Abstract
Recent studies have shown that plasma thrombopoietin (TPO) levels appear to be directly regulated by platelet mass and that removal of plasma TPO by platelets via binding to the c-Mpl receptor is involved in the clearance of TPO in rodents. To help elucidate the role of platelets in the clearance of TPO in humans, we studied the in vitro specific binding of recombinant human TPO (rhTPO) to human platelet-rich plasma (PRP), washed platelets (WP), and cloned c-Mpl. Using a four-parameter fit and/or Scatchard analysis, the approximate affinity of rhTPO for its receptor, which was calculated from multiple experiments using different PRP preparations, was between 128 and 846 pmol/L, with ∼25 to 224 receptors per platelet. WP preparations gave an affinity of 260 to 540 pmol/L, with ∼25 to 35 receptors per platelet, and erythropoietin failed to compete with 125I-rhTPO for binding to WP. Binding and dissociation studies conducted with a BiaCore apparatus yielded an affinity of 350 pmol/L for rhTPO binding to cloned c-Mpl receptors. The ability of PRP to bind and degrade 125I-rhTPO was both time- and temperature-dependent and was blocked by the addition of excess cold rhTPO. Analysis of platelet pellets by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that 125I-rhTPO was degraded into a major fragment of ∼45 to 50 kD. When 125I-rhTPO was incubated with a platelet homogenate at pH = 7.4, a degradation pattern similar to intact platelets was observed. Together, these data show that human platelets specifically bind rhTPO with high affinity, internalize, and then degrade the rhTPO.
THE PRESENCE OF thrombopoietic and megakaryopoietic activities in the plasma and serum of thrombocytopenic animals and patients has been well documented during the last 4 decades.1-3 Initially, it was speculated that these activities were due to thrombocytopenic-induced upregulation of a megakaryocyte lineage specific growth factor.1-3 After the isolation and cloning of thrombopoietin (TPO) and its receptor (c-Mpl), it is now clear that most, if not all, of these previously described activities can be attributed to TPO.4-8 Recently, using TPO-specific enzyme-linked immunosorbent assays (ELISAs), circulating levels of human TPO have been determined in plasma from cancer patients undergoing radiation and chemotherapy. In these patients, an inverse correlation between platelet number and plasma TPO levels has been observed.9 10 Given that TPO is likely to be the physiologic regulator of platelet production, these observations support the notion that TPO levels become elevated as a compensatory mechanism for thrombocytopenia.
In contrast to the regulation of erythropoietin (EPO), circulating levels of TPO are not modulated by changes in TPO gene expression. Alternatively, it has been proposed that platelet mass directly regulates TPO levels by sequestering plasma TPO.11,12 This was initially proposed by Kuter and Rosenberg,12 who showed that platelet transfusion into thrombocytopenic mice immediately reduced plasma TPO levels in these mice. They speculated that the reduction in TPO levels occurred via binding of TPO to c-Mpl on platelets. In support of this hypothesis, we have recently shown that TPO mRNA levels in the kidney and liver of c-Mpl–deficient mice are not elevated compared with control mice, although their plasma TPO levels are elevated ∼10-fold.13 Platelets from normal mice were able to bind and degrade TPO, whereas platelets from c-Mpl–deficient mice could not.14 Furthermore, when c-Mpl–deficient mice received a platelet transfusion, their TPO levels immediately decreased to normal levels.14 In the present study, the interaction of TPO with c-Mpl on human platelets is further characterized. Our data show that recombinant human TPO (rhTPO) binds to c-Mpl with high affinity and suggests that c-Mpl mediates the internalization and degradation of rhTPO by human platelets.
MATERIALS AND METHODS
Binding of 125I-rhTPO by human platelet-rich plasma (PRP).Full-length CHO-derived rhTPO was iodinated by the indirect iodogen method,5 15 purified by size-exclusion chromatography, and formulated in 10 mmol/L Tris, 0.15 mol/L NaCl, 0.01% Tween 20, pH 7.4 (TNT buffer). The specific activity of the radiolabeled material ranged from approximately 50 to 90 μCi/μg protein and approximately 40 to 80 pg per tube (total TPO) was used for each experiment. 125I-rhTPO prepared by this method generally retains ∼77% of its bioactivity, as determined by a receptor based ELISA (data not shown). Human PRP (obtained from Biological Specialty Corp, Colmar, PA) was prepared from citrated plasma collected from normal subjects and shipped overnight to the investigators. The PRPs were used approximately 24 hours after their preparation. Several separate PRP preparations were used for these experiments and the concentration of platelets ranged between 400 and 1,000 × 109/L. However, each separate experiment was performed using a single PRP preparation. Some binding experiments were performed on PRP prepared from Genentech employees and used within 3 hours of being drawn. Informed consent was obtained from all blood donors (both Biological Specialty Corp and Genentech donors). Each experiment presented in this study (except the BiaCore data) has been repeated at least once using a separate PRP and 125I-rhTPO preparation.
To determine the binding kinetics of TPO for platelets, 0.18 mL of PRP was incubated with 125I-rhTPO with varying amounts of cold rhTPO for 30 minutes at 37°C, washed with 0.75 mL buffer (4°C), and centrifuged at 14,000 rpm (Eppendorf Model 5415C; Eppendorf, Hamburg, Germany), and the platelet-associated radioactivity was determined. The amount of radioactivity in the platelets was used to determine receptor affinity using a four-parameter curve fit (KaleidaGraph; Synergy Software, Reading, PA), and the number of binding sites per platelet was calculated by Scatchard analysis.16
Binding of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO with or without cold rhTPO for 30 minutes at 37°C. Platelet-associated radioactivity (n = 2 per data point) was used to determine affinity data from a four-parameter fit and receptor number via Scatchard analysis. Data from multible binding experiments using different PRP and 125I-rhTPO preparations are presented in Table 1.
Binding of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO with or without cold rhTPO for 30 minutes at 37°C. Platelet-associated radioactivity (n = 2 per data point) was used to determine affinity data from a four-parameter fit and receptor number via Scatchard analysis. Data from multible binding experiments using different PRP and 125I-rhTPO preparations are presented in Table 1.
Expression of glycoprotein-D-Mpl (gD-Mpl).The entire coding region of human c-Mpl was obtained by polymerase chain reaction. An Xho I site was engineered in the 5′ polymerase chain reaction primer immediately adjacent to the first amino acid of the mature c-Mpl protein for in-frame fusion with the herpes simplex virus type 1 glycoprotein D.17 The gD-Mpl cDNA was subcloned in the pRK5 expression vector and transfected in 293 cells using the calcium phosphate method. Clones were selected in 0.4 mg/mL G418 and conditioned media were screened for gD-Mpl expression by immunoprecipitation using the anti-gD monoclonal antibody, 5B6.18 The best expressing clone was selected and gD-Mpl was purified using an anti-gD monoclonal affinity column.
Binding of rhTPO to gD-MPl.rhTPO was coupled via its carbohydrate moiety to the CM5 sensor chip using the aldehyde coupling protocol found in the BiaCore Methods Manual Supplement (Pharmacia Biosensor AB, Uppsala, Sweden). Binding and dissociation studies were run at five different concentrations (500, 100, 50, 10, and 5 nmol/L) of gD-Mpl.17 18 Binding constants were obtained from the slope of the plots versus time data using the BiaCore TM kinetics evaluation software as described by the manufacturer (Pharmacia, Uppsala, Sweden).
Preparation of washed human platelets.Washed platelets (WP) were prepared from blood samples from adult Genentech employees as previously described.19 Whole blood was collected into 3.8% Na Citrate (9:1 vol/vol) and PRP was prepared by centrifugation at 150g for 12 minutes. Prostaglandin I2 (PGI2 ) and apyrase were added to a final concentration of 300 ng/mL to 5 U/mL, respectively. The PRP was then centrifuged (Beckman Model GPR, Palo Alto, CA) at 1,600g for 5 minutes and resuspended in Tyrode's buffer containing 2% bovine serum albumin (BSA), 300 ng/mL PGI2 , and 0.2 U/mL apyrase. Platelets were centrifuged again and resuspended in Tyrode's-BSA. To allow for recovery from the PGI2 treatment, the washed platelets were allowed to stand in the dark for a minimum of 2 hours at room temperature before use.
Degradation of 125I-rhTPO by PRP.To determine the effect of time and temperature on platelet uptake of rhTPO, 0.18 mL of PRP was incubated with 125I-rhTPO for 0 to 120 minutes at 37°C or for 1 hour at 0°C, 10°C, 20°C, or 37°C. The PRP was then washed with 0.75 mL TNT buffer (4°C), centrifuged at 14,000 rpm in a Eppendorf microfuge. The platelet-associated radioactivity was separated over 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions overnight at 50 V. The gels were dried, exposed to x-ray film at −70°C, and analyzed by autoradiography. In another experiment, 0.17 mL of either PRP or WP was incubated with 125I-rhTPO at 37°C for 60 minutes and analyzed as described above.
To determine if the degradation of rhTPO by platelets was enzymatic, we prepared a platelet lysate. Human platelets were washed three times with Tyrode's buffer before homogenization and sonication in TNT buffer and 1 mmol/L CaCl2 (pH 7.4). Insoluble material was removed by centrifugation and aliquots of the lysate were incubated with 125I-rhTPO with or without protease inhibitors at 37°C for 5 hours. Degradation of 125I-rhTPO was analyzed using SDS-PAGE as described above.
RESULTS
Binding of 125I-rhTPO to PRP and WP.A displacement curve showing the binding of 125I-rhTPO to PRP in the presence of increasing concentrations of cold rhTPO for 30 minutes at 37°C is presented in Fig 1. When analyzed using a four-parameter fit, binding data indicated an affinity constant of ∼358 pmol/L. The receptor number determined by Scatchard analysis was ∼95 receptors per platelet. These data are consistent with rhTPO binding to a single class of receptors (c-Mpl) on platelets. It should noted that, at 37°C, binding of rhTPO to platelets results in some internalization and degradation of 125 I-rhTPO (see Figs 4 through 8), which may affect the binding calculations. However, when the binding was performed using washed platelets at 22°C, the binding constants and receptor numbers were very similar to PRP at 37°C (kd of ∼258 pmol/L and ∼25 receptors/platelet). These data suggest that less binding sites are available on the WP incubated at 22°C than on the platelets in PRP incubated at 37°C. In multiple experiments using different tracer and PRP preparations, similar numbers to the above were also determined (Table 1). Also, EPO was unable to compete with 125I-rhTPO for binding sites on platelets, suggesting that EPO does not cross-react with the c-Mpl receptor (data not shown).
Time-dependent degradation of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO for 1 to 120 minutes at 37°C. Platelet-associated radioactivity (n = 2 per timepoint) was then analyzed by SDS-PAGE and autoradiography. Nonincubated 125I-rhTPO dosing solution (DS) was also run in the last lane of the gel. Time is indicated on the top of the figure and approximate MW (determined from prestained MW markers) is presented on the right axis.
Time-dependent degradation of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO for 1 to 120 minutes at 37°C. Platelet-associated radioactivity (n = 2 per timepoint) was then analyzed by SDS-PAGE and autoradiography. Nonincubated 125I-rhTPO dosing solution (DS) was also run in the last lane of the gel. Time is indicated on the top of the figure and approximate MW (determined from prestained MW markers) is presented on the right axis.
Affinity Constants and Receptor Numbers Determined From Different PRP and 125I-rhTPO Preparations
| PRP Preparation . | Affinity (kd) . | Receptor No. . | ||
|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Human BSC* | 605 ± 270 | 219-814 | 128 ± 54 | 79-184 |
| Human GNE† | 369 ± 50 | 319-438 | 97 ± 15 | 75-105 |
| Human GNE† | 311 ± 244 | 125-670 | 171 ± 72 | 66-224 |
| Human GNE† | 391 ± 65 | 328-472 | 34 ± 13 | 24-53 |
| WP‡ | 367 ± 151 | 260-540 | 27 ± 8 | 20-35 |
| PRP Preparation . | Affinity (kd) . | Receptor No. . | ||
|---|---|---|---|---|
| . | Mean ± SD . | Range . | Mean ± SD . | Range . |
| Human BSC* | 605 ± 270 | 219-814 | 128 ± 54 | 79-184 |
| Human GNE† | 369 ± 50 | 319-438 | 97 ± 15 | 75-105 |
| Human GNE† | 311 ± 244 | 125-670 | 171 ± 72 | 66-224 |
| Human GNE† | 391 ± 65 | 328-472 | 34 ± 13 | 24-53 |
| WP‡ | 367 ± 151 | 260-540 | 27 ± 8 | 20-35 |
Represents mean data from four separate PRP preparations obtained from Biological Specialties Co using different 125I-rhTPO preparations run at different times.
Represents mean data from three separate experiments using PRP preparations from Genentech employees (n = 4) using different 125I-rhTPO preparations run at different times.
Represents mean data from three separate washed platelet preparations from Genentech employees using different 125I-rhTPO preparations run at different times.
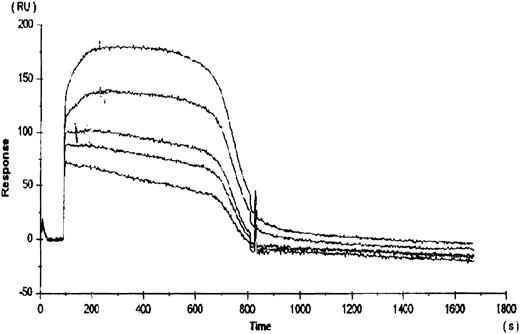
To compare the relative binding of rhTPO to platelets with the binding of rhTPO to the cloned c-Mpl receptor, rhTPO was immobilized to a BiaCore sensor chip via a c-terminal carbohydrate group. The subsequent binding and dissociation of several concentrations of gD-Mpl to the rhTPO was measured (Fig 2). The five curves presented in Fig 2 represent the dissociation kinetics for the different concentrations of added gD-Mpl. The calculated kd for gD-Mpl binding to rhTPO was ∼350 pmol/L, which is very similar to the value calculated by 125I-rhTPO binding to platelets. These data suggest that, under more physiologic conditions, the binding of 125I-rhTPO to platelets is similar to cold rhTPO binding to gD-Mpl.
Binding of rhTPO to recombinant c-Mpl (gD-Mpl). rhTPO was immobilized to a BiaCore sensor chip via a c-terminal carbohydrate group. The subsequent binding and dissociation of five different concentrations (500, 100, 50, 10, and 5 nmol/L) of gD-Mpl to the rhTPO was measured (represented by the 5 data curves). Binding constants were obtained from the slope of the plots versus time data using the BiaCore TM kinetics evaluation software.
Binding of rhTPO to recombinant c-Mpl (gD-Mpl). rhTPO was immobilized to a BiaCore sensor chip via a c-terminal carbohydrate group. The subsequent binding and dissociation of five different concentrations (500, 100, 50, 10, and 5 nmol/L) of gD-Mpl to the rhTPO was measured (represented by the 5 data curves). Binding constants were obtained from the slope of the plots versus time data using the BiaCore TM kinetics evaluation software.
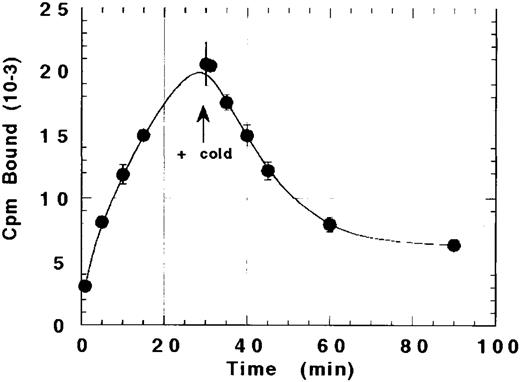
The ability of platelets to internalize rhTPO after binding was suggested by our inability to completely displace bound 125I-rhTPO with cold rhTPO. As shown in Fig 3, the amount of TPO bound to platelets increased over time to a maximum at 30 minutes. Excess cold (20 μg) rhTPO was then added to the reaction and incubated for another 60 minutes. Although the addition of a greater than 1,000-fold excess of cold TPO displaced approximately 60% to 70% of the bound 125I-rhTPO within 60 minutes, 30% to 40% of the bound TPO was not displaceable. These data suggest that a fraction of 125I-rhTPO bound by platelets at 30 minutes internalized.
Failure of cold TPO to completely displace bound 125I-rhTPO from platelets. Human PRP was incubated with 125I-rhTPO for 30 minutes before the addition of a greater than 1,000-fold excess of cold TPO. Platelet-associated radioactivity (n = 2 per timepoint) was sampled before and after the addition of cold rhTPO.
Failure of cold TPO to completely displace bound 125I-rhTPO from platelets. Human PRP was incubated with 125I-rhTPO for 30 minutes before the addition of a greater than 1,000-fold excess of cold TPO. Platelet-associated radioactivity (n = 2 per timepoint) was sampled before and after the addition of cold rhTPO.
The metabolic fate of the 125I-rhTPO removed by the platelets is presented in Figs 4 and 5. Analysis of platelet pellets by SDS-PAGE indicates that, after binding to platelets, 125-rhTPO is degraded into a lower molecular weight (MW) form (∼40 to 50 kD) in a time-dependent manner (Fig 4). The ∼40- to 50-kD fragment likely represents part of the glycosylated c-terminus of the molecule, because that is where the tyrosines are present. The variability in glycosylation of the CHO-derived molecule likely contributes to the diffuse nature of the proteolytic fragments of the 125I-rhTPO. The degradation of 125I-rhTPO was also temperature-dependent (Fig 5). Densitometric analysis of the above-mentioned autoradiographs indicated that both the total amount of 125I-rhTPO bound and the amount degraded increased over time to a maximum at approximately 60 minutes and at a temperature of 37°C (data not shown). The internalization and degradation of 125I-rhTPO was specific as it was blocked by addition of excess cold rhTPO (see Fig 7). Plasma alone was unable to degrade 125I-rhTPO, and no detectable 125I-rhTPO fragments were present in the plasma component of the PRP after incubation (data not shown). To compare the ability of platelets to degrade rhTPO in the absence of plasma, we incubated 125I-rhTPO for 60 minutes with either PRP or WP. The degradation pattern as analyzed by SDS-PAGE was similar between the PRP and the WP (data not shown). These data indicate that platelets are able to degrade 125I-rhTPO in the absence of any plasma components.
Temperature-dependent degradation of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO at 0°C, 10°C, 20°C, or 37°C for 60 minutes. Platelet-associated radioactivity (n = 3 per data point) was then analyzed by SDS-PAGE and autoradiography. Nonincubated 125I-rhTPO dosing solution (DS) was also run in the first lane of the gel. Temperature (in degrees Celsius) is indicated on the top of the figure and approximate MW (determined from prestained MW markers) is presented on the right axis.
Temperature-dependent degradation of 125I-rhTPO by human PRP. PRP was incubated with 125I-rhTPO at 0°C, 10°C, 20°C, or 37°C for 60 minutes. Platelet-associated radioactivity (n = 3 per data point) was then analyzed by SDS-PAGE and autoradiography. Nonincubated 125I-rhTPO dosing solution (DS) was also run in the first lane of the gel. Temperature (in degrees Celsius) is indicated on the top of the figure and approximate MW (determined from prestained MW markers) is presented on the right axis.
Effects of protease inhibitors on the binding and degradation of 125I-rhTPO by intact platelets. Human PRP was incubated with 125 I-rhTPO with or without cold rhTPO, calpain inhibitor I [Cal (−)], or iodoacetic acid (Iodo) for 60 minutes at 37°C. Binding and degradation of 125I-rhTPO was determined (n = 3 per experiment) as previously described. *Indicates significantly less (P < .05) than total binding (TB), as analyzed by ANOVA followed by a Tukey-Kramer test.
Effects of protease inhibitors on the binding and degradation of 125I-rhTPO by intact platelets. Human PRP was incubated with 125 I-rhTPO with or without cold rhTPO, calpain inhibitor I [Cal (−)], or iodoacetic acid (Iodo) for 60 minutes at 37°C. Binding and degradation of 125I-rhTPO was determined (n = 3 per experiment) as previously described. *Indicates significantly less (P < .05) than total binding (TB), as analyzed by ANOVA followed by a Tukey-Kramer test.
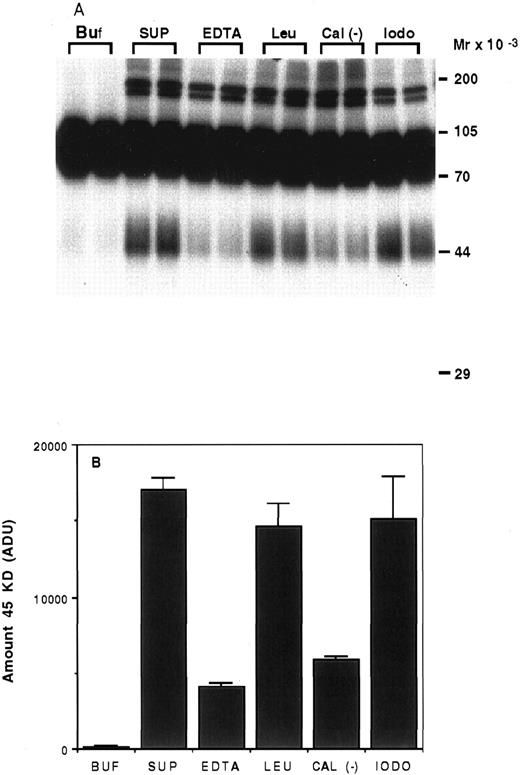
Characterization of the proteolysis of rhTPO by platelets.To determine the type of enzyme involved in the degradation of rhTPO by platelets, we incubated 125I-rhTPO with the soluble fraction of a platelet homogenate for 5 hours at 37°C with or without various protease inhibitors. As can be seen on Fig 6A, very little degradation was present when 125I-rhTPO was incubated in buffer alone (Buf ). When 125I-rhTPO was incubated with the platelet homogenate (SUP), a extra band of ∼45 kD was present, suggesting partial degradation. This band is similar in MW to the band produced by intact platelets (Figs 4 and 5). A densitometric analysis of the amount of the 45-kD degradation band is presented in Fig 6B. Some higher MW bands (>170 kD) are present in the autoradiograph and likely represent nonspecific aggregates of the tracer and proteins in the platelet lysate, because they are not present in the samples incubated in buffer alone (Buf ). The amount of the 45-kD band was decreased by the addition of either 5 mmol/L EDTA or 20 μg/mL calpain inhibitor to the reaction. The addition of 100 μg/mL leupeptin or 2 mmol/L iodoacetic acid had little effect on the proteolysis of 125I-rhTPO. These data suggest that the protease involved is a cation-dependent enzyme similar to calpain. Because calpain is present in platelets,20 we incubated 125I-rhTPO with 100 μg/mL pure porcine calpain. A degradation pattern similar to that observed with intact platelets was observed (data not shown).
Proteolysis of 125I-rhTPO by platelet lysates. Platelet lysates were prepared from washed PRP. Aliquots of the lysate were incubated with 125I-rhTPO with or without protease inhibitors at 37°C for 5 hours. Degradation of 125I-rhTPO was analyzed by SDS-PAGE and autoradiography. Approximate MW (determined from prestained MW markers) are listed on right axis (A). A densitometric analysis of the autoradiograph is presented in (B). Buf, buffer; SUP, supernatant; EDTA; Leu, leupeptin; Cal (−), Calpain inhibitor I; iodo, iodoacetic acid.
Proteolysis of 125I-rhTPO by platelet lysates. Platelet lysates were prepared from washed PRP. Aliquots of the lysate were incubated with 125I-rhTPO with or without protease inhibitors at 37°C for 5 hours. Degradation of 125I-rhTPO was analyzed by SDS-PAGE and autoradiography. Approximate MW (determined from prestained MW markers) are listed on right axis (A). A densitometric analysis of the autoradiograph is presented in (B). Buf, buffer; SUP, supernatant; EDTA; Leu, leupeptin; Cal (−), Calpain inhibitor I; iodo, iodoacetic acid.
The effect of calpain inhibitor and iodoacetic acid on the degradation of 125I-rhTPO by intact platelets is shown in Fig 7A and B. The addition of cold rhTPO blocked binding of 125I-rhTPO to platelets, calpain inhibitor did not effect binding to platelets, and iodoacetic acid significantly reduced binding. However, both inhibitors almost completely blocked degradation of 125I-rhTPO.
The fact that the calpain inhibitor blocked degradation of 125I-rhTPO in both intact platelets and platelet lysates suggests that an enzyme similar to calpain is involved. These data are in contast to that of iodoacetic acid, which blocks degradation in intact platelets, but not in platelet lysates.
DISCUSSION
Using TPO-specific assays, recent clinical data confirm an inverse relationship between platelet mass and TPO levels.9,10 Using an assay specific for full-length glycosylated rhTPO, it was also shown that the endogenous plasma form of TPO is the full-length molecule.21 These observations suggest that circulating TPO levels are regulated by platelet mass. de Gabriele and Penington22 first proposed a model for regulation of TPO by platelets. Kuter et al11,12 also proposed that platelet mass regulates TPO levels directly by acting as a sink for TPO. This is based on their observation that TPO levels in thrombocytopenic rabbits can be lowered almost immediately by platelet transfusions.12 This hypothesis is supported by the fact that TPO mRNA expression in the kidney and liver of c-Mpl–deficient mice is unchanged when compared with wild-type mice, even though TPO levels are significantly elevated.13 14
Our data clearly show that human platelets can both bind and degrade full-length rhTPO. The affinity constants derived from the binding data indicate that rhTPO's affinity for platelet receptors was similar to that for the cloned c-Mpl receptor construct, gD-Mpl. The binding constants were also similar for rhTPO binding to PRP at 37°C and WP at 22°C. These data show that platelets bind rhTPO, likely via c-Mpl with high affinity (∼350 pmol/L), and that this binding is saturable. Scatchard analysis predicted a low number of (∼23 to 224) binding sites per platelet. However, this estimate may be low, because c-Mpl binding sites on circulating platelets are likely partially occupied by endogenous circulating TPO.9,10 If we assume the number of available c-Mpl receptors is ∼25 to 200 per platelet and that the approximate number of platelets per liter of blood in humans is 200 × 109, then the predicted binding capacity would be ∼8 to 64 pmol per liter of blood. This approximation suggests that dosing rhTPO at a concentration of 8 to 64 pmol/L may result in the saturation of the available platelet binding sites, thus affecting the clearance of the nonbound rhTPO. One dramatic example of the effects of platelet binding on the clearance of rmTPO was observed in c-Mpl–deficient mice. Mice deficient in c-Mpl cleared a dose of radiolabeled rmTPO approximately fivefold slower than did normal mice.14 In addition, in preliminary mouse experiments, the clearance and metabolism of radiolabeled rmTPO was decreased when saturating amounts of cold rmTPO were coinjected (data not shown). Together, these data suggest that the clearance of exogenously administered TPO will be affected by both dose level and platelet numbers.
TPO has been shown to stimulate phosphorylation of cytoplasmic proteins in platelets via c-Mpl.23-25 These data would suggest that an active second messenger system exists in platelets and that TPO can activate intracellular enzymes. The fact that iodoacetic acid decreased the binding and degradation of 125I-rhTPO by platelets suggests that internalization is required for degradation. Similar results have been reported by Li and Stanley26 using human macrophages to study the internalization of colony stimulating factor-1 (CSF-1). Incubation of these cells with iodoacetic acid did not completely block binding, but instead blocked receptor dimerization and internalization of CSF-1.26 They suggested that iodoacetic acid inhibited internalization by capping free sulfhydral sites on the receptor, which in turn prevented covalent dimerization of the receptor complex. Receptor dimerization has been reported for other members of the cytokine receptor family.27 Based on receptor analogy, TPO binding may lead to a dimerization of c-Mpl8 before subsequent internalization and degradation. Many plasma proteins are taken up by platelets in the circulation.28 It is known that platelets can internalize other proteins via receptor-mediated processes.28-30
Although proteases have been identified in platelets,19 31 the identity of the enzymes involved in proteolyzing TPO is not clear. Platelet homogenates incubated at neutral pH displayed a TPO degradation pattern similar to that of intact platelets. This activity was inhibited by EDTA or calpain inhibitor, indicating that this specific proteolysis is catalyzed by an enzyme with an activity similar to that of calpain. Calpain is present in the cytosol of human platelets and, when activated, is involved in the presentation of glycoprotein IIb/IIIa receptors on the surface of platelets. In our experiments, pure porcine calpain (10 to 100 μg/mL) was able to proteolyze 125I-rhTPO in a pattern similar to that of intact platelets.
In conclusion, our data strongly suggest that human platelets have the ability to bind, internalize, and degrade rhTPO in vitro. This process is both time- and temperature-dependent and can be blocked by the addition of certain cysteine protease inhibitors. Our in vitro data suggest that human platelets should also have the capability to regulate plasma TPO levels in vivo. Because the binding of rhTPO by platelets is a saturable phenomenon, the clearance of TPO in humans may be dependent on the capacity of circulating platelets to bind and degrade it. Therefore, it may be possible in the future to predict clinical dosing of rhTPO based on the number of receptors per platelet and total number of circulating platelets present in the patient.
ACKNOWLEDGMENT
The authors thank M. Siegal, Dr W. Li, and L. Crandall for technical assistance; T. Lipari for his helpful suggestions; and Y. Lin for her editorial comments.
Address reprint requests to Paul J. Fielder, PhD, Department of Pharmacokinetics and Metabolism, Mail Stop 70, Genentech Inc, 460 Point San Bruno Blvd, South San Francisco, CA 94080.




![Fig. 7. Effects of protease inhibitors on the binding and degradation of 125I-rhTPO by intact platelets. Human PRP was incubated with 125 I-rhTPO with or without cold rhTPO, calpain inhibitor I [Cal (−)], or iodoacetic acid (Iodo) for 60 minutes at 37°C. Binding and degradation of 125I-rhTPO was determined (n = 3 per experiment) as previously described. *Indicates significantly less (P < .05) than total binding (TB), as analyzed by ANOVA followed by a Tukey-Kramer test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2782/4/m_bl_0002f7.jpeg?Expires=1769098286&Signature=HZ8hHmwKhNrW2NpA-6N95COIHey~M6W1ZtVQBNSsUnvNeQINdKqIXQT-JKG0B0ueG0eGm5EqZTpBdZujSFJfOPRTZPqcIoGHC3YMRWHBbb5H3IfgKyof59~JS67wvnPp9T5qQaqf41dnWjuRAiYcQyDtWN4kDVSJnK9Chk2xg7urCDNTIfg3LOl59CJDRpkEvycEJ8QLiT~gb999vdTacNesDC8g~ZXkZG5r5cNsApw2sWZ1LRyzuumXS0CflSd24imSVad8f8tcBcK2ZAE3Jhbt5AptVc21CbQB7MiJFSKSbnwLPj9zNVu7wwLCSZ~4YIdm-Lcrt5jNsSnJpVfyVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal