Abstract
The dysfunctional protein C from a thrombophilic patient heterozygote for a G1388 to A converting the codon for Arg−1 to His was purified from plasma and characterized. N-terminal amino acid sequence analysis of the light chain of the protein C demonstrated that the dysfunctional protein C is elongated with one amino acid, namely the mutated His. This finding is compatible with disruption by the mutated His of the original basic propeptidase recognition sequence (Arg−5-Ile-Arg-Lys-Arg−1), resulting in a shift of the cleavage site to a new position, Lys−2-His−1, which follows an alternative basic amino acid propeptidase recognition sequence (Arg−5-Ile-Arg-Lys−2). Because the mutation affects the propeptide that directs the γ-carboxylation converting Glu to Gla residues in the Gla domain, it was investigated whether the mutation impaired this reaction. Gla fragment obtained by cleavage of the dysfunctional protein C light chain with endoproteinase Asp-N was isolated by reverse-phase high-performance liquid chromatography, methylated, and subjected to N-terminal sequence analysis. The methylation step enabled the positive identification of Gla residues as well as the determination of the relative amount of Gla and Glu residues at each of the nine γ-carboxylation sites of the Gla domain. The analysis showed that all nine potential γ-carboxylation sites of the dysfunctional protein C were normally carboxylated. This result is compatible with the notion that position −1 is not a part of the recognition element for the γ-carboxylase. In conclusion, evidence is provided showing that the mutation leads to aberrant propeptide processing and secretion of dysfunctional normally carboxylated protein C extended with the mutated His.
PROTEIN C IN blood plasma is the precursor of the anticoagulant serine proteinase activated protein C that plays a role in maintaining the fluidity of blood and limiting the hemostatic coagulation process after tissue injury.1,2 Conversion of protein C to its active form takes place on the endothelial cell surface and is catalyzed by thrombin complexed with the membrane receptor thrombomodulin. Activated protein C exerts its anticoagulant function by proteolytic cleavage of the activated coagulation cofactors Va and VIIIa. The significance of the anticoagulant function of protein C is evident from the fact that individuals heterozygotic for protein C deficiency have an increased risk of developing venous thromboembolic disease in early adulthood, whereas homozygotes may develop severe thrombotic disease in the neonatal period.2,3 At least 160 different mutations have been identified in the protein C gene as the putative cause of protein C deficiency.4
Protein C belongs to the family of liver-synthesized plasma glycoproteins involved in blood coagulation that require vitamin K for their complete posttranslational modification.5 6 The other proteins are prothrombin, coagulation factors VII, IX and X, and protein S. The vitamin K–dependent coagulation proteins are composed of a series of modules, two of which are unique for this family of proteins and exhibit marked sequence homology. One is composed of about 20 amino acid residues (24 in protein C), a long propeptide in the leader sequence that governs the vitamin K–dependent posttranslational modification step. The other is composed of about 40 amino acid residues, a long adjacent module that is the target of the vitamin K–dependent posttranslational modification and forms the N-terminal Gla domain of the mature protein. This domain is pivotal for a Ca2+-dependent binding of the vitamin K–dependent coagulation proteins to phospholipid membranes where they exert their biological function.
During the posttranslational modification of the Gla domain 9 to 12 (9 in protein C), glutamic acid residues undergo γ-carboxylation, forming γ-carboxyglutamic acid (Gla) residues.7,8 This reaction is catalyzed by a vitamin K–dependent γ-glutamyl carboxylase, the action of which is directed by a conserved carboxylase recognition site in the N-terminal portion of the propeptide. The Gla residues ligate Ca2+ in the core of the Gla domain, resulting in a highly ordered structure in which the N-terminus is buried.9-11 This results in the exposure of hydrophobic amino acid side chains, which are important for membrane binding.9,12 13
After completion of γ-carboxylation, the propeptides are cleaved off to yield the mature proteins.14-16 This reaction is catalyzed by one or more subtilisinlike propeptidases that are similar or identical to those that cleave other secretory proproteins,17,18 such as pro-von Willebrand factor and proalbumin.19,20 They preferentially cleave peptide bonds that generally follow an Arg-Xxx-Lys/Arg-Arg recognition sequence. Naturally occurring missense mutations affecting the codons for the basic amino acids of the propeptidase recognition sequence of factor IX (Arg−4 to Gln/Leu/Trp, Lys−2 to Asn, Arg−1 to Ser),21 protein C (Arg−1 to Cys/His/Ser),4,22 and protein S (Arg−1 to His)23 all result in secretion of gene products to the blood with severely reduced functions. Some of the mutated proteins have been purified, and the molecular basis of their dysfunction has been characterized. The Arg−4 to Gln and Arg−1 to Ser mutations in the factor IX gene abolish propeptidase cleavage, resulting in the secretion of factor IX with retained full-length propeptide.24-27 Controversial data exist as to whether the defective propeptide processing is associated with impaired carboxylation.24,26,28 Recently it was reported that the naturally occurring Arg−1 to Ser mutation in the propeptide sequence of the protein C gene leads to the secretion of a dysfunctional molecule elongated in the N-terminus with the mutated Ser.22 Furthermore, the data suggested that γ-carboxylation was unaffected by the mutation. The gene products resulting from the two other mutations (Arg to Cys and Arg to His) identified at codon −1 of the protein C gene have been characterized.29-31 The data suggested that the dysfunctional proteins were insufficiently γ-carboxylated and that the Arg to Cys mutation resulted in elongation of the N-terminus of the abnormal protein with at least the mutated Cys.
The aim of the present work was to characterize the molecular basis of dysfunctional protein C purified from a patient with the Arg−1 to His mutation in the protein C gene. It is demonstrated that the abnormal protein is elongated with the mutated His and that all nine Glu residues of the Gla domain are normally γ-carboxylated.
MATERIALS AND METHODS
Case report.The study was conducted in accordance with the Helsinki Declaration of 1975 as revised in 1983 and approved by the Regional Ethical Committee.
The patient is a protein C–deficient man born in 1936. He was the only family member available for investigation. From the age of 25 years he has experienced several spontaneous attacks of phlebography-verified deep vein thrombosis affecting the calf, popliteal, and femoral veins on the right side and the calf, popliteal, femoral, and iliac veins on the left side. In the same period he has experienced several spontaneous attacks of superficial vein thrombosis on both crura. He has no concurrent disease. The mother of the patient, born in 1899, has no history of thromboembolic disease. The father of the patient died at 70 years of age, and no clinical information on him is available. The data documenting the plasma protein C–deficient state of the patient are presented in Results. The patient also was analyzed for other hemostatic risk factors for venous thromboembolism. He was found to be heterozygous for the G1691 to A (Leiden) mutation in the factor V gene known to be associated with resistance to activated protein C.2 Other parameters were within the normal range. They included the plasma concentrations of the anticoagulant proteins antithrombin and protein S (total and free), fibrinogen, plasminogen and plasminogen activator inhibitor 1, and the thrombin time and the activated partial thromboplastin time.
Collection and storage of blood samples.Blood for analysis of hemostatic parameters and DNA was collected as previously described.32 Blood for purification of protein C was collected from the patient or blood donors in Baxter CDP OPTIPAC (Baxter S.A., La Châtre, France) according to the manufacturer's instructions. Plasma was separated within 30 minutes by centrifugation at 22°C and 4,300 g for 7 minutes. It was stored at −80°C until purification of protein C.
Gene analysis.Isolation of genomic DNA from peripheral blood cells was performed as described.32
Polymerase chain reaction (PCR) of genomic DNA and analysis of PCR-amplified protein C and factor V DNA segments.All nine exons and splice junctions in the protein C gene were amplified by PCR, and the nucleotide sequences of the amplified products were determined as described.32,33 The presence of a G to A mutation at nucleotide 1691 in the factor V gene, which predicts an amino acid substitution in the factor V molecule (Arg506 to Gln), was determined as described.34
Assay of protein C and other biochemical assays.The plasma concentrations of protein C(coagulation) and protein C(enzymatic) were analyzed by Staclot Protein C and Stachrom Protein C, respectively (both Diagnostica Stago, Asnières, France). The concentration of protein C (immunological) in plasma or in purified samples was analyzed by an enzyme-linked immunosorbent assay as previously reported32 or by the same assay after substitution of the two different monoclonal antibodies by rabbit immunoglobulin G (IgG) (A 370) as capture antibody and horseradish peroxidase–conjugated rabbit IgG (P 374) as detecting antibody (both from DAKO, Glostrup, Denmark). The same results were obtained with monoclonal and rabbit antibodies, when both mutant and normal protein C were analyzed. Activated partial thromboplastin time, thrombin time, and the concentrations of coagulation factors II + VII + X (functional), total and free protein S, antithrombin, fibrinogen, plasminogen, and plasminogen activator inhibitor 1 were analyzed as described.32 35
Agarose gel electrophoresis and Western blotting.Analysis of protein C in plasma by agarose gel electrophoresis at pH 8.6 in the presence of 1.9 mmol/L CaCl2 followed by Western blotting was performed as described.32 Blotted protein C bands were visualized by using rabbit immunoglobulins against human protein C (A 370) and peroxidase-conjugated swine immunoglobulins against rabbit immunoglobulins (P 217) (both from DAKO).
Adsorption of plasma protein C on barium citrate.Protein C in plasma samples was adsorbed on barium citrate and eluted with a solution containing 0.2 mol/L EDTA as described by Suzuki et al,36 except for scale down of the procedure to a start volume of 2 mL plasma and the use of 2.5 mmol/L Pefabloc SC (Pentapharm Ltd., Basel, Switzerland) instead of 0.1 mmol/L diisopropyl fluorophosphate.
Protein purification.Protein C was purified from plasma by anion exchange chromatography at pH 7.5 using 0.4 mol/L NaCl for elution followed by adsorption to and elution from BaSO4 by 0.1 mol/L Na citrate, pH 5.8, and a second anion exchange chromatography at pH 6.5 using a linear gradient from 0.18 to 0.50 mol/L NaCl. The procedure was that described by Comp et al,37 except for a scale down of the start volume to 200 to 400 mL plasma and the use of Q Sepharose FF (Pharmacia, Uppsala, Sweden) instead of quaternary aminoethyl Sephadex Q-50. The fractions from the second anion exchange chromatography containing protein C were subjected to reverse-phase high-performance liquid chromatography (RP-HPLC) on a 2.1 × 150 mm C-4 column (214TP5215, Vydac, Hesperia, CA) eluted at 50°C at 0.2 mL/min with a 40-minute gradient from 30% to 50% of solvent B (0.1% trifluoroacetic acid in acetonitrile) in solvent A (0.1% trifluoroacetic acid in water) using a 1090 HPLC apparatus equipped with a diode array detector (Hewlett-Packard, Waldbronn, Germany). Absorbances at 214 and 280 nm were monitored. The purified protein C was reduced and the Cys residues derivatized using iodoacetamide as described by Charbonneau,38 and the heavy and light chains were separated by HPLC as described above.
Endoproteinase Asp-N cleavage of protein C light chain.The isolated light chains were dried and redissolved in 200 μL 10% acetonitrile in 40 mmol/L sodium phosphate, pH 8.0. Endoproteinase Asp-N (0.2 μg sequence grade from Boehringer Mannheim, Mannheim, Germany) was added, and the mixture was incubated for 18 hours at 37°C. The resulting fragments were isolated by RP-HPLC on a 2.1 × 150 mm C-8 column (208TP5215, Vydac) eluted in 45 minutes with a gradient from 5% to 50% B in A using the conditions described above.
N-terminal amino acid sequence analysis.The N-terminal amino acid sequences of 5 to 20 pmol aliquots of the purified peptides were determined using an automatic protein sequencer (Procise model 494A; Perkin Elmer, Norwalk, CT). All chemicals (except R4) and solvents were sequence grade or HPLC grade supplied by Perkin Elmer. The HPLC solvents were A3 (premix in 3.5% tetrahydro-furan) and B2 (acetonitrile/methanol).
Characterization of extent of γ-carboxylation of Glu residues.The extent of γ-carboxylation was determined by amino acid sequence analysis of N-terminal Gla fragments obtained by cleavage of the protein C light chain with endoproteinase Asp-N. Gla residues are not detected during standard amino acid sequence analysis procedures due to their hydrophilic character. To circumvent this problem, samples were methylated before sequence analysis.39 The methylation step enabled the positive identification of Gla residues as the more hydrophobic dimethylesters of PTH-Gla as well as the determination of the relative amount of PTH-Gla and PTH-Glu (both methylated) at each of the nine γ-carboxylation sites of the Gla domain. Methylation was performed by incubation of the dried samples for 2 hours under argon in 50 μL of 2 mol/L HCI in methanol. Furthermore, the ordinary R4 (25% trifluoroacetic acid) was substituted with 1 mol/L HCl in methanol. The solutions of HCl in methanol was produced by cooling 40 mL dry methanol to −80°C, then 3.4 mL or 6.8 mL (to produce 1 mol/L and 2 mol/L HCl, respectively) acethylchloride was added dropwise in an ice bath under constant agitation. The methylated PTH-Gla derivatives eluted just after PTH-Pro, whereas the methylated PTH-Asp and PTH-Glu derivatives eluted around 1 minute later than PTH-Ala and just after PTH-Tyr, respectively. The relative amounts of Gla and Glu were determined from the change in absorbance at 269 nm (ΔmAU) of the methylated PTH-Gla and PTH-Glu derivatives assuming that they had similar extinction coefficients.39 This assumption appears valid because the molar extinction coefficients of the standard amino acids except for Pro, Trp, and Lys are within a relatively narrow range (14,900 to 17,200).40
Data on Plasma Starting Material and Protein C Recovery During Purification of Normal and Patient Protein C by Anion Exchange Chromatography and BaSO4 Adsorption
| Material . | Patient . | Normal . | ||
|---|---|---|---|---|
| . | Portion 1 . | Portion 2 . | Portion 1 . | Portion 2 . |
| Plasma | ||||
| Volume (mL) | 230 | 260 | 200 | 400 |
| Protein C(immunological) concentration (arbitrary U/L) | 1.11 | 1.13 | 1.10 | 0.82 |
| Recovery of protein C(immunological) (%) | ||||
| First anion exchange chromatography step, eluate | 75 | 76 | 53 | 68 |
| BaSO4 eluate | 56 | 91 | 98 | 65 |
| Second anion exchange chromatography step, eluate | 126 | 57 | 76 | 85 |
| Material . | Patient . | Normal . | ||
|---|---|---|---|---|
| . | Portion 1 . | Portion 2 . | Portion 1 . | Portion 2 . |
| Plasma | ||||
| Volume (mL) | 230 | 260 | 200 | 400 |
| Protein C(immunological) concentration (arbitrary U/L) | 1.11 | 1.13 | 1.10 | 0.82 |
| Recovery of protein C(immunological) (%) | ||||
| First anion exchange chromatography step, eluate | 75 | 76 | 53 | 68 |
| BaSO4 eluate | 56 | 91 | 98 | 65 |
| Second anion exchange chromatography step, eluate | 126 | 57 | 76 | 85 |
Protein C was purified as described in Materials and Methods by anion exchange chromatography at pH 7.5 using 0.4 mol/L NaCl for elution followed by adsorption to and elution from BaSO4 by 0.1 mol/L Na citrate, pH 5.8, and a second anion exchange chromatography at pH 6.5 using a linear gradient from 0.18 to 0.50 mol/L NaCl. Each recovery value refers to the total amount of protein C(immunological) obtained from the previous purification step. One arbitrary unit of protein C(immunological) corresponds to the average amount of protein C in 1 L normal plasma.
Mass spectrometry.The proteolytic fragments were analyzed by matrix-assisted laser desorption mass spectrometry in a Biflex time-of-flight instrument (Bruker-Franzen, Bremen, Germany) in the linear mode. A proportion of the samples were dried and dissolved in 5 μL 0.1% trifluoroacetic acid in 30% acetonitrile, 0.5 μL aliquots of the dissolved samples were mixed with 0.5 μL 33 mmol/L α-cyano hydroxy cinnamic acid in acetonitrile/methanol (Hewlett Packard, Rockville, MD), and 0.5 μL of the mixture was applied to the target.
RESULTS
Identification of the mutation in the protein C gene.Nucleotide sequence analysis of exon III plus the adjacent splice junctions of the protein C gene showed that the patient is heterozygotic for a G to A transition at position 1388, converting the codon −1 for Arg (CGT) to His (CAT) at the propeptide cleavage site (not shown). No abnormalities could be demonstrated when the remaining exons plus splice junctions of the protein C gene were sequenced.
Characterization of mutated protein C in plasma.The concentration of protein C(coagulation) in plasma of the patient was reduced to 0.53 and 0.58 arbitrary units/L when measured at two occasions (95% reference interval, 0.70 to 1.30 arbitrary units/L), whereas the immunological and enzymatic concentrations of protein C were within normal ranges. These results are compatible with a type II protein C deficiency and confirm those of Gandrille et al,29 who investigated a family with the same Arg−1 to His mutation. Agarose gel electrophoresis followed by Western blotting showed that protein C in the plasma of the patient migrated as a single homogeneous band with the same mobility as protein C in plasma from normal subjects (data not shown), indicating that the dysfunctional protein C molecule does not exhibit gross abnormalities. When protein C in patient plasma was adsorbed on barium citrate and eluted with 0.2 mol/L EDTA, the recoveries of protein C(immunological) were 95% to 101% (n = 4). No protein C could be detected in the supernatant after the adsorption step. This demonstrated that both the normal and the dysfunctional protein C can adsorb to barium citrate and indicated that γ-carboxylation of the abnormal molecule is not affected or is only partly affected.
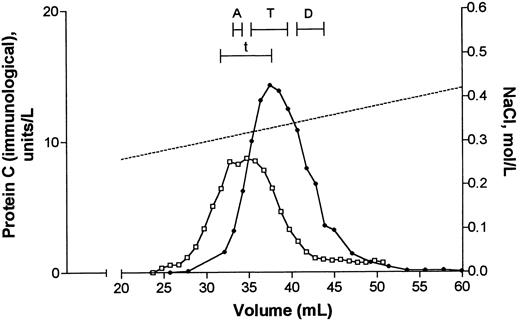
Purification of protein C.Protein C was purified from two portions of both patient plasma and normal plasma as described in Materials and Methods. Data on plasma starting material and recoveries of protein C(immunological) after the first anion exchange chromatography step, the barium sulphate adsorption/elution step, and the second anion exchange chromatography step are listed in Table 1. During the latter step the column was developed with a linear gradient from 0.18 to 0.5 mol/L NaCl. Figure 1 shows that protein C(immunological) from patient and normal plasma was eluted as a single peak with the same shape and at about the same molar concentrations of NaCl (approximately 0.32 mol/L).
Second anion exchange chromatography step during purification of protein C from plasma. Barium citrate eluate originating from 260 mL patient plasma (•) or 200 mL normal plasma (□) was applied to a column with 4 mL Q Sepharose FF. Elution of proteins was performed with a linear gradient from 0.18 to 0.50 mol/L NaCl (dotted line). Fractions (1 mL) were collected and analyzed for protein C(immunological). Fractions pooled and subjected to further purification by RP-HPLC (see Fig 2) are indicated by bars and denoted A, T, and D (patient) and t (normal subject). A quantity of 1 U/L of protein C(immunological) corresponds to the mean concentration in normal plasma.
Second anion exchange chromatography step during purification of protein C from plasma. Barium citrate eluate originating from 260 mL patient plasma (•) or 200 mL normal plasma (□) was applied to a column with 4 mL Q Sepharose FF. Elution of proteins was performed with a linear gradient from 0.18 to 0.50 mol/L NaCl (dotted line). Fractions (1 mL) were collected and analyzed for protein C(immunological). Fractions pooled and subjected to further purification by RP-HPLC (see Fig 2) are indicated by bars and denoted A, T, and D (patient) and t (normal subject). A quantity of 1 U/L of protein C(immunological) corresponds to the mean concentration in normal plasma.
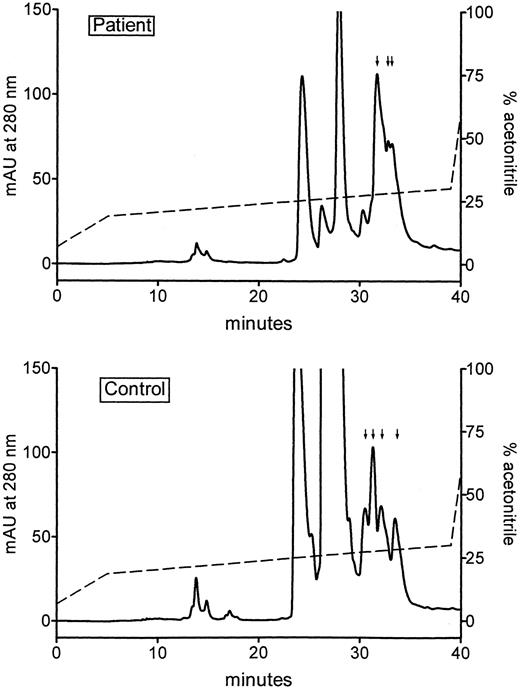
Protein C fractions eluted from the second anion exchange chromatography step were pooled as shown in Fig 1. Three pools were prepared of the protein C fractions from patients, corresponding to the ascending (A), top (T), and descending parts (D) of the protein C peak. Only one pool was prepared of the protein C fractions from normal subjects, corresponding to the top part (t) of the protein C peak. Subsequently protein C in each pool was further purified by RP-HPLC on a C-4 column, eluted with a gradient of acetonitrile. Analysis of each fraction showed that protein C(immunological) in all pools eluted at almost the same position. This is shown in Fig 2 for pools t and T from the normal subject and the patient, respectively. Figure 2 also shows that the elution profiles of the two pools measured at 280 nm, were comparable. This was also the case for the 214-nm profiles (not shown).
RP-HPLC analysis of pooled fractions from the Q Sepharose FF chromatography step (see Fig 1). The pooled top fractions, T and t, from the patient sample (upper panel) and the normal sample (lower panel), respectively, were applied to a C-4 column, eluted with a linear gradient of acetonitrile (dotted line), and the effluent monitored as the absorbance at 214 nm (data not shown) and 280 nm. Fractions were collected and analyzed for protein C(immunological). The fractions containing protein C (indicated by vertical arrows) were pooled and used for further studies.
RP-HPLC analysis of pooled fractions from the Q Sepharose FF chromatography step (see Fig 1). The pooled top fractions, T and t, from the patient sample (upper panel) and the normal sample (lower panel), respectively, were applied to a C-4 column, eluted with a linear gradient of acetonitrile (dotted line), and the effluent monitored as the absorbance at 214 nm (data not shown) and 280 nm. Fractions were collected and analyzed for protein C(immunological). The fractions containing protein C (indicated by vertical arrows) were pooled and used for further studies.
Determination of the N-terminal amino acid sequence of the mutated protein C.Amino acid sequence analysis of the RP-HPLC–purified protein C from pool t of the normal subject showed two sequences. They were identical with the N-terminal of protein C light and heavy chain, respectively.41 42 Analysis of the RP-HPLC–purified protein C from pools A, T, and D from the patient showed four sequences, two of which were the same as those found in the protein C from the normal subject. The third sequence started with a His and was followed by the normal sequence of the protein C light chain. The amounts of the normal and the His-extended light chains were each about half of the amount of the heavy chain, indicating that the patient plasma contained about equal amounts of normal protein C and mutated protein C elongated with the mutated His. The fourth sequence was compatible with the propeptide sequence of protein C starting at Thr−24, corresponding to a nonprocessed protein C variant with an intact propeptide attached. The relative amount of the fourth sequence compared with the normal light chain was 5% to 15% when the yields corresponding to cycle number 4 in the sequence analysis were compared. The low amount of the nonprocessed light chain prohibited further characterization of this variant. Subsequently, only protein C derived from pool T of the patient was further characterized.
For this purpose RP-HPLC–purified protein C from the patient was reduced and carboxyamidomethylated. This was followed by separation of the light and the heavy chains by HPLC on a reverse-phase C-8 column (not shown). Amino acid sequence analysis of the first 10 residues of the protein C light chain showed two parallel sequences in about equal amounts. One sequence was that of the light chain of normal protein C, whereas the other started with a His residue and was followed by the sequence of the light chain of normal protein C (Table 2).
N-Terminal Sequence Analysis of Protein C Light Chains Derived From Patient Plasma
| Cycle . | Normal Form . | Mutant Form . | ||
|---|---|---|---|---|
| . | Amino Acid . | Yield (pmol) . | Amino Acid . | Yield (pmol) . |
| 1 | Ala | 9.8 | His | 14.4 |
| 2 | Asn | 9.7 | Ala | 10.4 |
| 3 | Ser | 11.1 | Asn | 9.7 |
| 4 | Phe | 8.2 | Ser | 9.4 |
| 5 | Leu | 9.0 | Phe | 8.2 |
| 6 | (Gla)* | Leu | 9.5 | |
| 7 | (Gla)* | (Gla)* | ||
| 8 | Leu | 9.0 | (Gla)* | |
| 9 | Arg | 11.0 | Leu | 8.5 |
| 10 | His | 4.4 | Arg | 10.0 |
| Cycle . | Normal Form . | Mutant Form . | ||
|---|---|---|---|---|
| . | Amino Acid . | Yield (pmol) . | Amino Acid . | Yield (pmol) . |
| 1 | Ala | 9.8 | His | 14.4 |
| 2 | Asn | 9.7 | Ala | 10.4 |
| 3 | Ser | 11.1 | Asn | 9.7 |
| 4 | Phe | 8.2 | Ser | 9.4 |
| 5 | Leu | 9.0 | Phe | 8.2 |
| 6 | (Gla)* | Leu | 9.5 | |
| 7 | (Gla)* | (Gla)* | ||
| 8 | Leu | 9.0 | (Gla)* | |
| 9 | Arg | 11.0 | Leu | 8.5 |
| 10 | His | 4.4 | Arg | 10.0 |
Purified protein C from patient plasma was reduced and carboxyamidomethylated and the resulting heavy and light chains were separated by RP-HPLC. An aliquot of the fraction containing the light chain(s) was analyzed by sequence analysis of the first 10 residues. Two parallel sequences were seen in equal amounts as judged from the similar yields of amino acids obtained at comparable residue number positions. One sequence was identical with the normal protein C light chain and the other was the same but N-terminal extended with His. Values given are the yields obtained in the individual cycle corrected for background levels and lag in the following cycle.
No PTH-Glu derivatives could be detected at these residues, indicating that these sites were γ-carboxylated.
Characterization of Gla content of the mutated protein C.To investigate whether the Arg−1 to His mutation affected γ-carboxylation of protein C, the following approach was used. First, the protein C light-chain preparation from the patient was subjected to digestion with endoproteinase Asp-N. Then normal and mutated N-terminal Gla domain fragments containing all nine potential γ-carboxylation sites were partially separated from each other and completely separated from irrelevant fragments by RP-HPLC. Finally, the relative content of Gla and Glu residues at each of the nine γ-carboxylation sites in the partially separated N-terminal Gla domain fragments was estimated by amino acid sequence analysis. In order to detect both Gla and Glu derivatives in the sequencer, fragments were methylated before sequence analysis.
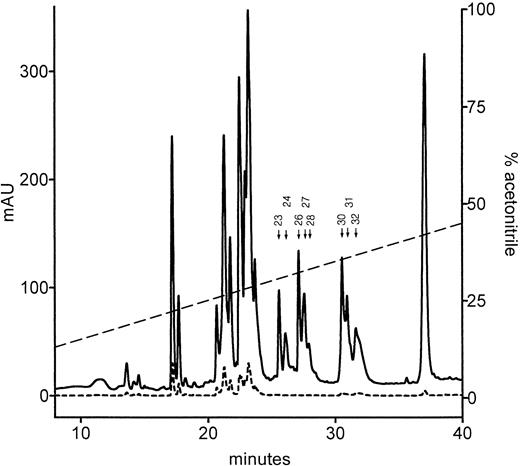
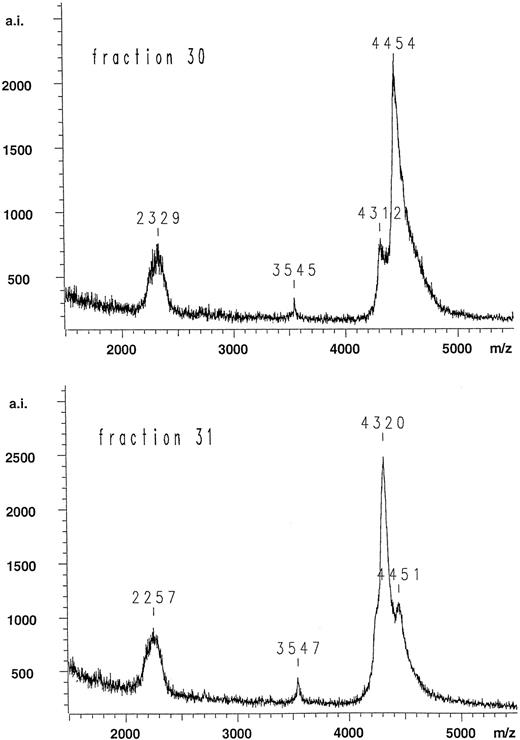
Figure 3 shows the HPLC elution profile of the fragments generated by endoproteinase Asp-N digestion of the light-chain preparation from the patient and separated on a reverse-phase C-4 column. The putative N-terminal Gla domain fragments of interest absorb at 214 nm, but not at 280 nm, because they do not contain Trp and Tyr residues. Eight fractions (23, 24, 26-28, and 30-32) fulfilled this criterion (Fig 3). All these fractions were subjected to mass spectrometry, and the resulting data were compared with the calculated molecular masses of all possible endopeptidase Asp-N cleaved fragments of the normal and mutated protein C light chain. The mass spectra of fractions 23 and 24 showed clear peaks corresponding to 2,699 and 2,700 D, respectively. Both values fit the calculated molecular mass of 2,698 D of the amino acid sequence composed of residues 48 to 70 that is located C-terminal to the Gla domain. Furthermore, the mass spectra of fractions 26, 27, and 28 showed clear peaks corresponding to 3,541, 3,542, and 3,544 D, respectively. All three values fit the calculated molecular mass of 3,538 D of the amino acid sequence composed of residues 48 to 78, including hydroxylated Asp at position 71.43 Elution at two or three positions of apparently identical peptides might be due to partial deamidation of Gln 49 (fraction 23 and 24) as well as a partial rearrangement of the susceptible Asp 71-Gly 72 bond to iso Asp-Gly (fractions 26, 27, and 28).44 These possibilities were not further investigated. No molecular mass could be obtained for fraction 32, and it contained only a low amount of sequenceable protein C light-chain N-terminus (most likely due to tailing from fraction 31) and was not investigated further. Mass spectra of fractions 30 and 31 both showed unusually broad peaks, indicating heterogeneity (Fig 4). Both contained two major components, with practically the same centroids having the m/z values 4,312/4,320 and 4,454/4,451, respectively. The proportions were very different, however, with the larger component dominant in fraction 30 and the smaller component dominant in fraction 31. Table 3 summarizes the range of possible molecular masses of the Gla domain depending on cleavage point of the endoproteinase Asp-N, inclusion of N-terminal His and degree of carboxylation. It is suggestive that the differences in molecular masses between the two components, 142/131 D, is close to the difference due to an extra His residue (137 D). This fits the results from the sequence analysis showing predominance of the mutated His-containing form in fraction 30 and predominance of the normal form in fraction 31. The peaks of fractions 30 and 31 span the ranges corresponding to 4,250 to 4,800 D and 4,200 to 4,600 D, respectively. The explanation of this heterogeneity could be a high degree of decarboxylation in the mass spectrometer of the labile Gla residues. Initial experiments with the Gla domain of prothrombin showed the same nature of mass spectra whether or not the samples were methylated before mass spectrometric analysis (data not shown). Subsequent amino acid sequence analysis of methylated samples verified the identity of these fragments. The ratio between the mutated and the normal N-terminal Gla domain sequence was 3:1 in fraction 30 and 1:2 in fraction 31 (data not shown) when determined from the yields of amino acids obtained at comparable residue number positions in cycles 1 to 6 (compare Table 2). Figure 5 shows an example of identification by RP-HPLC of the methyl esters of PTH-Glu and PTH-Gla corresponding to cycles 5 to 9 during sequencing of the Gla domain fragments in fraction 30. Table 4 summarizes the data on assessment of the Gla and Glu contents at each of the nine γ-carboxylation sites of the mutated and normal Gla domain fragments in fractions 30 and 31. It is noticed that the relative amount of Gla [median (range)] was 96% (88% to 100%) in fraction 30 (predominantly containing mutated Gla domain) and 91% (83% to 97%) in fraction 31 (predominantly containing normal Gla domain). Collectively, it is concluded from the data of Table 4 that there is no significant difference in the relative Gla content of the mutant and normal Gla domains.
RP-HPLC purification of Gla domain fragments resulting from endoproteinase Asp-N digestion of the protein C light chain originating from plasma of the patient. The peptides were eluted with a linear gradient of acetonitrile, and the effluent was monitored as the absorbance at 214 nm (full line) and 280 nm (broken line). The putative N-terminal Gla fragment(s) of interest absorb at 214 nm, but not at 280 nm, because they are free of Trp and Tyr residues. Peaks fulfilling this criteria are indicated by arrows and numbers. Mass spectra and sequence analysis of each of these fractions showed that the N-terminal Gla domain fragments of the light chain are present in fractions 30 and 31 (see Table 4 and Fig 4).
RP-HPLC purification of Gla domain fragments resulting from endoproteinase Asp-N digestion of the protein C light chain originating from plasma of the patient. The peptides were eluted with a linear gradient of acetonitrile, and the effluent was monitored as the absorbance at 214 nm (full line) and 280 nm (broken line). The putative N-terminal Gla fragment(s) of interest absorb at 214 nm, but not at 280 nm, because they are free of Trp and Tyr residues. Peaks fulfilling this criteria are indicated by arrows and numbers. Mass spectra and sequence analysis of each of these fractions showed that the N-terminal Gla domain fragments of the light chain are present in fractions 30 and 31 (see Table 4 and Fig 4).
Mass spectra of fractions 30 and 31 obtained after digestion of the patient sample protein C light chain with endoproteinase Asp-N (see Fig 3). The explanation of the values around m/z = 4,400 are discussed in the text. The peaks m/z = 2,329 and 2,257 are most likely the doubly charged species. There is no obvious explanation of the peaks with m/z = 3,545 and 3,547. m/z denotes ratio between mass of the molecular ion and charge, whereas a.i. denotes number of accumulated ions.
Mass spectra of fractions 30 and 31 obtained after digestion of the patient sample protein C light chain with endoproteinase Asp-N (see Fig 3). The explanation of the values around m/z = 4,400 are discussed in the text. The peaks m/z = 2,329 and 2,257 are most likely the doubly charged species. There is no obvious explanation of the peaks with m/z = 3,545 and 3,547. m/z denotes ratio between mass of the molecular ion and charge, whereas a.i. denotes number of accumulated ions.
Expected Molecular Masses of Gla-Domain Fragments Obtained by Endoproteinase Asp-N Digestion of Protein C Light Chain
| N-Terminal Amino Acid . | Gla Residues . | C-Terminal Amino Acid . | |
|---|---|---|---|
| . | . | Val34 . | Asp35 . |
| Ala1 | Intact | 4,569 | 4,684 |
| Decarboxylated | 4,173 | 4,288 | |
| His−1 | Intact | 4,706 | 4,821 |
| Decarboxylated | 4,310 | 4,425 | |
| N-Terminal Amino Acid . | Gla Residues . | C-Terminal Amino Acid . | |
|---|---|---|---|
| . | . | Val34 . | Asp35 . |
| Ala1 | Intact | 4,569 | 4,684 |
| Decarboxylated | 4,173 | 4,288 | |
| His−1 | Intact | 4,706 | 4,821 |
| Decarboxylated | 4,310 | 4,425 | |
RP-HPLC chromatograms showing an example of the identification of methylesters of PTH-Glu and PTH-Gla in the N-terminal Gla domain fragments of protein C originating from the patient (fraction 30 in Fig 3). The fragments were subjected to N-terminal sequence analysis after methylation. This enabled the specific detection of Gla and Glu residues and subsequent estimation of the relative amounts of the two residues at potential γ-carboxylation sites (see Table 4). Results from cycles 5 to 9 are shown. Two parallel sequences were identified corresponding to residues 5 to 9 (Leu-Gla-Gla-Leu-Arg) and residues 4 to 8 (Phe-Leu-Gla-Gla-Leu) of the normal and the His extended variant of protein C, respectively. Arrows at the bottom of the figure show the position of the identified residues. X-axis values are minutes and y-axis values are milli absorbance units at 269 nm.
RP-HPLC chromatograms showing an example of the identification of methylesters of PTH-Glu and PTH-Gla in the N-terminal Gla domain fragments of protein C originating from the patient (fraction 30 in Fig 3). The fragments were subjected to N-terminal sequence analysis after methylation. This enabled the specific detection of Gla and Glu residues and subsequent estimation of the relative amounts of the two residues at potential γ-carboxylation sites (see Table 4). Results from cycles 5 to 9 are shown. Two parallel sequences were identified corresponding to residues 5 to 9 (Leu-Gla-Gla-Leu-Arg) and residues 4 to 8 (Phe-Leu-Gla-Gla-Leu) of the normal and the His extended variant of protein C, respectively. Arrows at the bottom of the figure show the position of the identified residues. X-axis values are minutes and y-axis values are milli absorbance units at 269 nm.
Assessment of the Amounts of Gla and Glu Residues at Each of the Potential γ-Carboxylation Sites in the Gla Domain of Protein C From the Patient
| Cycle . | Gla(Glu)-residue . | Fraction 30 [mutant:normal (3:1)] . | Fraction 31 [mutant:normal (1:2)] . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Mutant . | Normal . | Glu . | Gla . | Relative amount of Gla (%) . | Glu . | Gla . | Relative amount of Gla (%) . |
| . | . | . | (Δ mAU) . | (Δ mAU) . | . | (Δ mAU) . | (Δ mAU) . | . |
| 6 | — | 6 | 0.00 | 1.10 | 100 | 0.06 | 2.21 | 97 |
| 7 | 6 | 7 | 0.07 | 4.33 | 98 | 0.13 | 2.74 | 95 |
| 8 | 7 | — | 0.11 | 3.66 | 97 | 0.08 | 1.06 | 93 |
| 14 | — | 14 | 0.02 | 0.29 | 94 | 0.08 | 0.63 | 89 |
| 15 | 14 | — | 0.05 | 1.25 | 96 | 0.06 | 0.53 | 90 |
| 16 | — | 16 | 0.01 | 0.62 | 98 | 0.04 | 0.72 | 95 |
| 17 | 16 | — | 0.09 | 1.51 | 94 | 0.11 | 0.77 | 88 |
| 19 | — | 19 | 0.00 | 0.43 | 100 | 0.07 | 0.67 | 91 |
| 20 | 19 | 20 | 0.09 | 1.25 | 93 | 0.07 | 0.84 | 92 |
| 21 | 20 | — | 0.05 | 1.30 | 96 | 0.04 | 0.50 | 93 |
| 25 | — | 25 | −0.01 | 0.10 | 100 | 0.03 | 0.24 | 89 |
| 26 | 25 | 26 | 0.06 | 0.43 | 88 | 0.11 | 0.53 | 83 |
| 27 | 26 | — | 0.05 | 0.96 | 95 | 0.04 | 0.34 | 89 |
| 29 | — | 29 | 0.01 | 0.34 | 97 | 0.03 | 0.36 | 92 |
| 30 | 29 | — | 0.06 | 1.01 | 94 | 0.03 | 0.24 | 89 |
| Cycle . | Gla(Glu)-residue . | Fraction 30 [mutant:normal (3:1)] . | Fraction 31 [mutant:normal (1:2)] . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Mutant . | Normal . | Glu . | Gla . | Relative amount of Gla (%) . | Glu . | Gla . | Relative amount of Gla (%) . |
| . | . | . | (Δ mAU) . | (Δ mAU) . | . | (Δ mAU) . | (Δ mAU) . | . |
| 6 | — | 6 | 0.00 | 1.10 | 100 | 0.06 | 2.21 | 97 |
| 7 | 6 | 7 | 0.07 | 4.33 | 98 | 0.13 | 2.74 | 95 |
| 8 | 7 | — | 0.11 | 3.66 | 97 | 0.08 | 1.06 | 93 |
| 14 | — | 14 | 0.02 | 0.29 | 94 | 0.08 | 0.63 | 89 |
| 15 | 14 | — | 0.05 | 1.25 | 96 | 0.06 | 0.53 | 90 |
| 16 | — | 16 | 0.01 | 0.62 | 98 | 0.04 | 0.72 | 95 |
| 17 | 16 | — | 0.09 | 1.51 | 94 | 0.11 | 0.77 | 88 |
| 19 | — | 19 | 0.00 | 0.43 | 100 | 0.07 | 0.67 | 91 |
| 20 | 19 | 20 | 0.09 | 1.25 | 93 | 0.07 | 0.84 | 92 |
| 21 | 20 | — | 0.05 | 1.30 | 96 | 0.04 | 0.50 | 93 |
| 25 | — | 25 | −0.01 | 0.10 | 100 | 0.03 | 0.24 | 89 |
| 26 | 25 | 26 | 0.06 | 0.43 | 88 | 0.11 | 0.53 | 83 |
| 27 | 26 | — | 0.05 | 0.96 | 95 | 0.04 | 0.34 | 89 |
| 29 | — | 29 | 0.01 | 0.34 | 97 | 0.03 | 0.36 | 92 |
| 30 | 29 | — | 0.06 | 1.01 | 94 | 0.03 | 0.24 | 89 |
Fractions 30 and 31 (see Fig 3) were methylated and subjected to N-terminal sequence analysis. The ratio between the mutant and normal N-terminal sequence was 3:1 in fraction 30 and 1:2 in fraction 31, as estimated from the yields of amino acids in the first six cycles (compare with Table 2, where equal amounts of the two forms were determined). Methylation of the samples allowed the positive identification of Gla residues (see Fig 5). The amounts of Gla and Glu at each potential γ-carboxylation site are expressed as the change in absorbance at 269 nm of methylated PTH-Gla and PTH-Glu derivatives. Assuming that the two PTH derivatives have comparable extinction coefficients, the relative amounts of Gla were calculated as Δ mAUGla/(Δ mAUGla + Δ mAUGlu). For further details, see Materials and Methods. The relative amount of Gla [median (range)] was 96% (88-100%) in fraction 30 and 91% (83-97%) in fraction 31. It is concluded from the data that there is no significant difference in the relative Gla content of the mutant and normal Gla-domains.
DISCUSSION
In the present study, we have characterized the molecular basis of dysfunctional protein C with no apparent anticoagulant activity, which was purified from plasma of a thrombophilic patient who is heterozygotic for a G1,388 to A mutation. This mutation converts the codon for Arg−1 (CGT) to His (CAT) in the propeptidase recognition sequence of the protein C. N-terminal amino acid sequence analysis demonstrated that the dysfunctional protein C is elongated with one amino acid, namely the mutated His. This finding is compatible with disruption by the mutated His of the original basic propeptidase recognition sequence (Arg−5-Ile-Arg-Lys-Arg−1) resulting in a shift of the cleavage site to a new position, Lys-2-His-1, that follows an alternative basic amino acid propeptidase recognition sequence (Arg−5-Ile-Arg-Lys−2). The elongation of the dysfunctional protein C with only one amino acid is consistent with the observation that the N-terminal protein C light chain from patients with the Arg−1 to His mutation and that from normal subjects have indistinguishable molecular masses when reduced samples are analyzed by sodium dodecylsulfate polyacrylamide electrophoresis followed by immunoblotting.29 Analogous to our data, Miyata et al22 demonstrated that a naturally occurring C1,387 to A mutation — converting the codon for Arg−1 to Ser — resulted in secretion to the blood of a dysfunctional protein C elongated with the mutated Ser. A third mutation converting Arg−1 to Cys was reported to give rise to a mutated protein C extended with at least the mutated Cys.29,30 This was based on the observation that the mutation was associated with a high molecular mass form of protein C in plasma. Upon reduction, this high molecular mass species disappeared, indicating that a disulfide bond linking Cys−1 to Cys in another plasma protein was broken. Naturally occurring missense mutations in the propeptide sequence of factor IX at position −4 converting Arg to Gln and at position −1 converting Arg to Ser completely abolish propeptidase cleavage, resulting in secretion of dysfunctional factor IX with retained full-length propeptide (18 amino acids long).24-27 Similarly, it has been reported that substitution of Arg by Ser at position −1 in human factor VII complementary DNA results in secretion of a dysfunctional recombinant factor VII with retained full-length propeptide (17 amino acids long) when studied in vitro in a mammalian cell expression system (baby hamster kidney cells).45 In the patient sample we found minor amounts of nonprocessed protein C with an intact propeptide attached. This nonprocessed protein C variant could not be identified in the normal subject sample. However, due to the larger signal-to-noise ratio in the sequence analysis, we cannot exclude the presence of minor amounts of nonprocessed protein C in the normal subject sample. The completely abolished propeptide cleavage of profactors IX and VII mutated at positions −1 (and −4) as opposed to the alternative propeptide cleavage of proprotein C mutated at position −1 can be explained by the fact that profactors IX and VII, unlike proprotein C, lack an alternative basic propeptidase recognition sequence in their propeptides (see Fig 5 in Miyata et al22 ).
Because the mutation reported here affects the propeptide that directs the γ-carboxylation converting Glu to Gla residues,7,8 it was of interest to investigate whether the mutation impaired this reaction. It was demonstrated that at each of the nine γ-carboxylation sites the mutant Gla domain was normally γ-carboxylated (Fig 5 and Table 4), which is compatible with the notion that position −1 is not a part of the recognition element for the γ-carboxylase.28,46,47 Gaussem et al31 studied Ca2+ binding and immunoreactivity of protein C in plasma from patients with the Arg−1 to His mutation. Immunoreactivity studies were performed with a monoclonal antibody that reacted with a specific sequence in the Gla domain in the absence of Ca2+ but poorly in the presence of Ca2+. They demonstrated that the mutant protein C had decreased Ca2+ affinity and that its binding to the monoclonal antibody was poorly inhibited by Ca2+. They interpreted their results as being due to impaired γ-carboxylation of the mutant protein. However, their findings also could be explained by an incomplete Ca2+-dependent folding of the Gla domain due to the additional His. Our data on γ-carboxylation agrees with those of Miyata et al22 on dysfunctional protein C elongated with mutated Ser at position −1. Amino acid sequence analysis showed no PTH-Glu derivatives at the 3 N-terminal γ-carboxylation sites (positions 7, 8, and 15), indirectly indicating that these sites were fully γ-carboxylated in the mutant protein C. They proposed that the Glu residues at the residual six γ-carboxylation sites were also normally γ-carboxylated. Their suggestion was based on the observation that their mutant protein C in analogy with the His−1 protein C mutant described here was precipitated by BaCl2 and eluted at the same concentration of NaCl as normal protein C when purified by ion-exchange chromatography. Controversial data exist on the extent of γ-carboxylation of factor IX with amino acid substitutions at positions −4 (Arg to Gln) and −1 (Arg to Ser).24-28
The N-terminal single amino acid extension of protein C mutated at position −1 (our data and those of Miyata et al22 ) and the full-length propeptide extension of factor IX or VII mutated at position −4 or −124,25,27,45 can fully explain the loss of anticoagulant and coagulant activity, respectively, of the mutated proteins. Structural studies of prothrombin and factor IX have shown that the Ca2+-dependent transition of the Gla domain to the fully stabilized and phospholipid-binding conformation is a two-step process.10-12,48 The first transition can be induced by Ca2+ as well as other divalent metal ions (such as Mg2+ and Mn2+), whereas the second transition is Ca2+-specific and is associated with burial of the N-terminus in the folded Gla domain structure. Given the similarity of the structures of the Gla domains of the vitamin K–dependent proteins, it is likely that the N-terminal amino acid extensions of the protein C, factor IX, and factor VII mutants mentioned above prevent the second transition. This is compatible with the observation that acetylation of the N-terminal Ala in prothrombin blocks the second transition.10 Furthermore, it is compatible with the observation that fully γ-carboxylated recombinant wild-type factor IX with retained propeptide is biologically inactive and binds Gla domain–specific monoclonal antibodies that recognizes the conformation induced by the first but not the second transition.49 When the propeptide is cleaved off, factor IX becomes fully active, and its Gla domain binds monoclonal antibodies that recognize the conformation induced by the second but not the first transition.
In summary we demonstrate that a G to A mutation in the protein C gene converting the Arg−1 codon to His results in a shift in the propeptidase-cleavage site to Lys−2-His−1 and secretion of a dysfunctional protein C that is N-terminal extended with the mutated His. γ-Carboxylation is not affected by the mutation.
ACKNOWLEDGMENT
We thank Allan Kastrup, Birgitte Lillethorup, and Berit Madsen for their excellent technical assistance.
Supported by grants from the Faculty of Health Sciences at University of Copenhagen, the Danish Medical Research Foundation, and the Novo Foundation.
Address reprint requests to Bent Lind, MD, Section for Hemostasis & Thrombosis, Department of Clinical Biochemistry KB 3-01-1, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal