To the Editor:
Paroxysmal nocturnal hemoglobinuria (PNH) is due to a somatic mutation of the PIG-A gene occurring in a hematopoietic stem cell. This results in a deficiency of all proteins that are normally attached to the cell membrane via a glycosyl phosphatidylinositol (GPI) anchor.1,2 There have been many reports of PIG-A mutations in patients with PNH3-6 and some reports of patients with more than one coexistent PNH clone.7,8 The majority of PIG-A mutations are small insertions or deletions that result in a frame-shift and early termination of the PIG-A product. In all of these cases there is no active PIG-A protein and thus a complete deficiency of GPI-linked proteins. In a minority of patients with PNH, the PIG-A mutation is a point mutation resulting in an amino acid substitution within the PIG-A protein. In these cases, a normal length PIG-A protein is produced that is either nonfunctional (complete deficiency of GPI-linked proteins; PNH type III cells) or has a markedly reduced level of activity (partial deficiency of GPI-linked proteins; PNH type II cells).9 Thus far, the point mutations resulting in PNH type III cells and PNH type II cells have not been reported in the same PIG-A codons.
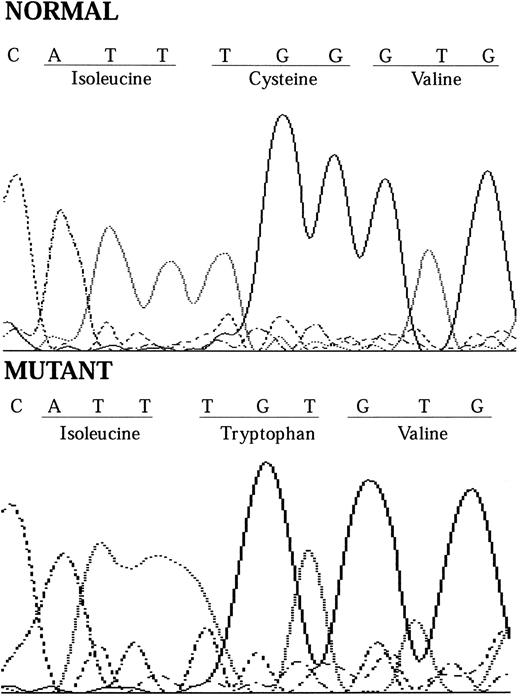
In December 1995, one of our group (P.H.) was a coauthor of an article published in BLOOD reporting the PIG-A mutations in a series of patients with PNH.10 One of these patients (HH12) had a PNH clone with partial deficiency of GPI-linked proteins (PNH type II cells) and was found to have a point mutation at position 548 of the PIG-A gene (TGT to TTT), resulting in a change of the cysteine residue at position 183 to phenylalanine. We now report the sequencing of the PIG-A mutation from a further patient with PNH who has a PNH clone with complete deficiency of GPI-linked proteins (PNH type III cells). He presented in 1988 with severe aplastic anemia and had a partial response to antilymphocyte globulin and cyclosporin A. He maintains adequate blood counts except when his dose of cyclosporin A is reduced. He has had no thrombotic or infectious complications but does have intermittent episodes of hemoglobinuria. Flow cytometric analysis shows that 36% of his red blood cells and 90% of his neutrophils are completely deficient in GPI-linked proteins and are thus derived from his PNH clone. He was found to have a point mutation at position 549 of the PIG-A gene (TGT to TGG) by sequencing of the genomic DNA from his neutrophils (Fig 1), with a resultant change of the cysteine residue at position 183 to tryptophan.
Chromatograms showing how the 1-bp substitution at nt 549 causes the cysteine amino acid residue at codon 183 to change from cysteine to tryptophan.
Chromatograms showing how the 1-bp substitution at nt 549 causes the cysteine amino acid residue at codon 183 to change from cysteine to tryptophan.
Thus, the alteration of the 183 cysteine to phenylalanine results in a PIG-A protein with a partial activity and a PNH type II phenotype, whereas the alteration of the same cysteine to tryptophan results in a PIG-A protein with no detectable function and a PNH type III phenotype. That different substitutions of the same amino acid can give rise to different PNH phenotypes suggests that this amino acid is important in the function or conformation of the PIG-A protein. Cysteine is a hydrophilic amino acid, whereas both phenylalanine and tryptophan are hydrophobic amino acids. Tryptophan is slightly more hydrophobic than phenylalanine and this probably explains why the change to tryptophan results in a type II phenotype and to phenylalanine results in a type III phenotype. Cysteine itself is often associated with the formation of disulfide bonds within a protein and these bonds confer additional stability to specific conformations of the proteins. That the substitution of this amino acid can cause such a marked reduction in the function of the PIG-A molecule and that the hydrophobicity of the resulting amino acid affects the degree of dysfunction suggests a crucial role for this amino acid, either in the structure or the function of the protein. In addition, the fact that this cysteine residue is highly conserved within the yeast and mouse homologues of the PIG-A protein further strengthens the cause for an important role of the 183 cysteine residue in the PIG-A protein.
This is the first description of different point mutations altering the same PIG-A codon to different alternative amino acids and causing a different phenotype of PNH. This finding indicates that the 183 cysteine is important to the function and/or conformation of the PIG-A protein.
ACKNOWLEDGMENT
The authors thank the Annette Fox Leukaemia Research Fund and the Friends of the Leukaemia Unit for their support of this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal