Abstract
CD44 is a widely distributed cell surface glycoprotein whose principal ligand has been identified as hyaluronic acid (HA), a major component of the extracellular matrix (ECM). Recent studies have demonstrated that activation through CD44 leads to induction of effector function in T cells and macrophages. In the current study, we investigated whether HA or monoclonal antibodies (MoAbs) against CD44 would induce a proliferative response in mouse lymphocytes. Spleen cells from normal and nude, but not severe combined immunodeficient mice, exhibited strong proliferative responsiveness to stimulation with soluble HA or anti-CD44 MoAbs. Furthermore, purified B cells, but not T cells, were found to respond to HA. HA was unable to stimulate T cells even in the presence of antigen presenting cells (APC) and was unable to act as a costimulus in the presence of mitogenic or submitogenic concentrations of anti-CD3 MoAbs. In contrast, stimulation of B cells with HA in vitro, led to B-cell differentiation as measured by production of IgM antibodies in addition to increased expression of CD44 and decreased levels of CD45R. The fact that the B cells were responding directly to HA through its binding to CD44 and not to any contaminants or endotoxins was demonstrated by the fact that F(ab)2 fragments of anti-CD44 MoAbs or soluble CD44 fusion proteins could significantly inhibit the HA-induced proliferation of B cells. Also, HA-induced proliferation of B cells was not affected by the addition of polymixin B, and B cells from lipopolysaccharide (LPS)-unresponsive C3H/HeJ strain responded strongly to stimulation with HA. Furthermore, HA, but not chondroitin-sulfate, another major component of the ECM, induced B-cell activation. It was also noted that injection of HA intraperitoneally, triggered splenic B cell proliferation in vivo. Together, the current study demonstrates that interaction between HA and CD44 can regulate murine B-cell effector functions and that such interactions may play a critical role during normal or autoimmune responsiveness of B cells.
CD44 IS A CELL SURFACE glycoprotein expressed by various lymphoid and nonlymphoid tissues.1,2 The CD44 molecule has been demonstrated to participate in lymphocyte adhesion to the matrix, lymph node homing, and lymphopoeisis.1,3 Recent studies have demonstrated that the CD44 molecule may also participate in lymphocyte activation. Studies from our laboratory demonstrated that antibodies against CD44 can trigger the lytic activity of the cytotoxic T lymphocytes (CTL), as well as the double-negative T cells that accumulate in the MRL lpr/lpr mice.4,5 Similarly, monoclonal antibodies (MoAbs) directed against CD44 molecules have been shown to either upregulate6,7 or downregulate8 anti-CD3 and anti-CD2 MoAb-induced proliferation of T cells. Furthermore, certain anti-CD44 MoAbs have also been shown to induce proliferation of resting human T cells in the absence of costimulation with anti-CD3 or anti-CD2 MoAbs.9-11 All of these data together demonstrate that activation via CD44 can trigger effector functions in human T lymphocytes. In addition, antibodies against CD44 have also been shown to activate human monocytes and enhance the natural killer (NK) cell-mediated cytotoxicity.12 13 Despite the recent demonstration that activation via CD44 can trigger effector function in T cells, NK cells, and macrophages, whether a similar activation via CD44 would lead to B-cell growth and differentiation has not been previously investigated.
Recently, hyaluronic acid (HA) has been reported to serve as an important ligand for CD44.14 HA is a major glycosaminoglycan component of the extracellular matrix (ECM). Based on the amino acid sequence deduced from cDNA sequencing, the conserved amino terminal region of CD44 was shown to have sequence similarity to HA binding domains of cartilage link and proteoglycan core proteins.15 Furthermore, it was reported that various cell types form homoaggregates in the presence of HA16 and a recombinant fusion protein of CD44 was shown to bind to HA.17 Having established that HA is the principal ligand for CD44, several recent studies have addressed whether ligation of CD44 via HA would lead to activation of lymphocytes. HA was shown to bind to CD44 on activated T cells and was demonstrated to act as a costimulatory ligand for CD44 in the activation of human T cell effector functions.9 Recently, it was also demonstrated that HA stimulates the growth of CD34+ umbilical cord blood cells to specifically differentiate into mature eosinophils.18
There is also growing evidence to suggest that interaction between HA and CD44 may regulate the functions of lymphoid and myeloid cells and that this interaction may play an important role in a variety of disease conditions. For example, large amounts of soluble CD44 were reported to be present in the sera of patients with advanced gastric and colon cancer.19 Also, at the site of arthritic joints, an inverse relationship between soluble CD44 in the synovial fluid and the number of immigrating blood cells was reported.13
In the current study, we investigated whether interaction between CD44 and HA would trigger the activation of murine lymphocytes, and we made a surprising finding that murine B cells, but not T cells, would respond strongly and directly to stimulation with soluble HA, as well as with MoAbs against CD44. The activated B cells underwent both proliferation and differentiation. Our data suggest that interaction between CD44 and HA may play an important role at sites of inflammation and participate in the regulation of autoimmune response.
MATERIALS AND METHODS
Mice.Adult female C57BL/6 mice, C3H/HeJ, Nude mice (≈8 months of age) and adult severe combined immunodeficient (SCID) mice were purchased from the National Institutes of Health, Bethesda, MD.
Preparation of cells and purification of lymphocytes.Mice were killed and spleens and lymph nodes were removed. Single cell suspension was prepared using a laboratory blender in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 10 mmol/L HEPES, 1 mmol/L glutamine and 50 μg/mL gentamycin as described.5 The FCS had less than 1 ng/mL of lipopolysaccharide (LPS) and was purchased from SUMMIT Biotechnology (Ft. Collins, CO). The red blood cells were lysed using ammonium chloride and the cells were resuspended in complete medium after three washings. To purify the T cells, lymph node cells were passed over nylon wool columns and the nonadherent cells were then treated with anti-Ia antibody for 30 minutes on ice, washed twice, and then treated with complement for 45 minutes at 37°C followed by washing three times.20,21 To purify the B cells, the spleen cells were treated with a combination of anti-CD3, anti-CD4, anti-CD8, anti-αβ T-cell receptor MoAbs for 30 minutes on ice and then treated with complement for 45 minutes at 37°C and washed three times.20 The purity of T- and B-cell preparations was checked by the ability of the cells to respond to specific mitogens as described in the Results.
Antibodies and chemicals.MoAbs were purified from the hybridomas as described recently.4,22 The following MoAbs were used: anti-CD4 (GK 1.5), anti-CD8 (53-6.72), CD44 (KM201), anti-CD3 (145.2C11), and anti-αβ TCR (H57-597). The MoAbs were purified and concentrated from hybridoma culture supernatants or obtained as ascites by growing the hybridomas in nude mice. F(ab′)2 fragments of anti-CD44 MoAbs were prepared by treatment with pepsin and passing the fragments over a protein A column.4 22 The anti-CD45R Abs (B220) used in this study were purchased from Pharmingen (San Diego, CA). HA, chondroitin-sulfate, and polymixin-B were purchased from Sigma Chemical Company (St Louis, MO).
Soluble CD44 fusion protein.The soluble CD44 receptor globulin (CD44 Rg) expressed plasmid was kindly provided by Dr Ivan Stamenkovic (Massachusetts General Hospital, Boston, MA). The CD44 Rg was prepared by transfection of COS cells and was concentrated and purified, as described.17
Proliferation assays.Whole spleen cells, purified T cells, or purified B cells were cultured in triplicate at a concentration of 6 × 105 cells/well, in 96-well, flat bottom tissue culture plates in a final volume of 200 μL of complete medium per well. Medium alone (control), T-cell mitogen (anti-CD3 MoAbs) or B-cell mitogens (LPS [100 μg/mL], anti-IgM antibodies [20 μg/mL]) were added to the wells and the cultures were incubated at 37°C for 48 hours. The cultures were pulsed with 3H-thymidine (2 μCi) 6 hours before harvesting. The cells were harvested using a semiautomated cell harvester (Skatron Inc, Sterling, VA) and the labeled DNA was counted in a liquid scintillation counter.
B-cell differentiation.Splenic B cells (1 × 106) were cultured in 96-well tissue culture plates in the absence or presence of LPS (50 μg/mL), HA (0.5 mg/mL), or anti-CD44 MoAbs (1:2 final dilution) in 0.2 mL of complete medium. After 4 to 5 days of culture, the supernatants were harvested, pooled, and tested for the presence of IgM antibodies using enzyme-linked immunosorbent assay (ELISA).
ELISA.IgM secreted in 4- to 5-day cultures was measured by an ELISA assay as described.21 Briefly, anti-IgM antibody was coated onto the microwells of an ELISA plate, and the wells were blocked with a blocking solution consisting of phosphate-buffered saline (PBS) containing 1% egg albumin. The wells were washed and standard concentrations of purified IgM or a 1:10 dilution of the test supernatant was added to the wells in triplicate. The bound Ig was quantitated by the addition of 1:400 dilution of alkaline phosphatase-conjugated goat antimouse IgM and then developed with p-nitrophenyl phosphate. The optical densities were measured on an ELISA reader at a wavelength of 410 and the concentrations of IgM were calculated by linear regression analysis.21
Studies on expression of CD44 and CD45R on B cells using immunofluorescence analysis.To investigate whether stimulation with HA would lead to increased expression of CD44 and decreased expression of CD45R, spleen cells and B cells enriched from normal C57BL/6 mice were cultured in the presence of medium or 0.1 mg/mL of HA for 24 or 120 hours. The cells were harvested and stained with fluorescein isothiocyanate (FITC)-anti–CD45R or phycoerythrin (PE)-anti–CD44 antibodies for 30 minutes on ice and then washed twice. Next, 10,000 cells were analyzed by a flow cytometer (Epics V, Model 752; Coulter Corp, Miami, FL).
Statistical analysis.The statistical comparison between experimental and control groups was performed using Student's t-test and P < .05 was considered to be significant.
RESULTS
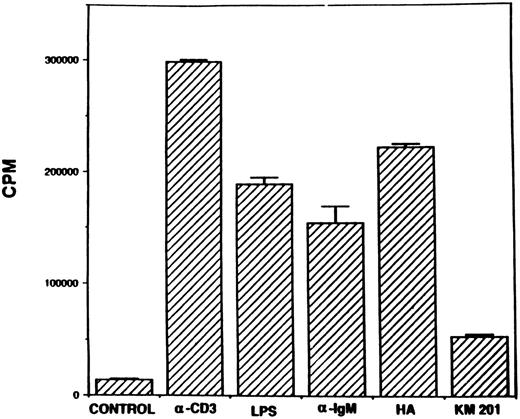
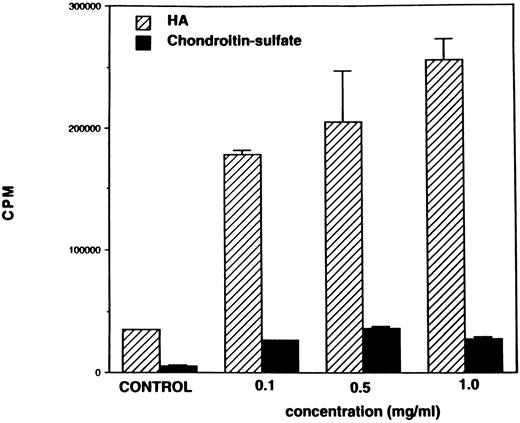
Stimulation with HA or MoAb against CD44 triggers spleen cell proliferation in vitro.Inasmuch as MoAbs against CD44 have been shown to induce human T-cell activation, as well as proliferation,9,10 we first addressed whether MoAbs against murine CD44 or HA would induce proliferation of naive murine spleen cells. In these studies, we used antibodies against CD44 obtained from hybridoma, KM201, because previous studies had demonstrated that these antibodies were specific for the hyaluronate binding site on CD44.3 To this effect, normal spleen cells from C57BL/6 mice were cultured in the presence of T- or B-cell mitogens, HA, or MoAbs against CD44, and the cell proliferation was measured after 48 hours of culture. As shown in Fig 1, stimulation with HA induced strong proliferation of spleen cells comparable to the proliferation induced by the T-and B-cell mitogens. Also, MoAbs against CD44 (KM201) were able to induce significant proliferation of spleen cells, although this response was markedly lower than the response seen with HA or other mitogens. When spleen cells were cultured with varying concentrations of HA, there was a dose-dependent increase in the proliferative responsiveness of spleen cells (Fig 2). Also, the response peaked at 48 hours (data not shown). Thus, in all subsequent studies, 0.5 or 0.1 mg/mL of HA was used and the proliferative response was studied at 48 hours. Interestingly, when similar concentrations of chondroitin-sulfate, another major component of the ECM, were tested, no significant proliferative responsiveness was detected (Fig 2).
Proliferative responsiveness of spleen cells to stimulation with HA and anti-CD44 antibodies. Spleen cells (6 × 105) from normal C57BL/6 mice were cultured in the absence (control) or presence of anti-CD3 (1:200 dilution), LPS (50 μg/mL), anti-IgM (20 μg/mL), HA (0.5 mg/mL), or anti-CD44 MoAbs (KM201, 1:10 final dilution). After 42 hours of culture, the cells were pulsed with 3H-thymidine and 6 hours later, the cells were harvested and 3H-thymidine incorporation in the DNA was measured. The vertical bars represent mean counts per minute (CPM) of triplicate cultures ± standard error (SE)
Proliferative responsiveness of spleen cells to stimulation with HA and anti-CD44 antibodies. Spleen cells (6 × 105) from normal C57BL/6 mice were cultured in the absence (control) or presence of anti-CD3 (1:200 dilution), LPS (50 μg/mL), anti-IgM (20 μg/mL), HA (0.5 mg/mL), or anti-CD44 MoAbs (KM201, 1:10 final dilution). After 42 hours of culture, the cells were pulsed with 3H-thymidine and 6 hours later, the cells were harvested and 3H-thymidine incorporation in the DNA was measured. The vertical bars represent mean counts per minute (CPM) of triplicate cultures ± standard error (SE)
Responsiveness of spleen cells to HA and chondroitin sulfate. Spleen cells (6 × 105) were cultured in the presence of increasing concentrations of HA or chondroitin sulfate (0.1 mg/mL, 0.5 mg/mL, 1.0 mg/mL) or medium alone (control). After 48 hours, cell proliferation was measured as described in the legend to Fig 1.
Responsiveness of spleen cells to HA and chondroitin sulfate. Spleen cells (6 × 105) were cultured in the presence of increasing concentrations of HA or chondroitin sulfate (0.1 mg/mL, 0.5 mg/mL, 1.0 mg/mL) or medium alone (control). After 48 hours, cell proliferation was measured as described in the legend to Fig 1.
T cells fail to respond to stimulation with HA or MoAb against CD44.We next addressed whether the HA or MoAb against CD44 were inducing the proliferation of T cells or B cells found in the spleen cell population. To this effect, T cells were purified by depletion of B cells and tested for the ability to respond to various mitogens as described in Fig 1. The data shown in Fig 3 demonstrated that the T cells responded to stimulation with anti-CD3 MoAbs, but not to LPS, thereby suggesting that the responding population consisted of T lymphocytes and did not have any contaminating B lymphocytes. Interestingly, the T cells were unable to respond to stimulation with HA or KM201 antibodies directed against CD44. It should be noted that the purified T cells consisted of non-B accessory cells such as macrophages and dendritic cells, which were responsible for giving rise to the proliferative response to soluble anti-CD3 MoAbs. To address whether the T cells required the presence of accessory cells such as B lymphocytes to respond to HA, we cultured the purified T cells in the presence of irradiated spleen cells as a source of accessory cells and observed that despite the addition of accessory cells, the splenic T cells were unable to mount a significant response to stimulation with HA or KM201 MoAbs (data not shown).
T cells fail to respond to HA. Purified T cells (B-cell depleted) from normal C57BL/6 mice were cultured in the presence of anti-CD3 (1:200), LPS (50 μg/mL), HA (0.5 mg/mL), or KM201(1:2), or medium alone (control). Cell proliferation was measured as described previously in the legend to Fig 1.
T cells fail to respond to HA. Purified T cells (B-cell depleted) from normal C57BL/6 mice were cultured in the presence of anti-CD3 (1:200), LPS (50 μg/mL), HA (0.5 mg/mL), or KM201(1:2), or medium alone (control). Cell proliferation was measured as described previously in the legend to Fig 1.
Inasmuch as, previous studies with human T cells have demonstrated that anti-CD44 MoAbs could act as a costimulus,6 7 we tested whether HA would act as a costimulus and trigger the proliferation of T cells in the presence of mitogenic or submitogenic concentrations of anti-CD3 MoAbs. The results from these studies suggested that HA was able to enhance the proliferation of T cells only modestly when stimulated with either mitogenic or submitogenic concentrations of anti-CD3 MoAbs (data not shown). Also, HA was unable to augment the proliferation of murine T cells stimulated with phorbol myristate acetate (data not shown). These data together suggested that HA was unable to act as a significant costimulus for murine T cells.
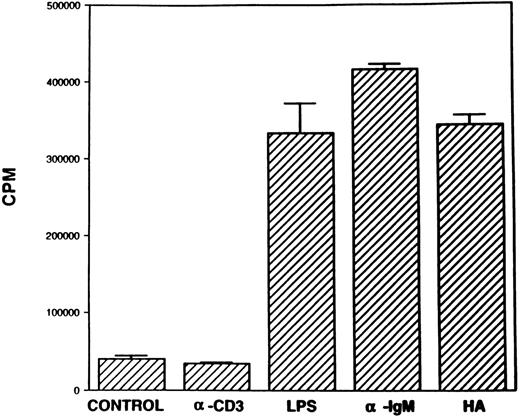
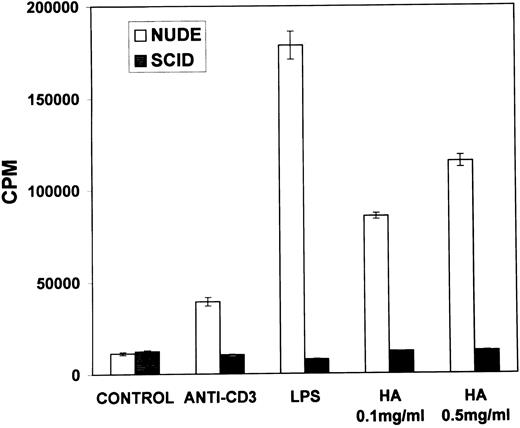
Proliferation of B lymphocytes in response to stimulation with soluble HA.We next addressed whether the B cells found in the spleen cell population were able to respond to HA. To this end, B lymphocytes were purified from the spleen cells by depleting T lymphocytes and were cultured in the presence of either B-cell or T-cell mitogens or with HA. The data shown in Fig 4 indicated that the purified B-cell population responded to B-cell mitogens, but not to the T-cell mitogens, thereby confirming the purity of the B lymphocytes. Furthermore, the B cells responded strongly to stimulation with HA comparable to other B-cell mitogens. These data, therefore, suggested that HA was stimulating the B, but not the T, lymphocytes and inducing proliferation. To further confirm that HA was activating B cells, but not the T cells, similar experiments were performed in nude and SCID mice. The data shown in Fig 5 demonstrated that nude mouse spleen cells responded strongly to stimulation with LPS and weakly to anti-CD3 MoAbs. Furthermore, the nude mouse spleen cells also proliferated strongly in response to stimulation with HA (Fig 5). The weak responsiveness of nude spleen cells when compared with normal spleen cells (Fig 1) to stimulation with anti-CD3 MoAbs may have resulted from a small number of T cells that appear in older nude mice. In contrast, spleen cells from SCID mice failed to respond to both T- and B-cell mitogens, as well as to HA (Fig 5). These data together corroborated that HA was able to induce proliferation of B cells.
Proliferation of B cells in response to stimulation with HA. B cells were purified from the spleen of normal C57BL/6 mice and cultured in the presence of T- or B-cell mitogens or with HA as described in the legend to Fig 3, and cell proliferation was measured.
Proliferation of B cells in response to stimulation with HA. B cells were purified from the spleen of normal C57BL/6 mice and cultured in the presence of T- or B-cell mitogens or with HA as described in the legend to Fig 3, and cell proliferation was measured.
Proliferation of spleen cells from immunodeficient mice in response to stimulation with HA. Spleen cells from nude or SCID mice were cultured in the presence of medium (control), T- or B-cell mitogens or with HA for 48 hours. Spleen cells from SCID mice were cultured in the presence of medium (control), T- or B-cell mitogens or with HA for 48 hours. The cell proliferation was measured as described in the legend to Fig 1.
Proliferation of spleen cells from immunodeficient mice in response to stimulation with HA. Spleen cells from nude or SCID mice were cultured in the presence of medium (control), T- or B-cell mitogens or with HA for 48 hours. Spleen cells from SCID mice were cultured in the presence of medium (control), T- or B-cell mitogens or with HA for 48 hours. The cell proliferation was measured as described in the legend to Fig 1.
HA-induced proliferation of B cells is independent of endotoxins.We also tested whether HA was able to serve as a costimulus to spleen cells cultured with mitogenic or submitogenic concentrations of LPS. The results from these studies showed that addition of HA to spleen cell cultures in the presence of mitogenic or submitogenic concentrations of LPS resulted only in an additive effect and failed to enhance the proliferative responsiveness of the splenic B cells (data not shown). These data also ruled out the possibility that HA may synergistically act with very low levels of endotoxins possibly found in FCS or HA containing medium. To further exclude the role of endotoxin in B-cell proliferation, polymixin B was added to the cultures. It was observed that while polymixin B blocked the LPS-induced B-cell proliferation, it failed to inhibit HA-induced B-cell activation (Table 1). Moreover, spleen cells from LPS-unresponsive C3H/HeJ strain responded strongly to stimulation with HA (Table 1). Together, these data excluded the potential role played by endotoxin in HA-induced B-cell proliferation.
Demonstration That Cells Responding to HA Are B Cells
| Mitogen . | Cell Proliferation (CPM ± SE)* . | |||||
|---|---|---|---|---|---|---|
| . | Experiment 1 . | Experiment 2 . | . | . | ||
| . | Absence of PB . | Presence of PB† . | C57BL/6 . | C3H/HeJ . | . | . |
| None | 3,041 ± 225 | 3,370 ± 865 | 5,552 ± 223 | 10,823 ± 466 | ||
| HA | 92,428 ± 510 | 80,958 ± 3,945 | 80,362 ± 1,809 | 111,222 ± 4,505 | ||
| LPS 1 μg/mL | 137,833 ± 7,909 | 5,283 ± 204 | 100,080 ± 837 | 22,569 ± 1,318 | ||
| LPS 5 μg/mL | 144,597 ± 6,893 | 18,491 ± 2,377 | 115,214 ± 8,048 | 37,718 ± 639 | ||
| LPS 10 μg/mL | 156,766 ± 3,383 | 42,527 ± 5,751 | 124,387 ± 2,694 | 43,423 ± 975 | ||
| Mitogen . | Cell Proliferation (CPM ± SE)* . | |||||
|---|---|---|---|---|---|---|
| . | Experiment 1 . | Experiment 2 . | . | . | ||
| . | Absence of PB . | Presence of PB† . | C57BL/6 . | C3H/HeJ . | . | . |
| None | 3,041 ± 225 | 3,370 ± 865 | 5,552 ± 223 | 10,823 ± 466 | ||
| HA | 92,428 ± 510 | 80,958 ± 3,945 | 80,362 ± 1,809 | 111,222 ± 4,505 | ||
| LPS 1 μg/mL | 137,833 ± 7,909 | 5,283 ± 204 | 100,080 ± 837 | 22,569 ± 1,318 | ||
| LPS 5 μg/mL | 144,597 ± 6,893 | 18,491 ± 2,377 | 115,214 ± 8,048 | 37,718 ± 639 | ||
| LPS 10 μg/mL | 156,766 ± 3,383 | 42,527 ± 5,751 | 124,387 ± 2,694 | 43,423 ± 975 | ||
Abbreviation: PB, polymixin B.
Spleen cells from C57BL/6 mice were cultured with various mitogens for 48 hours and cell proliferation was measured by 3H-Thymidine uptake assay. The results are expressed as mean counts per minute (CPM) ± SE.
PB was used at a concentration of 5 μg/mL.
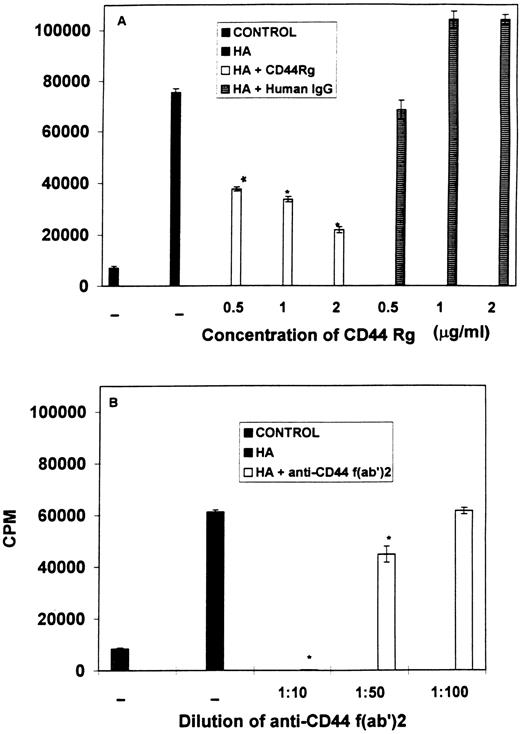
Role of CD44 in B-cell activation induced by HA.To further corroborate that the proliferation of B lymphocytes was triggered directly by HA via its binding to the CD44 molecule and to exclude the possibility of any contaminants being able to induce B-cell proliferation, we investigated the effects of addition of F(ab′)2 fragments of antibodies against CD44 (KM201) or soluble CD44 Rg to block the proliferation of spleen cells stimulated with HA. As seen in Fig 6(A), addition of soluble CD44 fusion protein to the cultures stimulated with HA, led to a dose-dependent inhibition of proliferation triggered by HA. Similar concentrations of human IgG used as a control, failed to cause significant inhibition. Furthermore, similar addition of F(ab′)2 fragments of KM201 MoAbs also caused a dose-dependent inhibition of HA-induced proliferation (Fig 6B). These data conclusively demonstrated that the spleen cells were responding directly to stimulation with HA and, furthermore, that CD44 was serving as the receptor for HA, leading to proliferation of the spleen cells.
Addition of F(ab)2 fragments of anti-CD44 MoAbs or soluble CD44 Rg blocks proliferation of spleen cells stimulated with HA. Spleen cells were incubated with medium (control) or stimulated with HA (A) in the presence of soluble CD44 Rg or human IgG or (B) in the presence of F(ab)2 fragments of anti-CD44 MoAbs alone (diluted 1:10, 1:50, 1:100 of hybridoma supernatants). Cell proliferation was measured as described in the legend to Fig 1. Bars with an asterix indicate statistically significant decrease when compared with the controls.
Addition of F(ab)2 fragments of anti-CD44 MoAbs or soluble CD44 Rg blocks proliferation of spleen cells stimulated with HA. Spleen cells were incubated with medium (control) or stimulated with HA (A) in the presence of soluble CD44 Rg or human IgG or (B) in the presence of F(ab)2 fragments of anti-CD44 MoAbs alone (diluted 1:10, 1:50, 1:100 of hybridoma supernatants). Cell proliferation was measured as described in the legend to Fig 1. Bars with an asterix indicate statistically significant decrease when compared with the controls.
Stimulation with HA leads to B-cell differentiation.We next investigated whether B cells stimulated with HA would undergo differentiation and produce IgM antibodies. To this effect, spleen cells were cultured in the presence of medium alone, LPS, HA, or anti-CD44 MoAbs. After culturing the cells for 4 days, the culture supernatants were harvested and the supernatants were analyzed for the concentration of IgM antibodies using an ELISA assay. The data depicted in Table 2 suggested that stimulation of spleen cells with HA or KM201 MoAbs triggered significant B-cell differentiation and secretion of IgM antibodies.
B-Cell Differentiation Following Stimulation With HA
| Stimulation . | Concentration of IgM (μg/mL)* . |
|---|---|
| Medium | 57.18 ± 13.91 |
| LPS | 160.75 ± 18.04 |
| HA 0.1 mg/mL | 100.85 ± 15.4 |
| HA 0.5 mg/mL | 123.97 ± 29.85 |
| KM201 | 89.50 ± 12.34 |
| Stimulation . | Concentration of IgM (μg/mL)* . |
|---|---|
| Medium | 57.18 ± 13.91 |
| LPS | 160.75 ± 18.04 |
| HA 0.1 mg/mL | 100.85 ± 15.4 |
| HA 0.5 mg/mL | 123.97 ± 29.85 |
| KM201 | 89.50 ± 12.34 |
Spleen cells from C57BL/6 mice were cultured with various mitogens and after 4 days, the culture supernatants were harvested and the concentration of IgM was measured using an ELISA.
It has also been shown that B-cell differentiation induces upregulation of CD44 and downregulation of CD45R.23 To this effect, spleen cells were cultured in the presence of medium alone or HA for 24 hours or 5 days. The cells were harvested and analyzed for the expression of CD44 and CD45R using flow cytometry. The data depicted in Fig 7 suggested that stimulation of spleen cells with HA triggered marked upregulation of CD44 and downregulation of CD45R on day 5 when compared with day 1 of culture, whereas cells cultured in medium alone failed to exhibit such changes (data not shown). These data are consistent with recent studies, which demonstrated that resting B cells that express lower levels of CD44 become strongly positive on activation and retain high level expression for more than eight divisions.23 Secondly, CD45R (B220), which is expressed at high levels on resting and activated B cells, was downregulated on B-cell differentiation,23 thereby suggesting that stimulation with HA induces B-cell differentiation.
Stimulation with HA triggers B-cell differentiation. Splenic B cells or whole spleen cells were cultured in the presence of HA (0.1 mg/mL) for (a) 24 or (b) 120 hours. The cells were harvested and splenic B cells were stained for the expression of CD44 and whole spleen cells were stained for CD45R using flow cytometry. The two histograms were overlayed for comparison. The mean channel number suggestive of the intensity of fluorescence for the histograms was as follows: CD44 (a) = 13.7, CD44 (b) = 85.9; CD45R (a) = 13.9, CD45R (b) = 7.09.
Stimulation with HA triggers B-cell differentiation. Splenic B cells or whole spleen cells were cultured in the presence of HA (0.1 mg/mL) for (a) 24 or (b) 120 hours. The cells were harvested and splenic B cells were stained for the expression of CD44 and whole spleen cells were stained for CD45R using flow cytometry. The two histograms were overlayed for comparison. The mean channel number suggestive of the intensity of fluorescence for the histograms was as follows: CD44 (a) = 13.7, CD44 (b) = 85.9; CD45R (a) = 13.9, CD45R (b) = 7.09.
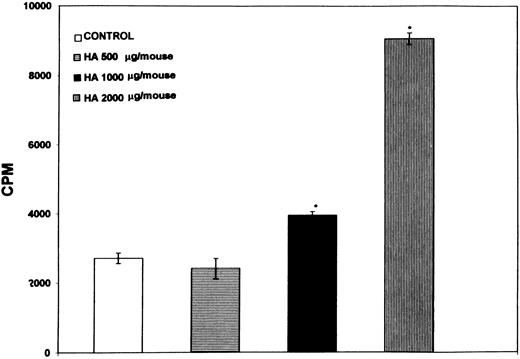
Administration of HA in vivo triggers spleen cell proliferation.Recent studies have demonstrated an increase in soluble CD44 or HA in disease conditions such as autoimmunity or tumor growth.13 19 Therefore, we tested whether administration of HA intraperitoneally would induce proliferation of B lymphocytes in vivo. Normal C57BL/6 mice were immunized with increasing concentrations of HA and 48 hours later, the number of cells undergoing proliferation were quantitated by direct addition of 3H-thymidine to the cultures and measuring the incorporation during the next 6 hours. As shown in Fig 8, injection of increasing concentrations of HA triggered a dose-dependent increase in the spontaneous proliferative responsiveness of spleen cells. Furthermore, by depleting T or B cells, it was observed that the proliferating cells were B cells, but not the T cells (data not shown). These data suggested that administration of HA was able to induce significant proliferation of B lymphocytes in vivo.
Spleen cells exhibit a dose-dependent spontaneous proliferative response to administration of HA in vivo. Normal C57BL/6 mice were injected with increasing concentrations of HA or PBS (control) and 48 hours later, the spleens were harvested. The number of cells undergoing spontaneous proliferation were quantitated by direct addition of 3H-thymidine to the cultures and measuring the incorporation during the next 6 hours. Bars with an asterix denote statistically significant difference when compared with the control.
Spleen cells exhibit a dose-dependent spontaneous proliferative response to administration of HA in vivo. Normal C57BL/6 mice were injected with increasing concentrations of HA or PBS (control) and 48 hours later, the spleens were harvested. The number of cells undergoing spontaneous proliferation were quantitated by direct addition of 3H-thymidine to the cultures and measuring the incorporation during the next 6 hours. Bars with an asterix denote statistically significant difference when compared with the control.
DISCUSSION
In the current study, we demonstrated that MoAbs against CD44 or HA, which serves as an important ligand for CD44, can directly activate B lymphocytes from normal mice. Interestingly, the HA failed to activate T lymphocytes significantly, despite the addition of accessory cells or submitogenic concentrations of anti-CD3 MoAbs. B lymphocytes activated with HA expressed increased levels of CD44, decreased levels of CD45R, and produced IgM antibodies, thereby indicating that they were able to undergo differentiation into antibody-secreting cells. Several experiments demonstrated that the B lymphocytes were directly responding to HA and not to any contaminants found in the culture medium and that the proliferation of B lymphocytes was induced by the interaction between HA and the CD44 molecule. Furthermore, administration of HA intraperitoneally into mice triggered proliferation of B cells in the spleen demonstrating that HA can serve as an important activation signal in vivo.
Several recent studies have demonstrated that the CD44 molecule in addition to participating in lymphocyte homing or lymphocyte adhesion to the ECM, can also participate in signal transduction, thereby regulating the activation of lymphocytes and monocytes.1,4-14 Earlier studies from our laboratory demonstrated that activation of CTLs via CD44 would lead to induction of cytotoxicity of the target cells.4 Furthermore, activation via CD44 could also lead to the induction of cytotoxicity in double-negative (DN) T cells found in autoimmune susceptible MRL lpr/lpr mice.5 We have also shown that the cytotoxicity induced by T cells can be inhibited in the presence of soluble HA, thereby suggesting that HA may serve as an important molecule involved in the target cell recognition by the cytotoxic T lymphocytes (unpublished observation, April 1995). Recent studies have demonstrated that antibodies against CD44 can either induce direct T-cell proliferation and interleukin (IL)-2 production9,10 or that anti-CD44 antibodies can act as a costimulus enhancing the proliferation of T cells stimulated with MoAbs against CD3 or CD2 molecules.6,7 In contrast to the human studies, in the current study, we observed that MoAb against CD44 or HA failed to induce significant T-cell proliferation in the murine model. This observation is in contrast to our earlier finding that MoAbs against CD44 can trigger the lytic machinery of the cytolytic T cells.4,5 These data, therefore, suggest that while stimulation via CD44 can lead to signal transduction and possible granule exocytosis, it is not sufficient to induce IL-2 or other autocrine growth factor production that is essential for T-cell proliferation. It is also possible that naive T cells, which express lower densities of CD44, may not be able to bind to HA,14 which may contribute to its inability to respond to stimulation with HA. In contrast to the murine T cells, the B lymphocytes were able to respond strongly to stimulation with anti-CD44 or HA by proliferation and differentiation. Previous studies from Miyake et al3 have demonstrated that interaction between CD44 on B lymphocyte precursors and an undefined ligand on bone marrow stromal cells is required for B-cell lymphopoeisis in vitro.
HA is one of the major glycosaminoglycans that forms the hydrated carbohydrate gel in which ECM glycoproteins are immersed.24 HA consists of a regular repeating sequence of two nonsulfated monosaccharides, D-glucuronic acid and N-acetyl-D-glucosamine. Unlike the major glycosaminoglycans found in the ECM such as chondroitin sulfate and heparin sulfate, HA is not covalently linked to proteins. Lymphoid cell lines, B-cell hybridomas, and activated B cells have all been shown to bind to purified HA and this binding can be blocked by MoAb against CD44.24 Several recent studies have suggested that the interaction between CD44 and HA may be critical for the differentiation of the progenitor cell population. MoAbs against CD44 have been shown to inhibit B-cell lymphopoeisis in long-term bone marrow cultures.3,25 Furthermore, HA was shown to stimulate the growth of CD34+ selected umbilical cord blood cells into specifically differentiated mature eosinophils.18 Also, HA binding via specific interaction with CD44 was shown to act as a costimulus for human T-cell effector functions9 and HA was also demonstrated to induce signal transduction as measured by intracellular Ca++ mobilization in a murine T-cell lymphoma line.26 These studies together demonstrate that the interaction between HA and CD44 may play a crucial role in the differentiation and activation of a variety of cells. CD44-HA interactions may also be important in thymic differentiation because HA accounts for more than 40% of the total glycosaminoglycan produced by the thymic epithelial cells.27
Previous studies have shown that normal B cells bind to HA to a lower degree than activated B cells, particularly those stimulated with IL-5.14,28 Also, there are strain differences in the functional state of CD44 molecules expressed by unstimulated B cells, which could influence HA binding.28 In the current study, the HA may have directly activated the HA-binding CD44+ B cells or alternatively HA may activate a few contaminating macrophages or T cells found in the purified B-cell population and trigger cytokines,11,12 which in turn can induce increased HA-binding ability of B cells and facilitate B-cell activation. In this context, it was striking to note that B cells cultured with HA expressed marked enhancement in the expression of CD44. Alternatively, in vitro culture of cells could enhance the HA-binding ability, as shown recently with human peripheral blood monocytes.29
Several lines of evidence suggested that the B lymphocytes were responding directly to HA via its binding to the CD44 molecule. The fact that the B cells were not responding to contaminants found in the HA preparation or endotoxin was demonstrated by several experiments. For example, F(ab)2 fragments of CD44 MoAbs or soluble CD44 fusion protein were able to compete with soluble HA and inhibit the proliferation of B lymphocytes. Secondly, the possibility that HA may act synergistically with low levels of endotoxins that may be found in the cultures or that the B cells were responding to the endotoxins was ruled out by demonstrating that HA was not able to act as a costimulus to mitogenic or submitogenic concentrations of LPS. Furthermore, polymixin B failed to inhibit HA-induced B-cell proliferation and B cells from C3H/HeJ mice responded strongly to stimulation with HA. These data together demonstrated that interaction between HA and the CD44 molecule was sufficient to trigger the activation of B lymphocytes.
Recent studies have demonstrated that the interaction between CD44 and HA may play an important role in a variety of disease conditions. For example, serum CD44 levels were reported to be significantly reduced in immunodeficient mice and elevated in tumor bearing mice.30 Furthermore, mice undergoing graft-versus-host reaction, as well as those having autoimmune disease, were shown to exhibit increased levels of CD44 in the serum.30 Serum containing high concentrations of CD44 were able to block the binding of HA to CD44 bearing hybridoma cells.30 Such data demonstrate that substantial quantities of CD44 are released into the circulation and this soluble CD44 may be able to interfere with the interaction between HA and CD44 expressed on lymphomyeloid cells. Increased levels of CD44 has been detected in patients with advanced gastric or colon cancer.17 Furthermore, it has also been demonstrated that in arthritic joints, there is an inverse relationship between soluble CD44 in the synovial fluid and the number of inflammatory blood cells found at that site.31 Such studies, therefore, indicate that interaction between HA and the CD44 molecule may play an important role in a variety of disease conditions. The fact that administration of HA in vivo also leads to activation of B lymphocytes, as seen in the current studies, further confirms the important role played by CD44 and HA. Thus, use of soluble CD44 at localized inflammatory sites may provide important avenues for the manipulation of the immune system in autoimmune diseases.
Supported in part by Grant No. IM#747 from the American Cancer Society (Atlanta, GA) and by Sigma Xi (Research Triangle Park, NC).
Address reprint requests to Prakash S. Nagarkatti, PhD, Department of Biology, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal