Abstract
Gene delivery via the hematopoietic stem cell (HSC) offers an attractive means to introduce antiviral genes into both T cells and macrophages for acquired immunodeficiency syndrome (AIDS) gene therapy. An amphotropic retroviral vector encoding a bicistronic gene coexpressing RevM10 and the murine CD8α′ chain (lyt2) was developed to transduce HSC/progenitor cells. After transduction of CD34+ cells isolated from human umbilical cord blood, the lyt2 molecule detected by flow cytometry was used to monitor the level of gene transduction and expression and to enrich RevM10-expressing cells by cell sorting without drug selection. Using this quantitative method, high levels of gene transduction and expression (around 20%) were achieved by high-speed centrifugation of CD34+ cells with the retroviral supernatant (spinoculation). After reconstitution of human bone marrow implanted in SCID mice (SCID-hu bone) with the transduced HSC/progenitor cells, a significant number of donor-derived CD14+ bone marrow cells were found to express the RevM10/lyt2 gene. Finally, replication of a macrophage-tropic human immunodeficiency virus–type 1 (HIV-1) isolate was greatly inhibited in the lyt2+/CD14+ cells differentiated from transduced CD34+ cells after the enrichment of lyt2+ population. Thus, the RevM10 gene did not appear to inhibit the differentiation of HSC/progneitor cells into monocytes/macrophages. The level of retrovirus-mediated RevM10 expression in monocytes/macrophages derived from transduced HSCs is sufficient to suppress HIV-1 replication.
GENE THERAPY or intracellular immunization has been proposed to provide an alternative treatment for human immunodeficiency virus–type 1 (HIV-1) disease.1-3 HIV-1 infects predominantly CD4+ T cells and monocytes/macrophages of the immune system.4,5 Thus, protection against HIV-1 infection has to be effective in the myeloid as well as in the lymphoid lineage. The hematopoietic stem cell (HSC) is an ideal target cell for introducing genes into multiple lineages of the immune system. HSCs can be isolated from a number of tissue sources, including bone marrow and peripheral blood, and are used to reconstitute all hematopoietic lineages in transplant recipients.6-8 However, HSC-based gene therapy has been hampered by ineffective protocols to enrich this rare cell population, by the inability to expand them in vitro, and by insufficient gene transfer technology. Recently, hematopoietic progenitor cells isolated from umbilical cord blood (UCB) have been shown to be efficiently transduced with murine leukemia virus (MLV)-based vectors.9-11 In addition, a retroviral vector encoding an anti–HIV-1 ribozyme has been shown to inhibit HIV replication in macrophage-like cells derived from the transduced stem/progenitor cells.12
The HIV-1 rev protein is critically required for the transport of unspliced HIV-1 mRNA into the cytoplasm and thus for the expression of HIV structural proteins.13,14 A trans-dominant mutant of HIV-1 rev, RevM10, has been shown to inhibit HIV-1 replication in peripheral blood lymphocytes (PBLs) without affecting the growth and functions of the transduced cells.15-19 A clinical trial using retrovirally modified PBLs is currently in progress to evaluate the RevM10 protein for acquired immunodeficiency syndrome (AIDS) gene therapy. However, it has not been demonstrated that RevM10 can inhibit macrophage-tropic HIV-1 replication in primary myeloid cells. In addition, it is unknown what effect (if any) RevM10 expression may have on the ability of transduced HSCs to differentiate into lymphoid or myeloid lineages.
To address these issues, we performed experiments to show that RevM10 can be efficiently transduced into cord blood–derived HSC/progenitor cells, which can give rise to primary myeloid cells expressing the RevM10 gene in an SCID-hu in vivo model and in cell cultures in vitro. We further demonstrate that sufficient levels of RevM10 expression can be achieved to suppress HIV-1 JR-FL replication in primary myeloid cells derived from retrovirally transduced human HSCs.
MATERIALS AND METHODS
Antibodies and human tissues/cells.Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies (MoAb) to human CD14 (LeuM3) and CD34 (HPCA-2) were purchased from Becton Dickinson (San Jose, CA). The antimurine CD8α chain (lyt2) antibody was purchased from Pharmingen (San Diego, CA). Isotype controls used were mouse myeloma IgG1 (MOPC21) and IgG3 (FLOPC21) obtained from Sigma (St Louis, MO). MoAbs against monomorphic or polymorphic determinants of HLA molecules were obtained from American Type Culture Collection (ATCC; Rockville, MD), including MA2.1 (HLA-A2), GAPA3 (HLA-A3), MB40.2 (HLA-B7), BB7.1 (HLA-B7), BB7.2 (HLA-A2), and W6/32 (HLA-A, B, C). Anti-Rev MoAb (8H12) was provided by H. Jaksche (Sandoz Forschungsinstitut, Vienna, Austria). Human tissues were obtained in compliance with regulations established by the state and federal governments. Human T-leukemia cell line CEM was obtained from ATCC.
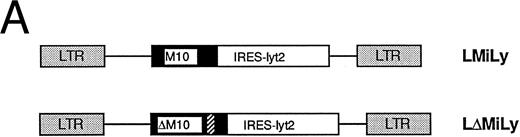
Retroviral vector construction and supernatant preparation.The LMiLY vector has been constructed as follows. The SV40-promoter-Neo gene in retroviral vector LMTNL20 was replaced with the murine CD8α′ gene (Lyt2)21 controlled by an internal ribosomal entry site (IRES).22 As shown in Fig 1A, the RevM10 gene is expressed from the Moloney murine leukemia virus long terminal repeat (LTR) and the cell surface reporter gene lyt2 is expressed from the same mRNA via an internal ribosomal entry site. The LΔMiLY vector is a similar vector encoding a RevM10-lyt2 mRNA with a mutation in the initiation codon of RevM10. The message from LΔMiLY expresses only the Lyt2 gene (Fig 1B). Retroviral vectors were transfected into the amphotropic packaging line PA317 and high-titer producer lines were screened. The viral supernatant was produced and titrated on NIH3T3 or human T-cell line, CEM, as described.23
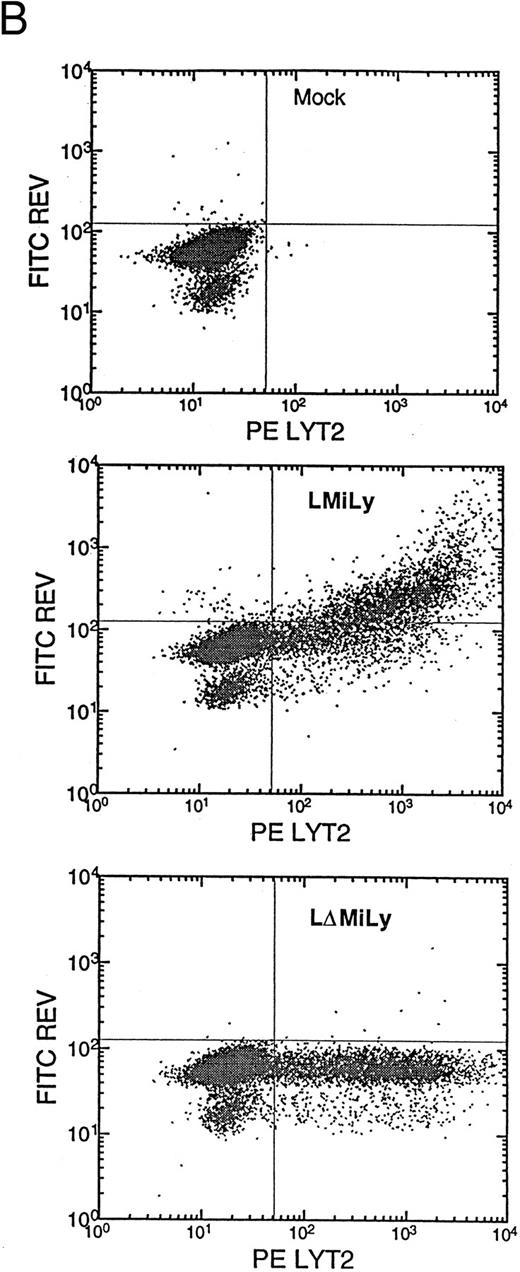
(A) Genomic structure of the retroviral vectors encoding a bicistronic mRNA coexpressing RevM10 and lyt2. LMiLy encodes the RevM10 gene and the murine CD8α′ gene under control of an IRES. LΔMiLy is identical to LMiLy, except that a mutation was introduced to eliminate the initiation codon of the RevM10 gene. The hatched region indicates a small insertion for distinguishing LMILy from LΔMiLy by polymerase chain reaction. IRES, internal ribosomal entry site. Lyt2, murine cDNA encoding the CD8α′ chain. (B) Coexpression of lyt2 and RevM10 in a transduced human T-cell line. CEM-ss cells were transduced with LMiLy or LΔMiLy or mock supernatant. Cell surface expression of lyt2 and intracellular expression of RevM10 were analyzed by FACS.
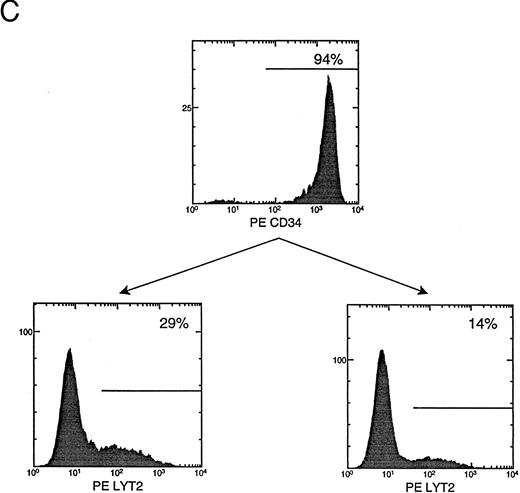
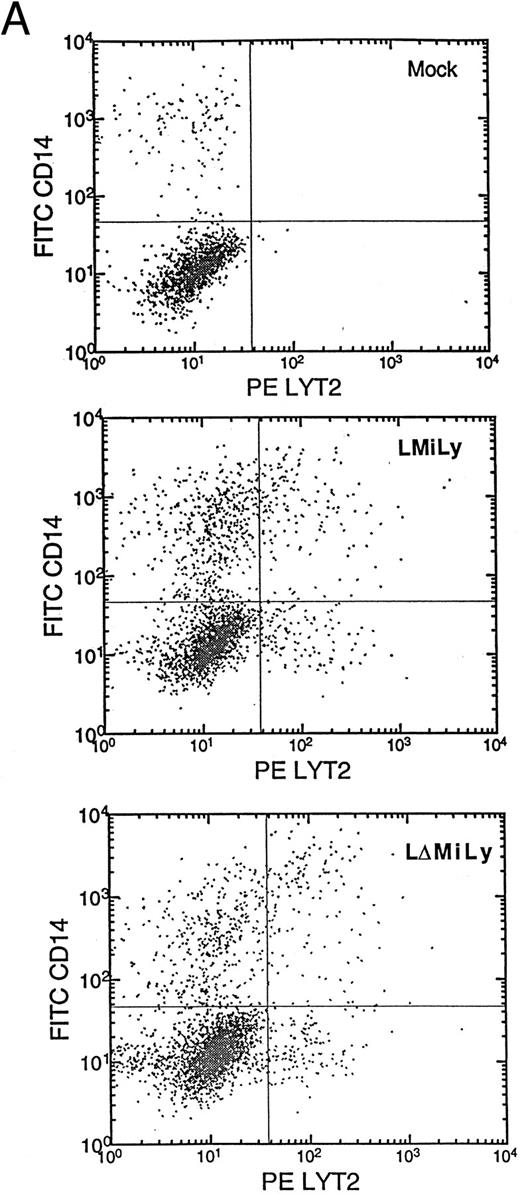
(C) CD34+ cell enrichment and transduction. Live cells (based on side scatter, size, and propidium iodide exclusion) were analyzed for their expression of CD34+ or lyt2 expression after transduction. The percentage of positive cells is indicated. The gate is based on isotype antibody control and/or mock-transduced cells. (Bottom left) LMiLy-transduced cells. (Bottom right) LΔMiLy-transduced cells.
(A) Genomic structure of the retroviral vectors encoding a bicistronic mRNA coexpressing RevM10 and lyt2. LMiLy encodes the RevM10 gene and the murine CD8α′ gene under control of an IRES. LΔMiLy is identical to LMiLy, except that a mutation was introduced to eliminate the initiation codon of the RevM10 gene. The hatched region indicates a small insertion for distinguishing LMILy from LΔMiLy by polymerase chain reaction. IRES, internal ribosomal entry site. Lyt2, murine cDNA encoding the CD8α′ chain. (B) Coexpression of lyt2 and RevM10 in a transduced human T-cell line. CEM-ss cells were transduced with LMiLy or LΔMiLy or mock supernatant. Cell surface expression of lyt2 and intracellular expression of RevM10 were analyzed by FACS.
(C) CD34+ cell enrichment and transduction. Live cells (based on side scatter, size, and propidium iodide exclusion) were analyzed for their expression of CD34+ or lyt2 expression after transduction. The percentage of positive cells is indicated. The gate is based on isotype antibody control and/or mock-transduced cells. (Bottom left) LMiLy-transduced cells. (Bottom right) LΔMiLy-transduced cells.
Isolation of CD34+ cells from UCB and spinoculation.Human UCB samples were collected and processed essentially as previously described.24 After separation by a Ficoll gradient, total mononuclear cells were stained with a CD34 antibody and selected with the CD34 Isolation Kit (Mini-MACS; Miltenyi Biotec, Auburn, CA) to a purity of ≥90%. The purified cells were cultured in the presence of interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), and stem cell factor (SCF; 50 ng/mL) for 3 days at 37°C. The expanded CD34+ cells were pelleted and resuspended in equal volume of retroviral supernatant and medium with 2× growth factors (plus 4 μg/mL of protamine sulfate) at a cell density of 5 × 105/mL. The resuspended cells were then spun in a centrifuge at 2,500g at room temperature for 3 hours as described.25 The cell pellet was resuspended in fresh medium with 1× growth factors and the transduction procedure was repeated the next day.
Fluorescence-activated cell sorting (FACS) analysis and lyt2/rev costaining.Transduced cells were cultured additionally for 48 hours and stained for CD34, CD14, and lyt2. In the case of myeloid differentiation in vivo, CD14/lyt2 or donor tissue HLA (MA2.1) were also performed. A FACScan (Becton Dickinson) was used to analyze the samples. To show coexpression of RevM10 and lyt2, CEM cells were transduced with either retroviral vectors or mock supernatant. Costaining of Rev and lyt2 was performed by first staining with anti-lyt2 FITC and followed by the standard intracellular staining for Rev.20
In vitro differentiation of CD34+ cells and challenge with HIV-1. After transduction, the CD34+ cells were cultured in the presence of 1× growth factors (IL-3/IL-6/SCF, 20/20/50 ng/mL, respectively) for 3 days. The lyt2+ cells (10% to 20%) were purified by FACS (>80% purity) and induced to differentiate toward monocytes/macrophages in the presence of IL-3 (20 ng/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL) for 6 days. Cells seeded in triplicate wells for each vector transduction (30,000 to 50,000 cells/well in 96-well plate) were challenged with HIV-1 JR-FL at 20,000 or 2,000 TCID50 U/well. Supernatant p24 levels were measured by an enzyme-linked immunosorbent assay kit (DuPont, Boston, MA) every 3 days (fresh medium added) for 30 days after infection.
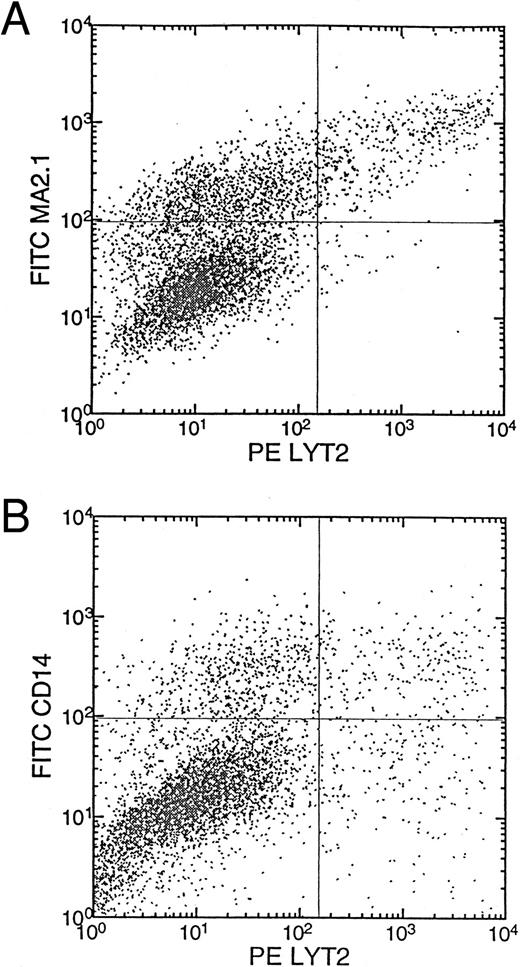
SCID-hu bone assay.Immunodeficient C.B-17 scid/scid (SCID) mice were used as recipients of human fetal bones to construct the SCID-hu bone mouse.26 SCID-hu bone mice of HLA-MA2.1–negative tissues at 6 to 8 weeks post transplantation were used as recipients of transduced HSCs. The transduced donor cells (HLA-MA2.1–positive) were injected into the human bone fragment implanted in the SCID mouse that received a single dose of whole body irradiation (325 rad) 4 to 6 hours before injection.27 A total of 1 × 105 transduced cells were injected per bone fragment. The engraftment and transgene expression were analyzed at 8 weeks postinjection. Donor cells (MA2.1+) were analyzed for their expression of lyt2 and CD14 markers.
RESULTS
Transduction of purified CD34+ cells with retroviral vectors coexpressing RevM10 and a cell surface reporter gene.Retroviral vectors were constructed that allow coexpression of RevM10 and a cell surface marker (murine CD8α′ chain or lyt2) from a bicistronic mRNA transcribed from the MLV LTR (Fig 1A). The two genes were supposed to coexpress in transduced cells from a transcript with IRES upstream of the lyt2 gene. This was confirmed in T-cell lines (Fig 1B) and in primary T cells (data not shown), because the levels of RevM10 expression directly correlated with lyt2 expression. Thus, lyt2 expression can be used as an indirect marker for retroviral gene transduction and RevM10 gene expression in target cells. CD34+ cells were purified from UCB samples to high purity (≥90%). Purified cells were transduced by centrifugation (spinoculation) and the expression of lyt2 was monitored. As shown in Fig 1C, high levels of lyt2 expression were achieved with both vectors. Therefore, the RevM10 gene can be transferred and efficiently expressed in UCB HSC/progenitor cells.
Differentiation of monocytes/macrophages from UCB HSC/progenitor cells transduced with the RevM10/lyt2 vectors in vivo.To address whether the retroviral gene transfer protocol and expression of RevM10 had a negative impact on the biologic functions of the transduced HSCs, transduced HSCs were injected into irradiated human bone fragments implanted in the SCID mouse (SCID-hu bone assay27 ). The SCID-hu bone assay measures the capability of long-term engraftment specific for HSCs of the CD34+Lin-Thy+ phenotype.27,28 For example, the CD34+Lin−Thy− phenotype in the mobilized peripheral blood was unable to generate engraftments in the bone implants even though they can efficiently form colonies in methylcellulose culture. As shown in Fig 2, efficient reconstitution occurred and a high percentage (16% to 54%) of the donor-derived cells (MA2.1+) expressed the RevM10/lyt2 gene (Table 1). Retrovirus-mediated gene expression was also detected in the human T cells after long-term reconstitution of human thymus fragments implanted in the SCID mouse (Plavec et al29 and unpublished results). Thus, RevM10/lyt2 can be efficiently expressed in myeloid cells derived from transduced CD34+ cells and at least some of the transduced HSCs were still able to reconstitute the human bone marrow and differentiate into myeloid and lymphoid lineages in vivo. Because of the qualitative nature of the SCID-hu bone assay in this study, a subtle effect of RevM10 on the myeloid differentiation process cannot be excluded.
Transduction of HSCs with long-term reconstitution activity in the SCID-hu bone mice. MA2.1+ donor CD34+ cells were transduced with LMiLy and injected into MA2.1− host bone fragments implanted in SCID mice. Donor cells were assayed for Lyt2 expression (A) and coexpression of CD14 and Lyt2 at 8 weeks posttransplantation (B). Control bone fragments were from irradiated SCID-hu bone mice not injected with any donor cells.
Transduction of HSCs with long-term reconstitution activity in the SCID-hu bone mice. MA2.1+ donor CD34+ cells were transduced with LMiLy and injected into MA2.1− host bone fragments implanted in SCID mice. Donor cells were assayed for Lyt2 expression (A) and coexpression of CD14 and Lyt2 at 8 weeks posttransplantation (B). Control bone fragments were from irradiated SCID-hu bone mice not injected with any donor cells.
Reconstitution and lyt2 Expression in the SCID-hu Bone Assay
| Bone . | UBC Cells* . | % Donor† . | % lyt2+ of Donor‡ . | % MA2.1+lyt2+ρ . | % CD14+lyt2+1-155 . |
|---|---|---|---|---|---|
| 1 | No cells | 4 | 86 | 4 | 3 |
| 2 | No cells | 4 | 100 | 4 | 3 |
| 3 | RevM10/lyt2 | 69 | 42 | 29 | 11 |
| 4 | RevM10/lyt2 | 72 | 54 | 39 | 8 |
| 5 | RevM10/lyt2 | 38 | 50 | 19 | 6 |
| 6 | RevM10/lyt2 | 18 | 52 | 9 | 4 |
| 7 | RevM10/lyt2 | 61 | 45 | 28 | 9 |
| 8 | RevM10/lyt2 | 50 | 19 | 9 | 11 |
| 9 | RevM10/lyt2 | 81 | 16 | 13 | 14 |
| 10 | RevM10/lyt2 | 73 | 23 | 17 | 11 |
| Bone . | UBC Cells* . | % Donor† . | % lyt2+ of Donor‡ . | % MA2.1+lyt2+ρ . | % CD14+lyt2+1-155 . |
|---|---|---|---|---|---|
| 1 | No cells | 4 | 86 | 4 | 3 |
| 2 | No cells | 4 | 100 | 4 | 3 |
| 3 | RevM10/lyt2 | 69 | 42 | 29 | 11 |
| 4 | RevM10/lyt2 | 72 | 54 | 39 | 8 |
| 5 | RevM10/lyt2 | 38 | 50 | 19 | 6 |
| 6 | RevM10/lyt2 | 18 | 52 | 9 | 4 |
| 7 | RevM10/lyt2 | 61 | 45 | 28 | 9 |
| 8 | RevM10/lyt2 | 50 | 19 | 9 | 11 |
| 9 | RevM10/lyt2 | 81 | 16 | 13 | 14 |
| 10 | RevM10/lyt2 | 73 | 23 | 17 | 11 |
CD34+ cells transduced with the retroviral vectors. No cells indicate irradiated bones with no injection.
% MA2.1+ cells from each reconstituted bone fragment. The no cell control shows staining background.
% lyt2+ cells of MA2.1+ cells indicating marking efficiency of the donor cells.
ρ % MA2.1+lyt2+ cells of total live, lymphoid, and myeloid blast cells.
% CD14+lyt2+ cells of total live cells.
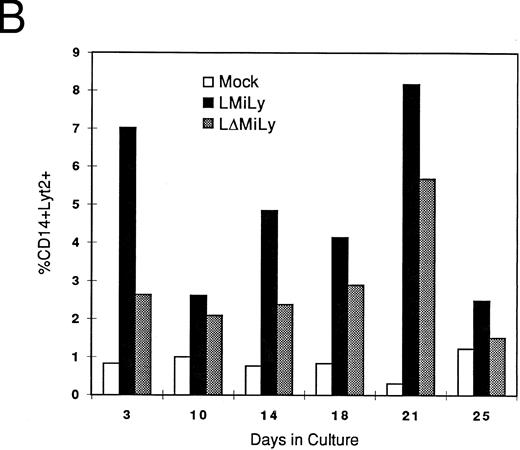
Inhibition of HIV-1 replication in the primary myeloid/macrophage cells transduced with the RevM10-expressing vector.Next, we established an in vitro differentiation system to derive myeloid cells from HSC/progenitor cells to evaluate RevM10 expression and anti-HIV efficacy in primary myeloid cells. As shown in Fig 3, myeloid cells expressing the lyt2 gene (CD14+lyt2+) can be generated in vitro by culturing transduced CD34+ cells in the presence of IL-3 and GM-CSF (Fig 3A and B). These cells expressed surface CD14 markers and showed a macrophage-like morphology when they adhered to plates (data not shown). The lyt2 gene was still expressed in these macrophage cells 4 to 5 weeks after the initiation of differentiation in the presence of IL-3 and GM-CSF. Thus, monocytes/macrophages expressing the RevM10/lyt2 genes can be derived in vitro from retrovirally transduced CD34+ cells. Furthermore, expression of RevM10 did not appear to change the efficiency (Fig 3A and B) of meyloid differentiation or the cell morphology of the differentiated cells (data not shown).
In vitro differentiation of myeloid cells after transduction. Mock- or vector-transduced CD34+ cells were differentiated for 10 days and analyzed for the myeloid marker CD14 and lyt2 expression (A). Kinetic analysis indicated stable lyt2 expression in the myeloid lineage cells in CD34+ cells transduced with either LMiLy or LΔMiLy (B). The presence of RevM10 gene product (LMiLY or LΔMiLy) did not affect the efficiency of differentiation.
In vitro differentiation of myeloid cells after transduction. Mock- or vector-transduced CD34+ cells were differentiated for 10 days and analyzed for the myeloid marker CD14 and lyt2 expression (A). Kinetic analysis indicated stable lyt2 expression in the myeloid lineage cells in CD34+ cells transduced with either LMiLy or LΔMiLy (B). The presence of RevM10 gene product (LMiLY or LΔMiLy) did not affect the efficiency of differentiation.
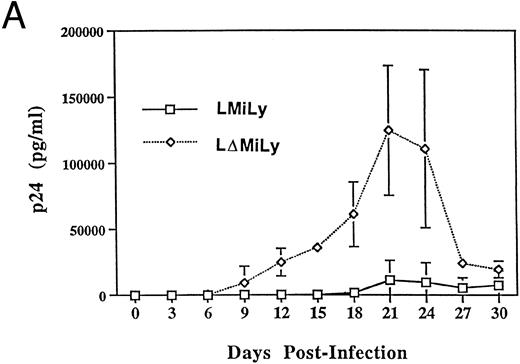
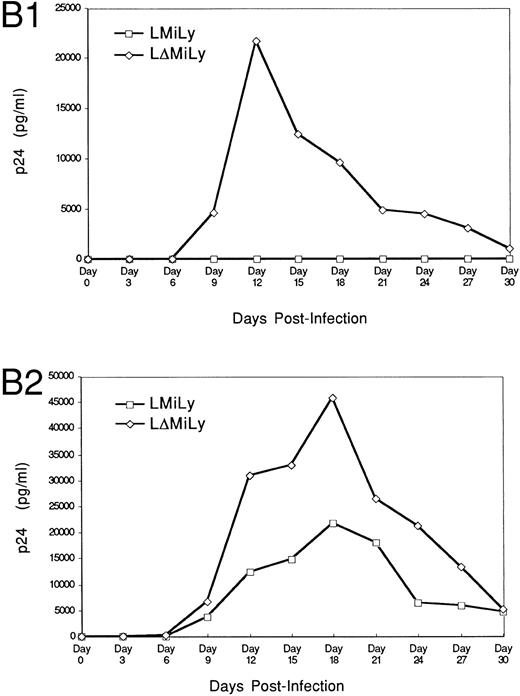
The bicistronic mRNA of the vector allows purification of RevM10-expressing cells based on lyt2 expression. To assess the effectiveness of RevM10 in inhibiting HIV-1 replication in these cells, lyt2+ cells were enriched by FACS to greater than 80% purity and differentiated into monocytes/macrophages in the presence of IL-3 and GM-CSF. When challenged with a macrophage-tropic HIV-1 isolate (JR-FL), high levels of HIV-1 replication were detected in the control cells (lyt2+ cells not coexpressing the RevM10 gene). However, very low levels of HIV replication were detected in the RevM10-expressing cells. The reduced replication of HIV-1 in the control cells at later time points after infection was due to the cytopathic effect of HIV-1 infection. High levels of HIV-1 replication in these cells led to cell fusion and cell death (data not shown). Experiments challenged with 2,000 TCID50 U/well (Fig 4B1) showed efficient protection. Experiment B1 and experiment B2 in Fig 4 were performed with lyt2+ cells from the same UCB tissue. Higher levels of HIV-1 infection were detected when 20,000 TCID50 U/well was used (Fig 4B2). RevM10 appeared to be less efficacious under this condition. The breakthrough replication of HIV-1 may be due to infection of untransduced (≈90% cells expressing lyt2) or nonexpressing cells. Therefore, RevM10 can be stably transduced into UCB HSC/progenitor cells to effectively inhibit HIV-1 replication in their myeloid progenies.
RevM10-modified primary myeloid cells showed resistance to HIV-1 replication. (A) Transduced CD34+ cells were enriched by FACS sorting to greater than 90% lyt2+. Fifty thousand sorted cells per well were differentiated in the presence of GM-CSF and IL-3 and infected with a macrophage-tropic HIV-1 isolate (JR-FL, 2,000 TCID50/well). p24 levels in the supernatant of JR-FL–infected primary cells were assayed for 30 days. Data were from triplicate wells and standard errors are shown. (B1) As in (A), 10,000 sorted Lyt2+CD34+ cells from a different UBC donor were challenged with HIV-1 JR-FL at 2,000 TCID50/well. (B2) was the same as (B1), except that the challenge was with JR-FL at 20,000 TCID50/well. Similar data were obtained with two other donor tissues (data not shown).
RevM10-modified primary myeloid cells showed resistance to HIV-1 replication. (A) Transduced CD34+ cells were enriched by FACS sorting to greater than 90% lyt2+. Fifty thousand sorted cells per well were differentiated in the presence of GM-CSF and IL-3 and infected with a macrophage-tropic HIV-1 isolate (JR-FL, 2,000 TCID50/well). p24 levels in the supernatant of JR-FL–infected primary cells were assayed for 30 days. Data were from triplicate wells and standard errors are shown. (B1) As in (A), 10,000 sorted Lyt2+CD34+ cells from a different UBC donor were challenged with HIV-1 JR-FL at 2,000 TCID50/well. (B2) was the same as (B1), except that the challenge was with JR-FL at 20,000 TCID50/well. Similar data were obtained with two other donor tissues (data not shown).
DISCUSSION
The target cells for HIV-1 infection in vivo are predominantly CD4+ T cells and monocytic (macrophage) cells. Therefore, all HSC-based anti-HIV gene therapies must be effective in both cell lineages. So far, most studies have been done in T cells (cell lines or phytohemagglutinin-activated primary T cells). RevM10 has been shown to be effective in reducing HIV-1 replication in T cells15-18 and is currently evaluated in a phase I clinical trial using RevM10-modified PBLs.19 30 In this study, we present data showing that RevM10 can be introduced into HSCs, which can give rise to myeloid progenies expressing RevM10 both in vivo and in vitro. Furthermore, RevM10 can inhibit HIV-1 replication in primary monocytes/macrophages derived from the transduced UCB stem/progenitor (CD34+) cells. This finding confirms that RevM10 is indeed a good candidate for HIV-1 gene therapy.
Many technical issues need to be overcome before human gene therapy can be widely used. Besides the problems specific to HSC-based gene therapy discussed in the introduction, AIDS gene therapy also has its unique difficulties. First, the HSC functions in HIV-1 patients may have been compromised.31-33 For example, it has been reported that colony-forming unit activity is reduced in CD34+ cells isolated from HIV-1–infected people.31,33-35 In some patients, although rare, the CD34+ cells appear to be infected by HIV-1.36-38 Second, the hematopoietic microenvironment may have been destroyed by HIV-1 infection.4 HIV-1 infection of human bone marrow-derived stromal cells have been shown to abolish their ability to support murine hematopoiesis.39 Thymic epithelial cells have also been shown to be infected and destroyed by HIV-1.40 Third, low levels of gene transduction and expression in a clinical setting may be ineffective, because HIV-1 infection can result in indirect cell killing of uninfected cells.41 42 A threshold of viral load reduction may have to be achieved to prevent the indirect cell killing. Alternatively, therapies preventing HIV-induced cell death may be necessary in combination with anti-HIV gene therapy.
One of the concerns with gene therapy in general is that the transgene may have potential adverse effects on normal cell functions. It has recently been reported that overexpression of the wild-type rev gene in human cells is cytotoxic.43 However, it has been shown that expression of RevM10 in human PBL has no adverse effect on the functions and growth of the transduced PBL.19 In our report, the primary myeloid cells derived from transduced HSCs appear to be phenotypically normal and ReM10 expression did not change the efficiency of differentiation or the cellular phenotype. HIV-1 infection occurs only if infected with a macrophage-tropic isolate. Challenges with T-tropic HIV-1 isolates failed to establish productive infection (data not shown). The long-term reconstitution of SCID-hu bone mice by the transduced HSCs indicated that the RevM10 gene product, at least in some transduced cells, did not inhibit HSCs from differentiating into the myeloid lineage. Because of the variations between different reconstituted SCID-hu mice, it is not possible to exclude the possibility of any subtle inhibitory effect of RevM10. The UCB CD34+ cells transduced with the retroviral vectors were also capable of giving rise to normal human T cells in long-term reconstitution of the SCID-hu Thy/Liv mouse (Akkina et al,44 Plavec et al,29 and unpublished results). Thus, HSCs with long-term reconstituting activity can be efficiently transduced and can differentiate into myeloid or lymphoid cell lineages with the expression of the RevM10 gene.
The MLV LTR has been shown to be a weak promoter in most murine primary cells or in mouse in vivo models.45-47 However, it seems to be a very active promoter in a number of human cell lines (Naviaux and Verma,47 Williams,48 Frazier and Garcia,49 and unpublished results). The data presented here further support the notion that the MLV LTR is an adequate suitable promoter for human HSC gene therapy. In our experience, MLV LTR not only supports transgene expression in stem/progenitor cells29 but also in differentiated T29 and myeloid cells (this report) derived from these progenitor cells in vivo. The HIV-1 infection of HSC-derived myeloid cells appears to be limited by both target cell frequency and initial viral doses (Fig 4). It seems to be a rare, inefficient process, especially with a low challenge dose of HIV-1. Using a higher dose of challenge, the degree of efficacy of RevM10 was found to decrease, as shown in Fig 4B2. Cell-to-cell transmission may be important to give rise to detectable viral production in the supernatant. The expression of revM10 in 80% to 90% of the target cells may be enough to efficiently block cell-to-cell transmission and replication, although initial infection of some target cells has happened. In addition, we have evidence that the MLV LTR can be upregulated by T-cell activation (Bonyhadi et al, unpublished observation). Thus, it is possible that this upregulation also occurs during the differentiation and/or HIV infection process. Therefore, MLV LTR-based retroviral vectors appear to be adequate gene transfer vehicles to express transgenes in human immune system via the human HSC.
The RevM10 gene has been tested in a number of studies in which it has been shown to inhibit HIV-1 replication in T cells. Comparison of a number of anti-HIV gene products to inhibit primary HIV-1 isolates has indicated that RevM10 is the most efficient in inhibiting HIV-1 replication in primary T cells.18 The anti-HIV efficacy data shown in this report suggest that RevM10 can also function efficiently in the primary myeloid cells. It is therefore conceivable that it will be effective in humans.
ACKNOWLEDGMENT
We thank Drs J.M. McCune, D. Baltimore, and B.P. Chen for critical reading of the manuscript and the SyStemix Comparative Medicine Group for their expert production of SCID-hu mice. We also thank I. Plavec and R. Rigg for the retroviral vectors and S. Chen for help with the SCID-hu bone assay.
The Progenesys Program at SyStemix is an HIV gene therapy Research and Development Collaboration jointly sponsored by Sandoz and SyStemix.
Address reprint requests to Hideto Kaneshima, MD, PhD, Progenesys Program, SyStemix, Inc, 3155 Porter Dr, Palo Alto, CA 94304.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal