Abstract
Topotecan (TPT) is a topoisomerase I (topo I) poison that has shown promising antineoplastic activity in solid tumors and acute leukemia. In the present study, a band depletion assay was used to evaluate the ability of TPT to stabilize topo I-DNA adducts in human leukemia cell lines and in clinical leukemia samples ex vivo. This assay showed that 50% of the cellular topo I in HL-60 human myelomonocytic leukemia cells became covalently bound to DNA at an extracellular TPT concentration of 4 μmol/L. In contrast, in 13 clinical specimens of human leukemia harvested before treatment of patients with TPT, the TPT concentration required to stabilize 50% of the cellular topo I in topo I-DNA complexes ranged from 3 to greater than 100 μmol/L (median, 30 μmol/L). Flow microfluorimetry showed that cellular TPT accumulation varied over only a twofold range and failed to provide evidence for transport-mediated resistance in the clinical samples. These observations raise the possibility that formation of topo I-DNA adducts is diminished in many specimens of refractory/relapsed acute leukemia by a mechanism that might alter topo I sensitivity to TPT.
TOPOTECAN (TPT) is a recently approved antineoplastic agent that stabilizes covalent complexes between the nuclear enzyme topoisomerase I (topo I) and DNA (reviewed in Slichenmyer et al,1 Chen and Liu,2 Potmesil,3 and Pommier et al4 ). Interaction of these topo I-DNA adducts with replication complexes results in the formation of small numbers of cytotoxic DNA double-strand breaks, which in turn set in motion events resulting in tumor cell apoptosis.5 6
TPT has shown promising activity in a number of solid tumors, including neuroblastoma, small cell lung cancer, platinum-resistant ovarian cancer, and squamous cell carcinoma of the head and neck (reviewed in Rowinsky et al7 ). Phase I trials in patients with refractory and relapsed leukemia have shown that the dose can be escalated at least threefold on two different schedules before dose-limiting nonhematologic toxicity is observed.8-10
Multiple mechanisms of resistance to TPT and the parent agent camptothecin (CPT) have been previously described in tissue culture cells (reviewed in Slichenmyer et al,1 Chen and Liu,2 Potmesil,3 and Pommier et al4 ). First, TPT accumulation is diminished in cells that overexpress one of several drug transporters, including P-glycoprotein and the more recently described mitoxantrone/TPT multidrug transporter.11-13 Second, cells with diminished topo I content form fewer topo I-DNA adducts and are resistant to this class of agents (reviewed in Slichenmyer et al,1 Chen and Liu,2 Potmesil,3 and Pommier et al4; see also Sorensen et al14 ). Finally, cells with alterations in topo I that affect the drug-induced stabilization of topo I-DNA adducts are resistant to this class of agents. Most previous studies dealing with this mechanism of resistance have focused on CPT-selected cell lines containing topo I mutations that affect the ability of the enzyme-DNA complex to be stabilized by CPT congeners (reviewed in Slichenmyer et al,1 Chen and Liu,2 Potmesil,3 and Pommier et al4 ). However, Goldwasser et al15 have recently reported that the concentration of CPT required to stabilize topo I-DNA adducts varies dramatically among previously untreated colon cancer cell lines, even though the cell lines have similar CPT accumulation and similar topo I content. These observations raise the possibility that some other difference in topo I, possibly a posttranslational modification, might also affect the ability of this class of agents to stabilize topo I-DNA adducts.
There are relatively few studies of these potential mechanisms of resistance in clinical specimens from patients receiving topo I-directed agents. As an adjunct to two phase I trials of TPT in acute leukemia, we studied the formation of TPT-stabilized topo I-DNA adducts in marrow mononuclear cells from patients receiving TPT-containing reinduction chemotherapy. For this purpose, we adapted a previously described approach termed a band depletion assay.12 16-18 This assay is based on the observation that topo I covalently bound to DNA migrates more slowly than free topo I on sodium dodecyl sulfate (SDS)-polyacrylamide gels. Accordingly, as increasing topo I-DNA adducts are formed in the presence of TPT, a corresponding decrease in the molecular weight (Mr ) ∼100,000 signal for topo I is observed on immunoblots. When this assay was applied to clinical leukemia samples, a wide variation in the extracellular TPT concentration required to convert half of the cellular topo I to covalent topo I-DNA complexes was observed. Interestingly, sequential assays performed in cells from a single patient harvested before administration of TPT and 8 weeks later at the time of leukemic regrowth indicated that the TPT concentration required to stabilize topo I-DNA adducts was higher at relapse, even though the cellular topo I content and TPT accumulation did not decrease. These observations suggest that measurements of topo I-DNA adduct formation rather than topo I content alone might be required to understand TPT resistance in the clinical setting.
MATERIALS AND METHODS
Reagents and antibodies.TPT was kindly provided by Dr Randall K. Johnson (SmithKline Beecham, King of Prussia, PA). ECL enhanced chemiluminescence reagents were from Amersham (Arlington Heights, IL). Sources of all other reagents were previously described.19
C-21 murine monoclonal anti-topo I was raised as previously described.20 The following murine monoclonal antibodies (kindly provided by the indicated investigators) were also used: Ki-S1 anti-topo IIα21 (Dr Udo Kellner, University of Kiel, Kiel, Germany), C-2-10 anti-poly(ADP-ribose) polymerase22 (Dr Guy Poirier, Laval University, Quebec City, Canada), and anti-histone H1 (Dr James Sorace, Veteran's Affairs Medical Center, Baltimore, MD). Chicken polyclonal antiserum to the nucleolar polypeptide B23/nucleophosmin was raised against gel-purified murine B23 as described.23 Affinity-purified peroxidase-coupled secondary antibodies were purchased from KPL (Gaithersburg, MD).
Cell isolation.Bone marrow samples were obtained from leukemia patients enrolled in two phase I trials of TPT between February 1992 and October 1995. Clinical and laboratory aspects of these phase I trials were performed according to protocols approved by Institutional Review Boards in compliance with policies of the US Department of Health and Human Services. As reported previously,9,10 all patients had blast crisis chronic myelogenous leukemia (CML-BC); acute myelogenous leukemia (AML) that regrew after therapy that included cytarabine and daunorubicin, usually in conjunction with amsacrine or etoposide24,25; or acute lymphocytic leukemia (ALL) that regrew after prednisone, vincristine, etoposide, L-asparaginase, daunorubicin, and cytarabine.26 Heparinized bone marrow aspirates were obtained from the posterior iliac crests of the patients before the initiation of TPT chemotherapy. To isolate fractions enriched in blasts, marrows were sedimented on ficoll-Hypaque step gradients (d = 1.079 and 1.119 g/mL) as previously described.24 Cells collected from the upper interface were diluted with buffer A (serum-free RPMI 1640 containing 10 mmol/L HEPES, pH 7.4), sedimented at 200g for 10 minutes, and resuspended in buffer A at a concentration of 1 to 3 × 106 cells/mL.
HL-60, KG1a, and K562 human leukemia cell lines were propagated in RPMI 1640 medium containing 10% (HL-60) or 5% (KG1a and K562) heat-inactivated fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 2 mmol/L glutamine. Before the band depletion assay, cells were collected on ficoll-Hypaque gradients (d = 1.119 g/mL) to remove nonviable cells, sedimented, and resuspended in buffer A as described above.
Band depletion assay.Assays were performed prospectively within 3 hours after marrow aspiration. Aliquots of marrow mononuclear cells or HL-60 cells in 1 mL buffer A were added to 1.5-mL microfuge tubes containing 0, 3, 10, 30, or 100 μmol/L TPT (final concentration) added in 5 μL dimethyl sulfoxide (DMSO). After samples were incubated at 37°C for 45 minutes, they were sedimented at 3,200g for 1 minute. Proceeding one tube at a time, supernatants were aspirated and pellets were solubilized by addition of 1 mL of lysis buffer27 containing 6 mol/L guanidine hydrochloride as well as 250 mmol/L Tris-HCl (pH 8.5 at 21°C), 10 mmol/L EDTA, 1% (vol/vol) freshly added β-mercaptoethanol, and 1 mmol/α-phenylmethyl sulfonyl fluoride (added immediately before use from a 100-fold stock prepared in anhydrous isopropanol) followed by brisk vortexing. Under these conditions, guanidine hydrochloride is a strong protein denaturant28,29 that traps preexisting covalent topo I-DNA complexes. After ≥4 hours of incubation at 20°C, samples were sonicated to shear the viscous DNA, treated with iodoacetamide to block free sulfhydryl groups, dialyzed at 4°C into 4 mol/L urea and then into 0.1% (wt/vol) SDS,27 lyophilized to dryness, and stored at −20°C. Immediately before electrophoresis, samples were solubilized at a concentration of 3 × 107 cell equivalents/mL by heating to 65°C for 20 minutes in SDS sample buffer consisting of 4 mol/L urea, 2% (wt/vol) SDS, 62.5 mmol/L Tris-HCl (pH 6.8), and 1 mmol/L EDTA. Aliquots containing 3 × 105 diluent- or TPT-treated cells were applied to adjacent wells of an SDS-polyacrylamide gel containing a 5% to 15% (wt/vol) acrylamide gradient. To provide a standard curve that was constant from gel to gel, aliquots containing 0.3 × 105, 0.75 × 105, 1.5 × 105, and 3.0 × 105 HL-60 cells were also applied to each gel. After electrophoresis, samples were electrophoretically transferred to nitrocellulose. The blots were stained with 0.1% (wt/vol) Fast Green FCF in 50% (vol/vol) methanol-5% (vol/vol) acetic acid to confirm efficient protein transfer and irreversibly affix the polypeptides, destained with 50% (vol/vol) methanol-5% (vol/vol) acetic acid, and treated with blocking solution consisting of 10% (wt/vol) powdered milk, 150 mmol/L NaCl, and 10 mmol/L Tris-HCl (pH 7.4 at 21°C). Blots were then probed with C-21 anti-topo I followed by peroxidase-coupled goat antimouse IgM using techniques previously described in detail.9,30 After the bound secondary antibody was detected using enhanced chemiluminescence, signals on the resulting x-ray film were digitized and quantitated (area × intensity) as previously described24 and then compared with the serial dilution of untreated HL-60 cells on each blot. This permitted determination of the relative topo I signal at Mr ∼100,000 in each sample at various drug concentrations.
Assessment of cellular TPT accumulation.TPT accumulation was examined by flow microfluorimetry.12 In brief, marrow mononuclear cells suspended at a concentration of 1 to 3 × 106 cells/mL in buffer A were treated with 25 μmol/L TPT for 30 minutes at 37°C and then immediately analyzed on a FACScan flow microfluorimeter (Beckton Dickinson, Mountain View, CA). Detection was performed using a 15-mW argon laser with a 488-nm excitation filter and a 585-nm emission filter with a bandwidth of 42 nm. Fifteen thousand events were collected for each sample. The mean fluorescence intensity was determined using software supplied by the manufacturer. Along with each marrow sample, KG1a cells were simultaneously subjected to the same assay.
RESULTS
Characterization of band depletion assay using human leukemia cell lines.A band depletion assay was used to study the TPT-induced stabilization of topo I-DNA adducts in intact leukemia cells. The basis for this assay is the observation that topo I covalently bound to DNA migrates more slowly than unbound topo I. Accordingly, as cells are incubated in increasing TPT concentrations and more covalent topo I-DNA complexes are stabilized, the topo I signal at Mr ∼100,000 on Western blots diminishes. By comparing the TPT-induced attenuation of this signal with the signal produced by a serial dilution of untreated cells, the percentage of initially detectable topo I that becomes covalently bound to DNA at each TPT concentration can be assessed.
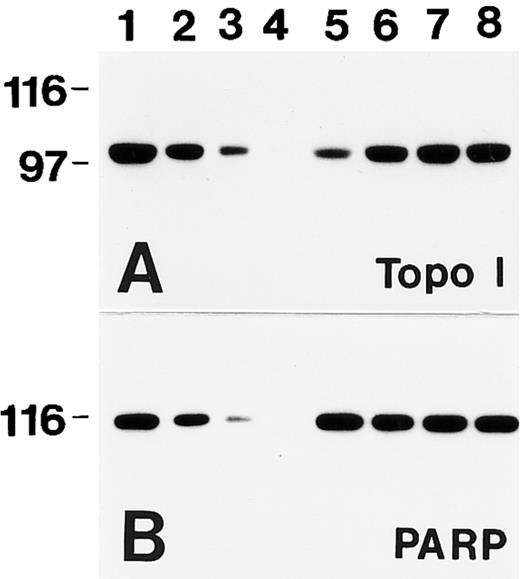
An example of this assay is shown in Fig 1. HL-60 cells were incubated with increasing concentrations of TPT for 45 minutes and then immediately lysed under denaturing conditions. Subsequent immunoblotting with a monoclonal anti-topo I antibody showed that the signal for topo I decreased as the concentration of TPT increased (Fig 1A, lanes 5 through 8). Comparison to a standard curve generated by serial dilution of the diluent-treated HL-60 cells (Fig 1A, lanes 1 through 4) permitted quantitation of the signal at Mr ∼100,000 in each TPT-treated sample (Fig 1F ). In these cells, 50% of the initially detectable topo I became covalently bound to DNA after treatment with 4 ± 2 μmol/L TPT (mean ± SD, n = 3). In contrast, immunoblotting failed to show any TPT-induced alteration in topo IIα (Fig 1B), an enzyme that performs a related reaction. Likewise, the signals for poly(ADP-ribose) polymerase and B23, two polypeptides with subnuclear distributions similar to that of topo I,30 31 were not altered by TPT treatment (Fig 1C and D). These controls show that the topo I signal is selectively diminished by TPT treatment.
TPT treatment results in selective depletion of the Mr ∼100,000 topo I signal. (A through E) HL-60 cells were treated with DMSO (lanes 1 through 4 and 9) or 100 μmol/L (lane 5), 30 μmol/L (lane 6), 10 μmol/L (lane 7), or 3 μmol/L (lane 8) TPT for 45 minutes, lysed under denaturing conditions, and prepared for SDS-polyacrylamide gel electrophoresis. Gels were loaded with polypeptides from 3 × 105 (lanes 1 and 5 through 9), 1.5 × 105 (lane 2), 0.75 × 105 (lane 3), or 0.3 × 105 cells (lane 4). After electrophoresis, samples were transferred to nitrocellulose. Blots were probed with antibodies that recognize topo I (A), topo IIα (B), poly(ADP-ribose) polymerase (C), B23 (D), or histone H1 (E). (F ) Relative topo I signal (from [A]) plotted as a function of TPT concentration.
TPT treatment results in selective depletion of the Mr ∼100,000 topo I signal. (A through E) HL-60 cells were treated with DMSO (lanes 1 through 4 and 9) or 100 μmol/L (lane 5), 30 μmol/L (lane 6), 10 μmol/L (lane 7), or 3 μmol/L (lane 8) TPT for 45 minutes, lysed under denaturing conditions, and prepared for SDS-polyacrylamide gel electrophoresis. Gels were loaded with polypeptides from 3 × 105 (lanes 1 and 5 through 9), 1.5 × 105 (lane 2), 0.75 × 105 (lane 3), or 0.3 × 105 cells (lane 4). After electrophoresis, samples were transferred to nitrocellulose. Blots were probed with antibodies that recognize topo I (A), topo IIα (B), poly(ADP-ribose) polymerase (C), B23 (D), or histone H1 (E). (F ) Relative topo I signal (from [A]) plotted as a function of TPT concentration.
Recent reports have suggested that prolonged incubation with CPT is accompanied by topo I degradation.32 This phenomenon does not appear to be responsible for the decreased topo I signal observed in Fig 1. First, the band depletion assay involves only 45 minutes of incubation with TPT, a time frame much shorter than the period reportedly required for CPT-induced topo I downregulation.32 Second, results depicted in Fig 2 provide direct evidence that the TPT-induced change in topo I signal does not reflect topo I degradation. In this experiment, samples treated with 10 μmol/L TPT (lane 5) were heated to 48°C for 1 to 3 minutes in the continued presence of TPT and then lysed under denaturing conditions (lanes 6 through 8). By inhibiting the cleavage step of topo I but not its religation activity, heat treatment would be expected to reverse the effect of TPT on the cleavage-religation equilibrium of the enzyme and thereby decrease the amount of topo I that is covalently bound to DNA when the denaturing buffer is added.17 Consistent with this prediction, heat treatment resulted in the prompt reappearance of the initial topo I signal on blots (Fig 2), a result that would not be expected if topo I had been degraded.
Band depletion does not reflect destruction of topo I. HL-60 cells were treated with diluent (lanes 1 through 4) or 10 μmol/L TPT for 45 minutes at 37°C (lanes 5 through 8) and sedimented at 3,200g for 1 minute. All of the supernatant except for 50 μL was removed. Cells were resuspended in the remaining 50 μL of supernatant and heated to 48°C for 0 minutes (lanes 1 through 5), 1 minute (lane 6), 2 minutes (lane 7), or 3 minutes (lane 8) before addition of lysis buffer. After preparation for electrophoresis as described in the Materials and Methods, samples containing polypeptides from 3 × 105 cells (lanes 1 and 5 through 8) or the serial dilution described in the legend to Fig 1 (lanes 2 through 4) were loaded in adjacent lanes. Blots were probed with antibodies to topo I (A) or poly(ADP-ribose) polymerase (B).
Band depletion does not reflect destruction of topo I. HL-60 cells were treated with diluent (lanes 1 through 4) or 10 μmol/L TPT for 45 minutes at 37°C (lanes 5 through 8) and sedimented at 3,200g for 1 minute. All of the supernatant except for 50 μL was removed. Cells were resuspended in the remaining 50 μL of supernatant and heated to 48°C for 0 minutes (lanes 1 through 5), 1 minute (lane 6), 2 minutes (lane 7), or 3 minutes (lane 8) before addition of lysis buffer. After preparation for electrophoresis as described in the Materials and Methods, samples containing polypeptides from 3 × 105 cells (lanes 1 and 5 through 8) or the serial dilution described in the legend to Fig 1 (lanes 2 through 4) were loaded in adjacent lanes. Blots were probed with antibodies to topo I (A) or poly(ADP-ribose) polymerase (B).
Altered stabilization of topo I-DNA complexes in fresh leukemia samples.The band depletion assay was used to prospectively examine the formation of topo I-DNA adducts in leukemic marrow samples. Pretreatment bone marrow aspirates were obtained from 27 of 31 leukemia patients enrolled in two phase I trials of TPT-containing chemotherapy. Cell counts were inadequate for performance of the present assay in 7 specimens, and the topo I level was below the limit of reliable quantitation in 7 specimens. Results obtained with the remaining 13 pretreatment samples, which contained 82% to 100% blasts, are shown in Fig 3 and summarized in Fig 4.
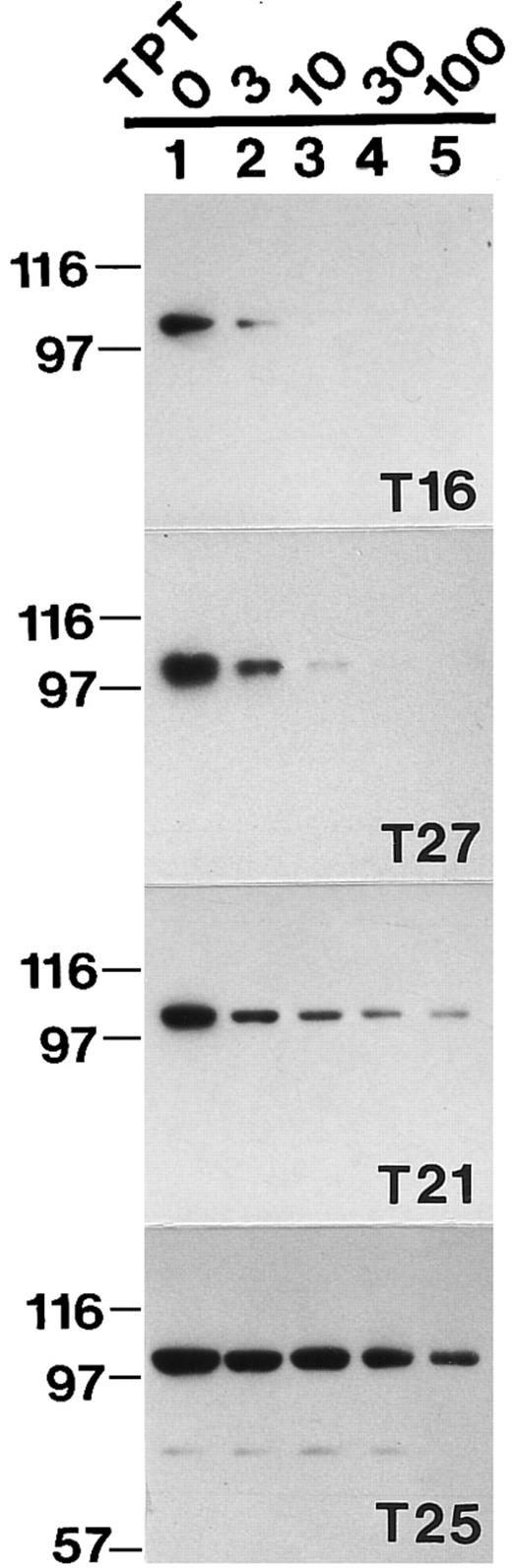
Topo I band depletion assay in clinical leukemia samples. Aliquots of freshly isolated blasts from 4 different leukemia patients were incubated at 37°C for 30 minutes in buffer A containing 0 (lane 1), 3 μmol/L (lane 2), 10 μmol/L (lane 3), 30 μmol/L (lane 4), or 100 μmol/L TPT (lane 5); lysed; and subjected to SDS-polyacrylamide gel electrophoresis followed by Western blotting with anti-topo I antibody.
Topo I band depletion assay in clinical leukemia samples. Aliquots of freshly isolated blasts from 4 different leukemia patients were incubated at 37°C for 30 minutes in buffer A containing 0 (lane 1), 3 μmol/L (lane 2), 10 μmol/L (lane 3), 30 μmol/L (lane 4), or 100 μmol/L TPT (lane 5); lysed; and subjected to SDS-polyacrylamide gel electrophoresis followed by Western blotting with anti-topo I antibody.
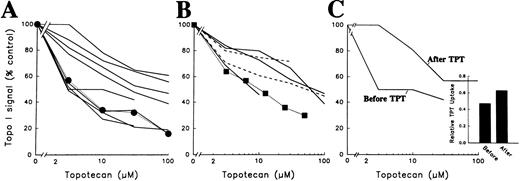
Quantitative analysis of band depletion assay in samples of relapsed/refractory acute leukemia and CML-BC. (A) Densitometry of the 8 AML samples examined by this method before TPT treatment (─ ) and of HL-60 cells subjected to the assay (•, ⋅⋅⋅⋅). To provide a densitometry standard, serial dilutions of control HL-60 cells were included on each blot as depicted in Figs 1 and 2. Truncated lines indicate that the signal was not quantifiable at the next drug concentration. (B) Densitometry of ALL ( ─ ), CML-BC samples (- - -), and K562 CML cells (▪, ⋅⋅⋅⋅) subjected to the same assay. (C) Densitometry of leukemia samples obtained from a single AML patient before and 8 weeks after TPT therapy. Both samples contained greater than 97% blasts. (Inset) TPT accumulation in the paired leukemia specimens before and 8 weeks after TPT therapy. Data are expressed as the mean fluorescence intensity of the clinical specimen divided by the mean fluorescence intensity of KG1a cells subjected to the same assay simultaneously.
Quantitative analysis of band depletion assay in samples of relapsed/refractory acute leukemia and CML-BC. (A) Densitometry of the 8 AML samples examined by this method before TPT treatment (─ ) and of HL-60 cells subjected to the assay (•, ⋅⋅⋅⋅). To provide a densitometry standard, serial dilutions of control HL-60 cells were included on each blot as depicted in Figs 1 and 2. Truncated lines indicate that the signal was not quantifiable at the next drug concentration. (B) Densitometry of ALL ( ─ ), CML-BC samples (- - -), and K562 CML cells (▪, ⋅⋅⋅⋅) subjected to the same assay. (C) Densitometry of leukemia samples obtained from a single AML patient before and 8 weeks after TPT therapy. Both samples contained greater than 97% blasts. (Inset) TPT accumulation in the paired leukemia specimens before and 8 weeks after TPT therapy. Data are expressed as the mean fluorescence intensity of the clinical specimen divided by the mean fluorescence intensity of KG1a cells subjected to the same assay simultaneously.
As was the case in HL-60 cells (Fig 1), treatment with TPT resulted in a dose-dependent decrease in the signal for topo I at Mr ∼100,000 in the clinical leukemia samples (Fig 3, lanes 2 through 5). However, there were marked differences between specimens. In some samples (eg, Fig 3, top panel), the topo I signal was markedly diminished at 3 μmol/L TPT, the lowest concentration examined. In contrast, much higher concentrations were required to achieve a similar decrease in the Mr ∼100,000 topo I signal in other samples (Fig 3, bottom panels). Blotting with antibodies to other nuclear polypeptides once again confirmed the equal loading of all lanes within a given panel and showed the specificity of this band depletion for topo I (data not shown).
Quantitation of the signals (see the Materials and Methods) on these and additional blots showed that the concentration of TPT required to deplete the topo I signal to 50% of initial values (the ED50 ) ranged from ∼3 μmol/L to greater than 100 μmol/L (Fig 4 A and B), with a median value of 30 μmol/L. There was no obvious relationship between the type of leukemia (AML v ALL v CML-BC) and the ED50 (Fig 4A and B), although the number of samples in each category was small. Likewise, there was no correlation between ED50 and age or leukocyte count (data not shown).
Although marrow aspirates were not routinely obtained from patients when their leukemias recurred after TPT therapy, paired samples (one obtained before TPT and the other obtained at the time of leukemic regrowth after TPT therapy) were available from 1 patient. Interestingly, comparison of these samples showed a marked increase in the TPT concentration required to stabilize topo I-DNA adducts at the time the leukemia regrew (Fig 4C).
The requirement for elevated TPT concentrations to stabilize the topo I-DNA adducts might reflect diminished TPT accumulation or altered ability of TPT to stabilize topo I-DNA adducts. To distinguish between these possibilities, whole cell TPT accumulation was examined by flow microfluorimetry. Previous experiments have shown that fluorescence increases in a linear fashion with TPT accumulation, that greater than 90% of the fluorescence observed under the assay conditions is due to TPT accumulation, and that alterations in TPT accumulation due to transport-mediated resistance can be readily detected with this technique.12 13 When this assay was applied to the paired leukemia specimens, there was no evidence for diminished TPT accumulation in the later specimen (inset, Fig 4C). Application of this assay to 8 additional pretreatment specimens (not shown) showed that the TPT accumulation in these specimens varied over a twofold range. These results suggest that differences in TPT accumulation do not account for the 30-fold range of TPT concentrations required to stabilize topo I-DNA adducts in intact cells.
DISCUSSION
The present study describes a quantitative approach for assessing the stabilization of covalent topo I-DNA complexes in intact leukemia cells and shows that the extracellular TPT concentration required to stabilize these complexes varies widely among different leukemia specimens. These observations provide a potential explanation for the previously described differences in TPT sensitivity observed in clinical leukemia specimens using a clonogenic assay9 and raise the possibility that assays other than measurement of cellular topo I content might be required to understand mechanisms of resistance to topo I-directed agents in the clinical setting.
Several aspects of TPT action have been previously examined in clinical specimens. Subramanian et al33 reported that topo I-DNA adducts could be quantitated by lysing cells under denaturing conditions, separating the DNA-protein complexes from unbound protein on cesium chloride gradients, and quantitating the topo I in the DNA-containing fractions by Western blotting with antibodies to topo I. When this assay was applied to peripheral blood samples from patients receiving TPT therapy for solid tumors, differences in the stabilization of topo I-DNA adducts were observed. However, this assay did not distinguish between differences in TPT serum concentrations, variations in cellular TPT accumulation, alterations in topo I content, or differences in sensitivity of topo I-DNA adducts to TPT-induced stabilization, all of which are factors that might contribute to the observed variation in topo I-DNA complexes stabilized.
Ellis et al34 measured the stabilization of covalent topo I-DNA complexes in intact cells by lysing the cells under denaturing conditions, collecting the topo I-DNA complexes on nitrocellulose filters, and hybridizing the filters to radiolabeled alu sequences to quantitate the topo I-bound DNA. Application of this assay to clinical leukemia specimens showed that the quantity of topo I-DNA adducts formed in different specimens varied widely at the same extracellular TPT concentration ex vivo.8 This observation indicated that differences in cellular TPT pharmacology in different human leukemia specimens might contribute to altered topo I-DNA adduct formation, but could not discern whether the variations from specimen to specimen were due to differences in drug accumulation, topo I content or sensitivity of topo I-DNA adducts to TPT-induced stabilization.
In the present study, a different approach was used to study stabilization of topo I-DNA adducts in intact cells. Samples were incubated with increasing concentrations of TPT ex vivo, then lysed under denaturing conditions and subjected to Western blotting for topo I. The formation of topo I-DNA covalent complexes in this assay is manifest as a decrease in the Mr ∼100,000 topo I signal. Control studies performed using human leukemia cell lines showed that the TPT-induced band depletion was specific for topo I (Fig 1) and was highly reproducible, with an ED50 of 4 ± 2 μmol/L TPT in HL-60 cells.
An advantage of the band depletion assay is that it distinguishes between low intracellular topo I-DNA complexes as a consequence of low topo I levels versus low topo I-DNA complexes due to other causes. However, this assay does have several potential limitations. First, because of the potential difficulty in isolating single-cell suspensions, this assay does not appear to be suitable for solid tumors. Second, the detection of topo I-DNA adducts in this assay requires TPT concentrations that are 100- to 1,000-fold higher than the concentrations of TPT required to inhibit cell proliferation during continuous drug exposures (cf, Fig 1 and Rowinsky et al9 ). This requirement for higher TPT concentrations arises because the band depletion assay requires the stabilization of large numbers of topo I-DNA complexes to produce a signal (loss of the Mr ∼100,000 band on Western blots), whereas cytotoxicity can result from the stabilization of small numbers of complexes if these complexes are converted into cytotoxic lesions (reviewed in Slichenmyer et al,1 Chen and Liu,2 and Froelich-Ammon and Osheroff35 ). Nonetheless, previous results in tissue culture cells have provided evidence that differences in the TPT concentration required to stabilize topo I-DNA adducts accurately reflect differences in TPT sensitivity in cytotoxicity assays.12
When this assay was applied to clinical leukemia samples from patients with relapsed and refractory acute leukemia, greater than 30-fold differences were observed in the extracellular TPT concentration required to stabilize 50% of the cellular topo I as covalent topo I-DNA complexes (Fig 4). The median ED50 was 30 μmol/L, a value much higher than the ED50 in human leukemia cell lines. To distinguish between the possibility of variations in cellular TPT accumulation versus differences in the ability of TPT to stabilize topo I-DNA complexes, cellular TPT accumulation was examined by flow microfluorimetry. TPT accumulation varied over a twofold range, a variation that does not appear large enough to account for the differences in TPT concentration required to stabilize topo I-DNA complexes. Instead, it appears that there might be differences in sensitivity of topo I to TPT-induced stabilization of cleavable complexes. It is possible that these differences reflect variations in a posttranslational modification of topo I such as phosphorylation or poly(ADP-ribosyl)ation (reviewed in Slichenmyer et al1 ). However, we cannot at present rule out the possibility of previously undetected allelic variation in topo I or even subtle somatic mutations in topo I as a consequence of previous therapy. Whatever the cause of the differences in TPT concentration required to stabilize topo I-DNA complexes, the present observations show that measurement of topo I levels alone might not give an accurate representation of TPT action in clinical samples. Instead, pharmacodynamic assays such as the band depletion assay or the other assays discussed above might be required to study individual variations in the formation of enzyme-DNA adducts after treatment with topo I-directed agents.
ACKNOWLEDGMENT
We thank Drs U. Kellner and J. Sorace for their kind gifts of antibodies; W. Berry, C. Buckwalter, T. Kottke, and L. Prichard for assistance with some of the specimens; the nurses, physician's assistants, and housestaff of the John Hopkins Oncology Center Adult Leukemia Service for their expert patient care that helped make these studies possible; and D. Strauss for editorial assistance.
Supported in part by a grant from the American Cancer Society (DHP-46). S.H.K. is a Leukemia Society Scholar.
Address reprint requests to Scott H. Kaufmann, MD, PhD, Division of Oncology Research, Guggenheim 1342C, Mayo Clinic, 200 First St, SW, Rochester, MN 55905.

![Fig. 1. TPT treatment results in selective depletion of the Mr ∼100,000 topo I signal. (A through E) HL-60 cells were treated with DMSO (lanes 1 through 4 and 9) or 100 μmol/L (lane 5), 30 μmol/L (lane 6), 10 μmol/L (lane 7), or 3 μmol/L (lane 8) TPT for 45 minutes, lysed under denaturing conditions, and prepared for SDS-polyacrylamide gel electrophoresis. Gels were loaded with polypeptides from 3 × 105 (lanes 1 and 5 through 9), 1.5 × 105 (lane 2), 0.75 × 105 (lane 3), or 0.3 × 105 cells (lane 4). After electrophoresis, samples were transferred to nitrocellulose. Blots were probed with antibodies that recognize topo I (A), topo IIα (B), poly(ADP-ribose) polymerase (C), B23 (D), or histone H1 (E). (F ) Relative topo I signal (from [A]) plotted as a function of TPT concentration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.2098/3/m_bl_0014f1ae.jpeg?Expires=1767715106&Signature=XBTKWob8xSqy~y~~hC9uxPoMemWduOErytE6iIel3o3jaCOC5j0~iaK3MmlSC6tyfEMaDN6dndcu3DjVC0D2uVRlgyx2mRMC5kOSkOGJADL-AvUCHmpPnm3zYAEoNROX1iA-VrW09C61nk81u3lVZKgtpxQJAEsXos7hNhcrhiv~FN7pWnxKfmh59U8~bCxiIOqhUlcue5Y9T2I2xOS1vDSKzkVX3lic94x4DmV-k59ck-HttU2lSUWB~7PEKMNWTFbM-hMZWOctJvHVO9re4bUhpg9ipa0EkLXrNTK5FO3XioAhzKfu~tMU5lUdNaLZG4Ql6~0Bk6RQKPnH0pUSEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. TPT treatment results in selective depletion of the Mr ∼100,000 topo I signal. (A through E) HL-60 cells were treated with DMSO (lanes 1 through 4 and 9) or 100 μmol/L (lane 5), 30 μmol/L (lane 6), 10 μmol/L (lane 7), or 3 μmol/L (lane 8) TPT for 45 minutes, lysed under denaturing conditions, and prepared for SDS-polyacrylamide gel electrophoresis. Gels were loaded with polypeptides from 3 × 105 (lanes 1 and 5 through 9), 1.5 × 105 (lane 2), 0.75 × 105 (lane 3), or 0.3 × 105 cells (lane 4). After electrophoresis, samples were transferred to nitrocellulose. Blots were probed with antibodies that recognize topo I (A), topo IIα (B), poly(ADP-ribose) polymerase (C), B23 (D), or histone H1 (E). (F ) Relative topo I signal (from [A]) plotted as a function of TPT concentration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.2098/3/m_bl_0014f1f.jpeg?Expires=1767715106&Signature=S~55ZiRBBIr73rmVbxVzmATwaiC-SMKm~9wK6aOvtH-rXldjLlz2XrNdxjYaBLLIOpb8NFQ1CmleMOyoB-XYQaIUO5Dsfqk1V5cfqLwHQtEUgx~rWIFDUouLTC76V08ZJ2rAU1vIkr7RmnYrTJfPWK0XoedBc2oq55lNxGyiisfjd4xniwcZS7hlnAkh7pexFP-feESFaECjZo3ueI5nDwNWP92-p4xH7a5hD2aELMbgqgs5uF6EJDLDr3U0lbxso3MPhLIJErz4-zwvJifr65pjEV-pEfGpgNIT30jL~8IjufpIrxsC9ZNf~YA5uu89WPj7ZyR7Mdm9I7mlRbi73Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal