaccine consisting of tumor Ig protein conjugated to the carrier protein KLH and emulsified in an immunologic adjuvant. As part of a dose finding study, “incomplete” adjuvant was used in the first nine patients and “complete” adjuvant (including Thr-MDP) was given to the remainder of the patients. Toxicity of these injections consisted of local skin reactions (erythema, tenderness, induration), myalgias/arthralgias, and fever and were increased in the group receiving adjuvant containing Thr-MDP (Table 2). These side effects were transient (lasting 24 to 48 hours) and were related primarily to the dose of the Thr-MDP component of the adjuvant. The maximum tolerated dose of Thr-MDP was determined to be 0.4 mg/dose (data not shown). One patient developed an elevation of transaminases after the first treatment with vaccine containing complete adjuvant. These abnormalities resolved within 1 week. This patient received no further vaccinations, has remained well in over a year of follow-up, but was not included in this analysis. No other subjects were removed from the study. Since no difference in immunologic responses could be found between patients vaccinated with “incomplete” or “complete” adjuvant, the data have been combined for all analyses.
Immunologic responses.Serum was collected before and 2 weeks following each vaccination and analyzed for the presence of anti-Id and anti-KLH antibodies. Cellular proliferative responses were measured before each vaccine treatment and one month following the last treatment. As shown in Table 3, 20 of 41 patients (49%) developed specific anti-Id antibody and/or PBMC proliferative responses. A predominance of antibody (17 out of 20, 85%) versus cellular proliferative (7 out of 20, 35%) responses were seen with this vaccine preparation. In general, these anti-Id responses were initially detected after the third or fourth immunization, and a maximum response usually occurred after the fourth or fifth vaccination. Anti-Id antibody responses were sustained for several months. The ability of this vaccine preparation to induce antitumor Id immune responses was influenced by the patient's pre-vaccine disease status. Seventy-one percent (15 out of 21) of the patients who were in a complete remission developed specific antitumor Id responses whereas only 25% (5 out of 20) of patients with residual disease did so. A comparison of patient characteristics between those patients mounting an immune response and those who did not failed to identify any other differences between the two groups (Table 1).
Immune Responses
| Pre-Vaccine Status . | Anti-Idiotype . | Anti-KLH . | ||||
|---|---|---|---|---|---|---|
| . | Ab . | Cell . | Both . | Ab . | Cell . | Both . |
| Tumor absent (N = 21) | 10 | 1 | 4 | — | — | 21 |
| Tumor present (N = 20) | 3 | 2 | 0 | — | 2 | 18 |
| 13 | 3 | 4 | — | 2 | 39 | |
| Total immune responses | 20/41 | 41/41 | ||||
| Pre-Vaccine Status . | Anti-Idiotype . | Anti-KLH . | ||||
|---|---|---|---|---|---|---|
| . | Ab . | Cell . | Both . | Ab . | Cell . | Both . |
| Tumor absent (N = 21) | 10 | 1 | 4 | — | — | 21 |
| Tumor present (N = 20) | 3 | 2 | 0 | — | 2 | 18 |
| 13 | 3 | 4 | — | 2 | 39 | |
| Total immune responses | 20/41 | 41/41 | ||||
In contrast, anti-KLH responses developed in all patients. Forty-one out of 41 (100%) of patients developed PBMC cellular proliferative and 39 out of 41 (95%) developed antibody responses to the KLH protein. Anti-KLH responses were initially detected after the second or third immunization, were boosted with subsequent treatments, and were sustained for many months. The kinetics and the intensity of anti-KLH cellular and antibody responses were indistinguishable between the patients who were in a complete remission and those who had residual tumor before vaccine treatments (data not shown).
In some cases, cryogenically stored prevaccine tumor cells were available on patients who mounted anti-Id antibody responses. Staining of tumor cells with hyperimmune serum was attempted; however, technical difficulties prevented conclusive evidence of specific tumor staining. In these cases, the tumor's native surface Ig prevented the use of certain isotype specific anti-human Ig detection reagents since these reagents were capable of binding the tumor directly. In addition, nonspecific staining of tumor cells occurred from tumor B-cell Fc receptor binding of normal Ig present in the patient's immune serum.
Patient serum, obtained after completion of vaccine therapy, was examined for the development of rheumatoid factor. No patient developed a rheumatoid factor in response to this treatment. One patient (age 70) had a pre-existing, clinically assymptomatic rheumatoid factor, which remained unchanged following vaccine therapy.
Clinical outcome.Twenty-one patients were in complete remission and 20 had residual disease following chemotherapy given before vaccine treatments. The median follow-up is 7.3 years (range 2.1 to 17.6 years) from initial diagnosis, 5.3 years (range 1.2 to 8.6 years) from the time of last chemotherapy before vaccine treatment, and 4.6 years (range 1 to 7.9 years) from the start of vaccine treatments.
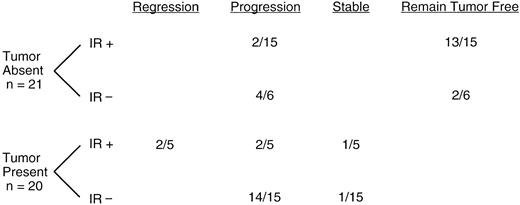
Fifteen patients in remission before vaccine therapy made specific immune responses. Of these (Fig 1), 13 remain in remission, and two had progression of their disease 5.2 and 1.8 years after the last chemotherapy given before vaccine treatment. Six of the patients in remission did not mount an immune response. Four of these patients have had progression of their disease (0.8, 1.1, 1.3, and 3.1 years after the last chemotherapy given before vaccine treatment) and two have remained tumor-free. Five of the patients who had residual disease before vaccine therapy made an immune response. Two of these patients underwent complete tumor regression in association with the development of a specific immune response, one has remained stable, and two have progressed. Of the two patients who underwent regression, one remained in complete remission for 7.9 years and one died of an unrelated cause 2.5 years from the time of last chemotherapy before vaccine treatment. Fifteen patients had residual disease before vaccine therapy but did not mount an immune response. Fourteen of these patients had progression of their disease and only one has remained stable. Four of these patients died, one of progression of his original tumor and three of transformed lymphoma. The kinetics and magnitudes of the anti-Id responses varied considerably from patient to patient; and since the number of patients making an anti-Id immune response who later progressed is small, no correlation between clinical outcome and the strength of the immune response could be made.
Clinical responses. Clinical responses associated with vaccine treatment are tabulated. Patients were subgrouped according to pre-vaccine disease status (tumor absent or tumor present) and to anti-Id immune response to vaccine (IR+, immune response or IR−, no immune response).
Clinical responses. Clinical responses associated with vaccine treatment are tabulated. Patients were subgrouped according to pre-vaccine disease status (tumor absent or tumor present) and to anti-Id immune response to vaccine (IR+, immune response or IR−, no immune response).
A second biopsy was obtained after disease progression in four patients who made an immune response and nine patients who did not. In all cases, cell surface tumor Ig protein was still present. Genetic analysis of matched prevaccine and relapsed tumor samples were performed. In all cases examined (3 of the 4 cases in which a patient relapsed after mounting an immune response), the tumors were clonally related as shown by sequence analysis of the Ig VH genes (data not shown).
Survival and freedom from disease progression.Five patients on this study have expired. Kaplan-Meier analyses of overall survival from the time of diagnosis were performed. The median survival of all 41 patients has not yet been reached and is significantly longer when compared to the median survival of nonvaccinated, case-matched historical controls (P < .0001, data not shown) or to all patients (n = 811) seen at this institution since 1963 with the diagnosis of FM or FSC NHL and with stage III or IV disease (median 10.9 years, P = .0029, data not shown). Kaplan-Meier analyses of freedom from disease progression (FFP) from the time of last chemotherapy before vaccine treatment were performed. The median duration of FFP of all 41 patients is 4.4 years. As presented above, antitumor Id immune responses were not induced in all patients. Therefore, an analysis of the data dividing patients into those mounting an antitumor Id immune response versus those who did not was performed. The median duration of FFP of patients who developed an antitumor Id immune response is 7.9 years whereas the median duration of FFP of patients who did not develop an immune response is 1.3 years (P < .0001, data not shown). The FFP of patients who did not mount a measurable anti-Id immune response is similar to that of historical case matched controls (data not shown).
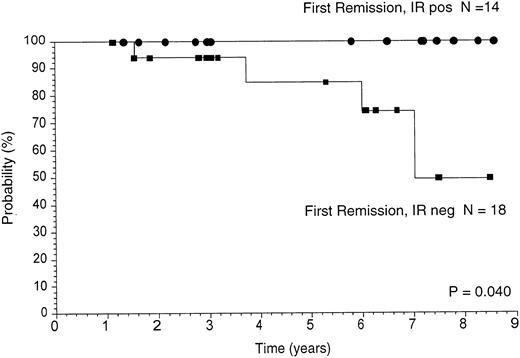
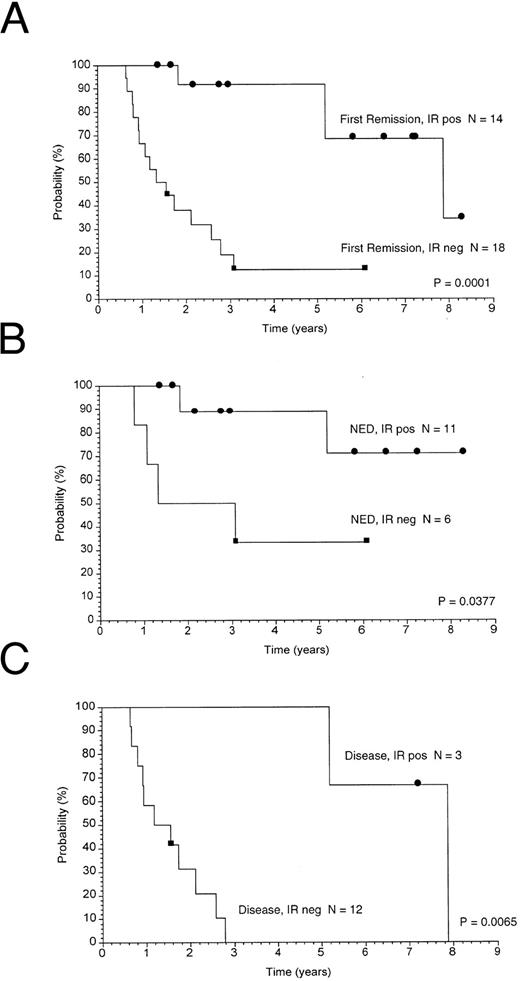
Thirty-two patients were in first remission and nine were in subsequent remissions before beginning vaccine treatments. The first remission group of patients were similar with regard to histology (FSC or FM) and stage (III or IV), and none were treated with transplant. Therefore, survival and freedom from disease progression data were analyzed separately for these patients. Kaplan-Meier analyses of overall survival from the time of last chemotherapy are shown in Fig 2. The median survival of patients mounting an antitumor Id immune response is significantly longer than those patients not mounting an immune response (median not yet reached v 7 years, P = .04) or to historical case matched controls (data not shown). FFP from the time of last chemotherapy before vaccine treatment is shown in Fig 3. Clinical outcome based on immune response was analyzed and is shown in Fig 3A. The median duration of FFP of patients who developed an antitumor Id immune response is 7.9 years whereas the median duration of FFP of patients who did not develop an immune response is 1.3 years (P = .0001). These results for the 32 patients in first remission are virtually identical to those cited above for the entire 41 patients. As presented in Table 3, the likelihood of making a specific immune response was related to the prevaccine remission status. Therefore, separate analyses were performed on the group in clinical remission (Fig 3B) and on the group with disease present at the time of vaccination (Fig 3C). Though the patient numbers become small, in each of these subgroups, the development of an antitumor Id immune response is associated with a longer duration of FFP. Therefore, regardless of the presence or absence of disease before vaccine therapy, the development of an antitumor Id immune response is associated with a better clinical outcome.
Overall survival. Kaplan-Meier analyses of overall survival calculated from the time of last chemotherapy given before vaccine treatments are shown. The survival of the 32 first remission patients is shown dividing the group into those mounting an antitumor Id immune response (IR+) versus those who did not (IR−).
Overall survival. Kaplan-Meier analyses of overall survival calculated from the time of last chemotherapy given before vaccine treatments are shown. The survival of the 32 first remission patients is shown dividing the group into those mounting an antitumor Id immune response (IR+) versus those who did not (IR−).
Freedom from disease progression. Kaplan-Meier analyses of freedom from disease progression calculated from the time of last chemotherapy given before vaccine treatment are shown. (A) Shows the 32 first remission patients divided according to the development of an anti-Id immune response (IR+, immune response or IR−, no immune response). Separate analyses were performed to examine the role of the presence or absence of disease before vaccine therapy. The patient population subgrouped according to prevaccine disease status is shown in (B, NED, no evidence of disease) and (C, residual disease).
Freedom from disease progression. Kaplan-Meier analyses of freedom from disease progression calculated from the time of last chemotherapy given before vaccine treatment are shown. (A) Shows the 32 first remission patients divided according to the development of an anti-Id immune response (IR+, immune response or IR−, no immune response). Separate analyses were performed to examine the role of the presence or absence of disease before vaccine therapy. The patient population subgrouped according to prevaccine disease status is shown in (B, NED, no evidence of disease) and (C, residual disease).
DISCUSSION
This study reports the long-term results of our continuing efforts to develop a form of active immunotherapy as a treatment of patients with non-Hodgkin's B-cell lymphoma who had achieved the maximal benefit from chemotherapy. A vaccine based on the unique Ig protein expressed by each patient's tumor was produced using hybridoma technology and manufactured at the same time the patient received cytoreductive chemotherapy. After recovery from chemotherapy, patients received their vaccine treatments.
Following vaccine treatments, specific antitumor Id immune responses were detected in 20 out of 41 (49%) of patients. In 2 patients with residual disease before vaccine therapy, complete tumor regression occurred in association with the development of antitumor Id immunity, and one patient remained disease-free 7.9 years after the last chemotherapy treatment. All patients appeared to be immunocompetent as shown by their ability to mount equivalent immune responses against the KLH protein. No autoimmune phenomena have been observed after therapy.
The median duration of follow-up is now 5.3 years after the last chemotherapy given before vaccine treatment (4.6 years after the start of vaccine therapy). The FFP and survival of those patients mounting an antitumor Id immune response is significantly longer compared to those who did not develop an immune response or to that of nonvaccinated, historical controls (data not shown). These results were strikingly similar when the analyses were performed on the relatively homogenous group of patients who were vaccinated in their first clinical remission. The FFP of those patients mounting an antitumor Id immune response is significantly longer compared to those who did not develop an immune response (Fig 3A), and this association remains valid regardless of the presence or absence of disease before vaccine treatments (Fig 3B and 3C). Moreover, patients mounting an anti-Id immune response have had a survival advantage. In this group of patients, the median survival is significantly improved when compared those patients not mounting an anti-Id immune response (Fig 2). Therefore, within all these groups, the development of an antitumor Id immune response appeared to be the most important factor identified, which was associated with a better clinical outcome.
The immune responses measured in this trial consisted of antibody responses (17 out of 20, 85%) and cellular proliferative responses (7 out of 20, 35%). The relative importance of antitumor Id antibody versus T-cell immune responses is unknown. In lymphoma, anti-Id antibodies alone are capable of inducing cell death, presumably through the direct induction of apoptosis or indirectly through complement fixation or antibody dependent cellular cytotoxicity mechanisms.5,22,23 In clinical trials, the passive administration of such antibodies can lead to tumor regression and long-term remissions.9-11 T-cell immune responses may also play an important role in the clinical outcome seen in this trial. PBMC proliferative responses are a convenient method of measuring cellular immunity but may primarily reflect CD4+ T-cell responses. Therefore, this assay could be underestimating the number of cellular responses. In a complementary study of a subset of these same patients, cytotoxic T cells were measured; their precursor frequency against autologous tumor cells was found to increase in association with vaccine treatments; and this increase was associated with a better clinical outcome.24
The human immune system has long been postulated to be capable of protecting the body from the uncontrolled growth of cells that have undergone malignant changes. The assumption is that the repertoire of the immune system includes the ability to recognize small differences between malignant and normal cells. According to this view, uncontrolled malignant growth occurs because tumor cells lack the ability to deliver the accessory signals required to trigger an effective immune response. Active immunotherapy with a vaccine represents an attempt to present tumor antigens in a more immunogenic form and correct these deficiencies in tumor surveillance. In this study, a vaccine consisting of tumor Ig protein chemically coupled to the foreign carrier protein KLH and emulsified in an immunologic adjuvant was used, and approximately half of the vaccinated patients made an antitumor Id immune response. In a separate study, we were able to induce strong anti-Id cellular immune responses in all patients treated with a vaccine preparation consisting of autologous dendritic cells pulsed with tumor Ig protein.25 Taken together, these results indicate that the immune systems of patients with lymphoma can be induced to recognize malignant cells if the correct signals are provided, and new vaccine preparations may be able to deliver such signals more effectively. Therefore, the failure to mount an antitumor Id immune response may be because of the strength of the vaccine rather than an inherent defect of the patient's immune system.
Late tumor relapses have occurred in vaccine treated patients who mounted anti-Id immune responses. In all such cases in which a second biopsy was obtained, the cell surface Ig protein was still present, indicating that relapse was not caused by a loss of the target. Sequence analysis of matched prevaccine and relapse tumor samples have indicated that the Ig VH gene remains genetically similar. At this time, it is not possible to determine whether point mutations seen are the result of selection or the natural progression of mutational changes which may be found with this disease. Importantly, however, point mutations are unlikely to destroy all idiotypic epitopes present on a large protein such as this. Thus, improvements in the potency of vaccine preparations may lead to stronger and more comprehensive anti-Id immune responses and even better clinical effects.
The long-term clinical results of this trial indicate a strong association between the development of specific antitumor Id immune responses and a better clinical outcome. Nevertheless, a direct causal relationship is not proven here. It is possible that the ability to mount an antitumor Id immune response is only a marker for individual patients who would do better clinically even without additional therapy. All of our patients were able to develop equivalent anti-KLH responses; and though this measures a response to a strong immunogen, it confirms the presence of a certain level of similiar immune function in each patient. This would indicate that the outcome of patients cannot be explained by differences in general immune competence. However, proof of benefit from vaccination will require prospectively randomized trials.
Currently, the only significant limitation for the general use of this treatment is the need to custom manufacture tumor Ig protein for each patient. Therefore, strategies for the rapid production of custom vaccines as well as improved methods of vaccine delivery of weak antigens to the immune system are actively being explored for use in future trials.
ACKNOWLEDGMENT
The authors thank Dr Byron Brown (Department of Health Research and Policy, Stanford University) for advice in the procedures for identifying the historical matched controls and on statistical methods, Samuel Brain for the use of his statistical analysis programs, and John Allen for his assistance in database searches.
Supported by Grants No. CA34233 and CA33399 from the National Cancer Institute. F.J.H. was supported by the James S. McDonnell Foundation Program for Molecular Medicine in Cancer Research (St. Louis, MO). C.B.C. was supported in part by the Kurt and Senta Herrmann Foundation (Zurich, Switzerland) and by the SIAK Farmitalia Foundation (St Gallen, Switzerland). R.L. is an American Cancer Society Clinical Research Professor.
Address reprint requests to Ronald Levy, MD, the Division of Oncology, Room M207, Stanford University Medical Center, 300 Pasteur Dr, Stanford, CA 94305.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal