Abstract
In patients with thrombotic thrombocytopenic purpura (TTP), excessive intravascular platelet aggregation has been associated with appearance in plasma of unusually large von Willebrand factor (vWF ) multimers. These extremely adhesive vWF multimers may arise due to deficiency of a “depolymerase” cleaving vWF to smaller molecular forms, either by reducing the interdimeric disulfide bridges or by proteolytic degradation. We studied the activity of a recently described vWF-cleaving protease in four patients with chronic relapsing TTP. Diluted plasma samples of TTP patients were incubated with purified normal human vWF in the presence of a serine protease inhibitor, at low ionic strength, and in the presence of urea and barium ions. The extent of vWF degradation was assayed by electrophoresis in sodium dodecyl sulfate-agarose gels and immunoblotting. Four patients, that included two brothers, with chronic relapsing TTP displayed either substantially reduced levels or a complete absence of vWF-cleaving protease activity. In none of these patient plasmas was an inhibitor of or an antibody against the vWF-cleaving protease established. Our data suggest that the unusually large vWF multimers found in TTP patients may be caused by deficient vWF-cleaving protease activity. Deficiency of this protease may be inherited in an autosomal recessive manner and seems to predispose to chronic relapsing TTP. The assay of the vWF-cleaving protease activity may be used as a sensitive diagnostic tool for identification of subjects with a latent TTP tendency.
THROMBOTIC thrombocytopenic purpura (TTP) is an uncommon illness characterized by thrombocytopenia, microangiopathic hemolytic anemia, fever, renal dysfunction, and central nervous system ischemia resulting from formation of platelet thrombi within the microvasculature. TTP was formerly considered to be a progressive disease with high mortality. The introduction of vigorous plasma exchange with fresh frozen plasma has markedly improved prognosis.1,2 TTP can occur in several members of the same family.3,4 The inheritance pattern of the familial forms of TTP is usually autosomal recessive.5 A rare variant of TTP, known as chronic relapsing TTP, is characterized by frequent episodes recurring after symptom-free intervals. The pathogenesis of TTP is unknown and the disease may be considered as a syndrome triggered by different causes. Most evidence suggests that endothelial cell injury is primarily involved in the sequence of events leading to manifestation of TTP.
Unusually large von Willebrand factor (vWF ) multimers have been observed in plasma from patients with chronic relapsing forms of TTP.6,7 vWF is a plasma glycoprotein synthesized in endothelial cells and megakaryocytes. From the storage organelles (Weibel-Palade bodies) of the endothelial cells, vWF is secreted primarily in the form of unusually large vWF multimers6,8,9 that become partially degraded by protease(s) in the circulation.9-12 In normal human plasma, vWF is found as a series of multimers ranging in size from about 500 to 20,000 kD. vWF mediates the initial platelet adhesion to the subendothelium of the damaged vessel wall at high shear rates. Only the highly polymeric forms of vWF are hemostatically active, ie, they normalize prolonged bleeding time and stop bleeding in patients with von Willebrand disease. The binding affinity for collagen13 and the platelet receptors, glycoproteins Ib and IIb/IIIa,14 is markedly increased in the largest multimeric forms of vWF. Although the circulating vWF in normal plasma does not show detectable binding to platelet receptors, interaction with platelets can be induced by nonphysiologic modulators, such as ristocetin and botrocetin, and by fluid shear stress. Unusually large vWF multimers secreted from endothelial cells have been shown to be more effective than the largest forms of vWF in normal plasma in binding to platelets and in inducing platelet aggregation under conditions of high fluid shear.15 It has been suggested16,17 that TTP and the closely related hemolytic uremic syndrome (HUS) are disorders associated with an excessive release from the endothelial cells of the unusually large vWF multimers and/or impaired degradation of these highly multimeric forms of vWF by a hitherto not identified “depolymerase.” Recently a specific protease has been partially purified from human plasma that cleaves purified human vWF to fragments produced by in vivo proteolysis.18 19
In the present study we found a deficient activity of the vWF-cleaving protease in the plasma of four patients with chronic relapsing TTP, suggesting that this deficiency may be associated with recurring episodes of TTP.
MATERIALS AND METHODS
Blood samples were anticoagulated in plastic tubes with vol of 0.106 mol/L Na3-citrate. Platelet-poor plasma was recentrifuged for 15 minutes at 3,000g at 25°C and stored at −20°C until tested. A citrated normal human plasma pool (NHP) was prepared from 42 healthy male subjects and stored at −70°C. vWF antigen (vWF:Ag) was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial rabbit antiserum against human vWF (RAHu/FVIII; Nordic, Tilburg, The Netherlands). Ristocetin cofactor activity (vWF:RCof ) was assayed in an optical aggregometer (Upchurch, Leicester, UK) at 37°C using washed paraformaldehyde-fixed human platelets in cacodylate-buffered saline. Platelet counts and hemoglobin determinations were performed by routine laboratory methods.
Multimer analysis of vWF in patient plasmas was performed in sodium dodecyl sulfate (SDS)-1% agarose gels (SeaKem HGT(P), FMC, Rockland, ME) according to Ruggeri and Zimmerman.20 Plasma samples containing 0.0025 units of vWF:Ag were applied onto each lane. Electrophoretically separated vWF multimers were electroblotted to nitrocellulose membranes and detected using rabbit antiserum against human vWF (A0082; Dako, Glostrup, Denmark) and alkaline phosphatase-labeled goat antibodies against rabbit IgG (D0487; Dako).
The activity of the vWF-cleaving protease in patient plasma samples was assayed at low ionic strength and in the presence of urea as previously described.18 Protease-free vWF was prepared by gel filtration on Sepharose CL-2B (Pharmacia-LKB, Uppsala, Sweden) of a cryoprecipitate obtained from fresh frozen normal human plasma. Early elution fractions in 0.13 mol/L NaCl/0.01 mol/L Na3-citrate/0.01 mol/L Tris-HCl, pH 7.4, that comprised the largest forms of plasma vWF (vWF:Ag concentration about 5 U/mL) and contained no measurable protease activity, were used as protease substrate. Dilutions of NHP were used for calibration of the protease assay in patient plasmas. Serine protease inhibitor Pefabloc SC (Boehringer, Mannheim, Germany) was added at 10 mmol/L final concentration to NHP or to patient plasmas, and further dilutions of these plasmas were made with 0.15 mol/L NaCl/0.01 mol/L Tris-HCl, pH 7.4 (TBS) containing 1 mmol/L Pefabloc. The vWF-cleaving protease was activated by a 5-minute incubation of diluted plasmas at 37°C with 10 mmol/L BaCl2 . One hundred microliters of the incubation mixture was added immediately after activation to 50 μL of purified vWF, and the resulting solution was carefully transferred onto a hydrophilic filter membrane (VSWP, 25-mm diameter; Millipore, Bedford, MA) floating on the surface of 50 mL dialysis buffer (1.5 mol/L urea/5 mmol/L Tris-HCl, pH 8.0) in a screw-cap plastic tube. The tube was closed and incubated for 24 hours in a dry oven at 37°C. The incubation mixture was then removed from the membrane and the reaction stopped by addition of 10 μL 0.2 mol/L EDTA, pH 7.4. Multimeric size of vWF was assayed by SDS-electrophoresis in 1.4% SeaKem HGT(P) agarose. After electrotransfer to nitrocellulose, vWF multimers were detected with peroxidase-conjugated rabbit antibodies against human vWF (P0226; Dako) as previously described.18 Analysis of digested vWF substrate that had been reduced with 13 mmol/L dithiothreitol (15 minutes, 60°C) was performed by electrophoresis on SDS-5% polyacrylamide gels according to Laemmli21 followed by electrotransfer to nitrocellulose and detection of the intact vWF subunit and of its proteolytic fragments using a rabbit antiserum against human vWF (A0082; Dako) and alkaline phosphatase-labeled goat antibodies against rabbit IgG (D0487; Dako).
Screening for inhibitors of the vWF-cleaving protease in patient plasmas was performed by measuring protease activity in 1:1 (vol/vol) mixtures of NHP and patient plasma. The presence in patient plasmas of antibodies against the protease was examined by dot-blot analysis using a preparation of vWF-cleaving protease purified from normal human plasma18: 3 μL of undiluted protease solution was applied onto nitrocellulose, overlayed for 4 hours at room temperature with plasma dilutions (1:500 or 1:50), and bound antibodies were detected with alkaline phosphatase-labeled goat-antihuman IgG (AHI0305, dilution 1:500; BioSource, Camarillo, CA).
Patients
Patient A1, a white man born in 1964, was first hospitalized in 1985 because of vomiting and abdominal pain. He was somnolent, slightly icteric, and showed some petechiae on his feet. Coombs-negative hemolytic anemia with a hemoglobin level of 10.8 g/dL (normal range, 13.5 to 16.8 g/dL), a platelet count of 15 × 109/L (normal range, 125 to 320 × 109/L), schistocytes on the peripheral blood smear, a plasma hemoglobin level of 0.035 g/L (normal level, <0.01 g/L), and serum creatinine level of 846 μmol/L (normal range, 59 to 116 μmol/L) led to the diagnosis of HUS/TTP. With hemodialysis and symptomatic treatment the patient fully recovered. Seven months later, after a febrile illness and acetylsalicylic acid intake, he had macrohematuria and a slight increase of serum creatinine level to 120 μmol/L. In 1987, 1988, and May 1989, he had bouts of intravascular hemolysis and thrombocytopenia, preceded by a respiratory infection in one instance and by surgical correction of a nasal septal deviation in another. In October 1989 he had hematuria and renal failure necessitating hemodialysis, and subsequently suffered an ischemic stroke with paresis of his left arm with protracted and only partial recovery. The following four bouts of TTP/HUS in 1990 through 1991 were each controlled with three plasmapheresis sessions using fresh frozen plasma for exchange. After the 10th bout of TTP/HUS in 1992, accompanied by focal epilepsy, two prophylactic plasmapheresis sessions every 4 months were instituted. Because replacement of prophylactic plasmapheresis by infusions of 4 U of fresh frozen plasma every 4 months led to a relapse 9 months later, regular plasmapheresis was resumed. He now suffers from debilitating psychoorganic syndrome with aggressive behavior. His platelet count is permanently below normal and lactate dehydrogenase levels are higher than normal, suggesting ongoing platelet consumption and low-grade microangiopathic hemolysis. Magnetic resonance imaging of the brain in January 1996 showed multiple infarctions in the right hemisphere and a volume reduction of the right temporal lobe.
Patient A2, brother of patient A1, was born in 1975 and was hospitalized at the age of 2 years because of severe hemolytic anemia. In 1992 he suffered from hemolysis and mild renal failure accompanied by abdominal discomfort, nausea and headache, and recovered spontaneously. In 1993 he was diagnosed as having TTP on the basis of hemolytic anemia (hemoglobin level, 11.9 g/dL) with red blood cell fragmentation and thrombocytopenia (platelet count, 20 × 109/L) without renal or neurologic involvement. After three plasmapheresis sessions with exchange by fresh frozen plasma he rapidly recovered. Another bout of microangiopathic hemolytic anemia and pronounced thrombocytopenia again necessitated plasmapheresis in 1994.
The parents (A3, A4) and the sister (A5) of patients A1 and A2 are clinically asymptomatic.
Patient B is a native of Yugoslavia, born in 1958. In April 1992, he was hospitalized with microangiopathic hemolytic anemia and thrombocytopenia. Hemoglobin level was 5.3 g/dL, platelet count 14 × 109/L, serum creatinine level 142 μmol/L, and the blood smear showed ++ fragmentocytes. TTP/HUS was diagnosed, and he was subjected to plasma exchange therapy for 5 days using a total of 12 U of fresh frozen plasma for replacement. Prednisolone (100 mg/d) was also administered. Nine days later, signs of intravascular hemolysis and thrombocytopenia recurred. Five further plasmapheresis sessions using 26 U of fresh frozen plasma were instituted, eventually leading to full recovery. In July 1994, TTP recurred without elevation of serum creatinine. Plasma exchange therapy during 22 days using a total of 174 U of fresh frozen plasma, prednisolone (200 mg intravenously [IV] daily), and three doses of vincristine (totally 5 mg IV) were administered, and complete remission was achieved. In February 1996 a new bout of TTP again necessitated plasma exchange therapy using 155 U of fresh frozen plasma during 8 consecutive days. In addition, the patient received IV corticosteroids and arginine aspartate (4 × 1 g/d orally), and subsequently recovered. The patient's family history is uneventful.
Patient C is a white woman born in 1963 and originating from Portugal. In 1987 during the first trimenon of her first pregnancy she developed headaches that were accompanied by fever, and had speech difficulty. Coombs-negative hemolytic anemia (hemoglobin level, 7.5 g/dL; normal range 12.1 to 15.4 g/dL) with 21% fragmentocytes on the smear, thrombocytopenia (platelet count, 10 × 109/L), lactate dehydrogenase level of 4,790 U/L (normal level, <480 U/L), bilirubin level of 56 μmol/L (normal range, 3 to 26 μmol/L), and proteinuria level up to 2.5 g/L with normal creatinine values led to a diagnosis of TTP. She received daily infusions of up to 12 U of fresh frozen plasma, vitamin E, and high-dose IV methylprednisolone. After 2 weeks, volume overload, arterial hypertension, and pulmonary edema necessitated the institution of plasmapheresis therapy during 8 days with a total of 40 U of fresh frozen plasma exchanged. Prednisone and vitamin E were given, and the further course of pregnancy and delivery in April 1988 was uneventful. From 1990 through 1993, six further episodes of TTP were successfully managed using fresh frozen plasma infusions, steroids, and vitamin E. Hysterectomy was performed in October 1994. The patient suffers from chronically recurring headache and intermittently occurring petechial spots. She was last hospitalized in May 1996 for a severe bout of TTP that necessitated infusions of fresh frozen plasma. The eight siblings of patient C are asymptomatic.
RESULTS
Blood samples for assays of vWF and vWF-cleaving protease were taken on two occasions from five members (A1-A5) of the family A, as well as from patients B and C. The first samples of patients A1 and B, characterized by decreased platelet counts, were obtained at the beginning of a relapse, whereas all other blood samples were taken during clinical remission. High levels of vWF:RCof (range, 1.22 to 2.83 U/mL) and vWF:Ag (range, 0.95 to 1.87 U/mL) were found in the TTP patients (Table 1), as well as in the asymptomatic members of family A. There was no obvious association between the platelet count and the ratio of vWF:RCof/vWF:Ag indirectly representing the relative proportions of the most active large molecular weight forms of vWF. The hemoglobin levels were normal or slightly decreased in the first samples of patient A1 and his asymptomatic mother A4.
Laboratory Data in TTP Patients
| Patient . | Born . | Blood Sample . | Date . | Platelets . | vWF:RCof . | vWF:Ag . | vWF:RCof/Ag . | Hb (g/dL) . |
|---|---|---|---|---|---|---|---|---|
| . | . | . | (mo/d/yr) . | (×10−9/L) . | (U/mL) . | (U/mL) . | . | . |
| A1, propositus, TTP | 1964 | 1 | 1/4/96 | 79 | 2.06 | 1.87 | 1.10 | 12.4 |
| 2 | 4/24/96 | 110 | 1.94 | 1.81 | 1.07 | 13.5 | ||
| A2, brother, TTP | 1975 | 1 | 1/4/96 | 175 | 2.83 | 1.81 | 1.56 | 14.4 |
| 2 | 5/4/96 | 247 | 1.42 | 1.40 | 1.01 | 14.9 | ||
| A3, father, asymptomatic | 1939 | 1 | 1/4/96 | 244 | 1.62 | 1.53 | 1.06 | 14.6 |
| 2 | 4/5/96 | 244 | 1.50 | 1.36 | 1.10 | 16.2 | ||
| A4, mother, asymptomatic | 1941 | 1 | 1/4/96 | 295 | 1.88 | 1.58 | 1.19 | 11.9 |
| 2 | 4/5/96 | 271 | 1.73 | 1.79 | 0.97 | 13.5 | ||
| A5, sister, asymptomatic | 1967 | 1 | 12/27/95 | 265 | 1.26 | 1.27 | 0.99 | 14.5 |
| B, propositus, TTP | 1958 | 1 | 2/20/96 | 45 | 1.67 | 1.76 | 0.95 | 14.0 |
| 2 | 4/24/96 | 278 | 1.22 | 0.95 | 1.28 | 15.0 | ||
| C, proposita, TTP | 1963 | 1 | 3/1/93 | 159 | 1.38 | 1.77 | 0.78 | 13.7 |
| 2 | 4/22/96 | 187 | 1.53 | 1.31 | 1.17 | 13.7 | ||
| Normal range | — | — | — | 125-320 | 0.5-1.5 | 0.5-1.5 | 0.64-1.36* | 13.5-16.8 (M) |
| 12.1-15.4 (F) |
| Patient . | Born . | Blood Sample . | Date . | Platelets . | vWF:RCof . | vWF:Ag . | vWF:RCof/Ag . | Hb (g/dL) . |
|---|---|---|---|---|---|---|---|---|
| . | . | . | (mo/d/yr) . | (×10−9/L) . | (U/mL) . | (U/mL) . | . | . |
| A1, propositus, TTP | 1964 | 1 | 1/4/96 | 79 | 2.06 | 1.87 | 1.10 | 12.4 |
| 2 | 4/24/96 | 110 | 1.94 | 1.81 | 1.07 | 13.5 | ||
| A2, brother, TTP | 1975 | 1 | 1/4/96 | 175 | 2.83 | 1.81 | 1.56 | 14.4 |
| 2 | 5/4/96 | 247 | 1.42 | 1.40 | 1.01 | 14.9 | ||
| A3, father, asymptomatic | 1939 | 1 | 1/4/96 | 244 | 1.62 | 1.53 | 1.06 | 14.6 |
| 2 | 4/5/96 | 244 | 1.50 | 1.36 | 1.10 | 16.2 | ||
| A4, mother, asymptomatic | 1941 | 1 | 1/4/96 | 295 | 1.88 | 1.58 | 1.19 | 11.9 |
| 2 | 4/5/96 | 271 | 1.73 | 1.79 | 0.97 | 13.5 | ||
| A5, sister, asymptomatic | 1967 | 1 | 12/27/95 | 265 | 1.26 | 1.27 | 0.99 | 14.5 |
| B, propositus, TTP | 1958 | 1 | 2/20/96 | 45 | 1.67 | 1.76 | 0.95 | 14.0 |
| 2 | 4/24/96 | 278 | 1.22 | 0.95 | 1.28 | 15.0 | ||
| C, proposita, TTP | 1963 | 1 | 3/1/93 | 159 | 1.38 | 1.77 | 0.78 | 13.7 |
| 2 | 4/22/96 | 187 | 1.53 | 1.31 | 1.17 | 13.7 | ||
| Normal range | — | — | — | 125-320 | 0.5-1.5 | 0.5-1.5 | 0.64-1.36* | 13.5-16.8 (M) |
| 12.1-15.4 (F) |
Abbreviations: M, male; F, female.
As determined from measurements in 145 subjects with normal vWF values.
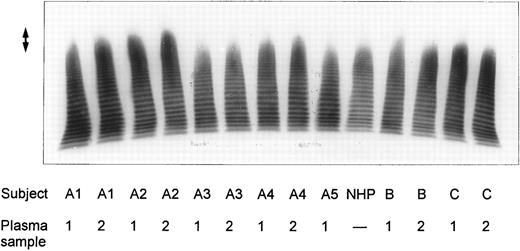
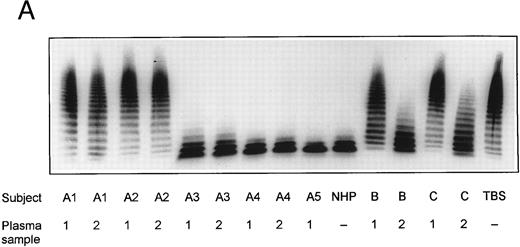
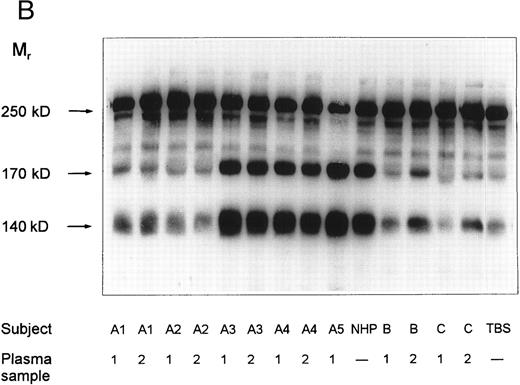
SDS-agarose-electrophoretic analysis of vWF (Fig 1) shows an increased prevalence of unusually large vWF multimers in patients A1 and A2 with chronic relapsing TTP, suggesting that in vivo proteolytic processing of vWF in both patients may be disturbed. The unusually large vWF multimers are increased also in patients B and C, albeit to a minor extent. The degradation of purified vWF by vWF-cleaving protease present in patient or normal plasma is shown in Fig 2A. Our results indicate that the proteolytic activity is completely absent in all four plasma samples of patients A1 and A2: no degradation of vWF substrate was observed, as in the control incubation mixture containing TBS instead of plasma. The asymptomatic parents (A3 and A4) and sister (A5) had an essentially normal vWF-cleaving protease activity. An almost complete lack of vWF-degrading activity was noted in the first samples taken from patients B and C, whereas the activities in the second plasma samples of patients B and C were estimated to be less than 20% and less than 5% to 10% of NHP, respectively, as judged from the calibrating agarose gel shown in Fig 3A.
Multimeric analysis of plasma vWF in SDS-1% agarose gel. Equal amounts of vWF:Ag (0.0025 U vWF:Ag/lane) from four patients with chronic relapsing TTP (A1, A2, B, and C), from asymptomatic family members (A3, A4, and A5) and of pooled NHP were applied on top of the gel and the vWF multimers were detected by immunostaining. The double-sided arrow (↕) denotes the unusually large vWF multimers. Plasma samples from each patient were obtained on two separate occasions at dates given in Table 1.
Multimeric analysis of plasma vWF in SDS-1% agarose gel. Equal amounts of vWF:Ag (0.0025 U vWF:Ag/lane) from four patients with chronic relapsing TTP (A1, A2, B, and C), from asymptomatic family members (A3, A4, and A5) and of pooled NHP were applied on top of the gel and the vWF multimers were detected by immunostaining. The double-sided arrow (↕) denotes the unusually large vWF multimers. Plasma samples from each patient were obtained on two separate occasions at dates given in Table 1.
vWF-cleaving protease activity in plasma from patients with chronic relapsing TTP, from asymptomatic family members and in NHP. Each plasma was diluted 1:20 with TBS/1 mmol/L Pefabloc before incubation with purified vWF. The SDS-1.4% agarose gel (A) shows multimeric patterns of the vWF substrate after incubation with either diluted plasma samples or with Tris-buffered saline (TBS). Analysis of the disulfide-reduced incubation mixtures was performed by SDS-polyacrylamide gel electrophoresis (B). The arrows indicate positions of the intact vWF subunit (250 kD) and of its proteolytic fragments (170 kD and 140 kD).
vWF-cleaving protease activity in plasma from patients with chronic relapsing TTP, from asymptomatic family members and in NHP. Each plasma was diluted 1:20 with TBS/1 mmol/L Pefabloc before incubation with purified vWF. The SDS-1.4% agarose gel (A) shows multimeric patterns of the vWF substrate after incubation with either diluted plasma samples or with Tris-buffered saline (TBS). Analysis of the disulfide-reduced incubation mixtures was performed by SDS-polyacrylamide gel electrophoresis (B). The arrows indicate positions of the intact vWF subunit (250 kD) and of its proteolytic fragments (170 kD and 140 kD).
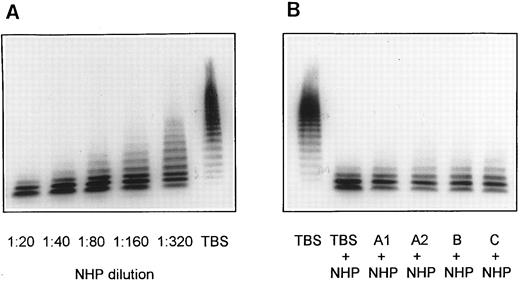
Screening for inhibitors against vWF-cleaving protease in patient plasmas. NHP dilutions 1:20, 1:40, 1:80, 1:160, and 1:320 were used for calibration of the vWF-cleaving protease assay (A). Mixtures of equal volumes of NHP and patient plasmas were diluted with 9 vol of TBS/1 mmol/L Pefabloc and contained, accordingly, a 1:20 dilution each of normal and patient plasma (B).
Screening for inhibitors against vWF-cleaving protease in patient plasmas. NHP dilutions 1:20, 1:40, 1:80, 1:160, and 1:320 were used for calibration of the vWF-cleaving protease assay (A). Mixtures of equal volumes of NHP and patient plasmas were diluted with 9 vol of TBS/1 mmol/L Pefabloc and contained, accordingly, a 1:20 dilution each of normal and patient plasma (B).
SDS-polyacrylamide gel electrophoretic analysis of disulfide-reduced vWF substrate, following incubation with diluted patient plasmas, is shown in Fig 2B. Minor amounts of proteolytic fragments (Mr = 170 and 140 kD) of the vWF subunit (Mr = 250 kD) in the lane denoted as TBS (incubation with Tris-buffered saline) are due to the presence of cleaved subunits in the vWF preparation used as substrate. These fragments were not notably increased after incubation of vWF with plasma samples of patients A1 and A2 or with the first samples of patients B and C. A slight increase in the intensity of the proteolytic fragments was observed in digestion mixtures with the second samples of patients B and C, whereas the vWF subunit was extensively degraded after incubation with NHP or with plasmas A3, A4, and A5. These results adequately reflect the multimeric patterns shown in Fig 2A and confirm that the disappearance of the large multimers is caused by proteolytic degradation of vWF substrate rather than by cleavage of disulfide bridges linking the protomeric subunits of vWF.
In a screening test for the presence of an inhibitor of the vWF-cleaving protease, all four plasmas (first samples of patients A1, A2, B, and C) that had been found to be devoid of vWF-cleaving protease activity were mixed with an equal volume of NHP and subjected to protease assay as described above. The resulting multimeric pattern of the digested vWF is very similar to that obtained with normal plasma diluted 1:2 with TBS (Fig 3B). This observation indicates that the patient plasmas A1, A2, B, and C contain no rapidly acting inhibitor of the vWF-cleaving protease. In additional experiments, all plasma samples of patients with TTP were tested for antibodies directed toward the vWF-cleaving protease by dot-blot analysis. No binding of such antibodies to an immobilized purified vWF-cleaving protease preparation was established at patient plasma dilutions 1:500 or 1:50 using enzyme-labeled goat antibodies against human IgG (data not shown).
DISCUSSION
TTP is a disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, renal dysfunction, fever, and neurologic symptoms. Several groups have attempted to identify the platelet aggregating factor responsible for formation of disseminated platelet thrombi. A 37-kD protein that caused aggregation of normal platelets was found in plasma of patients with acute TTP.22 A cysteine protease shown to induce platelet aggregation was identified in the sera of patients with TTP during the acute phase of the disease.23 Furthermore, unusually large vWF multimers capable of aggregating platelets under fluid shear stress were implicated in the pathogenesis of TTP.15
In the present study, high concentrations of vWF:Ag were found in plasmas of all our patients with chronic relapsing TTP. Increased levels of vWF in TTP patients have also been observed by other investigators.6,7 It has been proposed6,17 that in the course of TTP the injured endothelial cells secrete increased amounts of vWF. Although the SDS-agarose gel electrophoretic analysis showed the presence of unusually large vWF multimers in plasmas from our patients with chronic relapsing TTP, their ratio of vWF:RCof/vWF:Ag was in the normal range. Similar observations were reported by Mannucci et al,7 who described seven patients with unusually large vWF multimers and a mean ratio of vWF:RCof/vWF:Ag of 1.02. The ratio of vWF:RCof/vWF:Ag is apparently not a reliable parameter for documenting the presence of unusually large vWF multimers. It is conceivable that the in vitro agglutination of formalin-fixed platelets by unusually large vWF multimers is not markedly different from that induced by the large vWF multimers found in normal plasma.
Unusually large multimeric forms of vWF were noted in all plasmas from patients A1, A2, and C, whereas they were less prominent in plasma samples from patient B. The first plasma sample of patient B was obtained at the beginning of a relapse. Moake et al6 observed that the extremely large vWF multimers disappeared from plasma of TTP patients during relapse and reappeared during remission. Because the unusually large vWF multimers may be consumed in the process of platelet aggregation, their presence in plasma is not an obligatory finding throughout the entire illness in patients with TTP. Thus, the unusually large vWF forms were detected in only 21 of 86 plasma samples from four patients with drug-associated TTP.24
Infusion of fresh frozen plasma into patients with chronic relapsing TTP was found to be effective in preventing or reversing relapses.25,26 A tentative model for these observations predicted that an activity in fresh frozen plasma and in its cryosupernatant fraction promotes rapid in vivo disappearance of unusually large vWF multimers from the plasma of patients with chronic relapsing TTP.26 It has been postulated that in normal individuals a “depolymerase” is responsible for the conversion of unusually large vWF multimers to smaller polymers normally found in the circulation. This enzyme was proposed to be a protease or a disulfide bond reductase cleaving the interdimeric disulfide bridges, thereby eliminating the unusually large vWF multimers that spontaneously react with platelets and thus lead to formation of platelet thrombi within the microvasculature.6,16,17,26 Since in some patients with chronic relapsing TTP the underlying abnormality was controlled clinically by immunosuppressive therapy, a putative autoantibody directed against the “depolymerase” was proposed to be responsible for the presence of unusually large vWF multimers in plasma and for frequent relapses in these TTP patients.27
A specific vWF-cleaving protease has been recently purified from normal plasma.18,19 The proteolytic degradation of vWF by this protease was enhanced at low ionic strength or in the presence of urea,18 but also following incubation of vWF with guanidinium chloride or under conditions of high fluid shear stress.19 It has been postulated that shear stress of flowing blood modulates the conformation of vWF and enhances its susceptibility to proteolysis.28 The protease involved in vWF degradation was reported to generate fragments of 140 kD and 176 kD28 that were indistinguishable from those found in normal plasma.18 It is conceivable that the peptide bond 842Tyr-843Met in the intact subunit of vWF is protected from proteolytic attack by the specific vWF-cleaving protease, but becomes exposed under conditions affecting the protein conformation, eg, at low ionic strength, in the presence of urea or guanidinium chloride, and — physiologically relevant — at high shear rates occurring in the circulation.
In the present work we investigated whether the presence of unusually large vWF multimers in patients with TTP may be associated with a deficiency of the vWF-cleaving protease. Our results have shown deficiency of the vWF-cleaving protease activity in all four examined patients with chronic relapsing TTP. In family A, two brothers with clinical symptoms of TTP were completely lacking the vWF-cleaving protease activity, whereas in both asymptomatic parents and in the asymptomatic sister virtually normal protease activity was found. These observations tentatively suggest that the deficiency of vWF-cleaving protease in this family may be inherited in an autosomal recessive manner.
In our search for a fast-reacting protease inhibitor, we found no significant depression of the protease activity when normal plasma and patient plasma were mixed at a ratio of 1:1 (vol/vol) and the degradation of vWF substrate by these plasma mixtures was evaluated. Furthermore, dot-blot analysis provided no evidence for the presence of antibodies directed against the vWF-cleaving protease in any of the four patients with chronic relapsing TTP. Our results suggest that these patients have a deficiency of the vWF-cleaving protease activity. An immunologic assay will be required to decide whether this defect is caused by the absence of the protease antigen or by the presence of a functionally abnormal proteolytic enzyme.
In conclusion, our study suggests an impaired or completely lacking activity of the vWF-cleaving protease in four patients with chronic relapsing TTP. We propose that subjects with a deficiency of the vWF-cleaving protease display impaired degradation of the unusually large vWF multimers, which are capable of aggregating platelets in the circulating blood resulting in platelet thrombi characteristic of TTP. We believe that the described assay of the vWF-cleaving protease activity may be useful in the diagnosis of chronic relapsing TTP as well as for establishing a vWF-cleaving protease deficiency as an important risk factor for TTP in the hereditary form of the disease.
ACKNOWLEDGMENT
We are indebted to Jörg Gmür, MD, for allowing us to report on patient C treated at the University Hospital Zürich.
Supported by grants from the Swiss National Science Foundation (Grant No. 3200-37435.93), from the Central Laboratory, Blood Transfusion Service, Swiss Red Cross, and from IMMUNO, Vienna, Austria.
Address reprint requests to Miha Furlan, PhD, Central Hematology Laboratory, University Hospital, Inselspital, CH-3010 Bern, Switzerland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal