Abstract
Transfusion-associated graft-versus-host disease (TA-GVHD) is one of the most serious adverse effects of blood transfusion. It is generally thought to be caused by the infused lymphocytes. Donor-derived cytotoxic T lymphocytes (CTLs) directed against the recipient's HLAs, which have escaped the recipient's immune system and are proliferating, are considered to attack recipient organs and tissues. Despite the seriousness of the disease, the precise mechanism of its development remains unclear and no definitive treatment has been developed. With the aim of developing an effective treatment, we established and characterized T-cell clones from peripheral blood lymphocytes (PBLs) of a TA-GVHD patient. Three types of clones were established. Type I clones were CD8+ and specifically lyse cells that express HLA B52. Type II clones were CD4+, specifically lysed cells that express HLA DR15, and proliferated in response to stimulation with cells that express DR15. Type III clones were also CD4+, showed no cytotoxic activity toward any HLA-expressing cells, and proliferated in response to stimulation with cells that express DR15. Furthermore, we found that the Fas/Fas-ligand (Fas-L) system is involved in the cytotoxicity of the type I and II clones and that the type III clones produce and secrete a large amount of tumor necrosis factor β (TNFβ) after antigen stimulation. Based on our results, these three types of clones can be classified into two categories: those that have the ability to induce GVHD directly by cytolysis and that show no cytotoxic activity and those that have the ability to cause GVHD indirectly through secretion of cytotoxic lymphokines.
THE CLINICAL MANIFESTATIONS of transfusion-associated graft-versus-host disease (TA-GVHD) include fever, rash, hepatitis, diarrhea, bone marrow aplasia, and pancytopenia.1,2 The symptoms are severe and progress rapidly, and the mortality rate of TA-GVHD is said to be greater than 90%.3,4 It is generally thought that cytotoxic T lymphocytes CTLs that have escaped the host immune system and have been stimulated by allogeneic host HLAs are the principal effector cells of TA-GVHD that might attack host organs and tissues.5-7 However, the precise mechanisms of the development of the disease remain unclear. Moreover, despite the severity of the disease, no effective treatment has been developed. The recent report of successful treatment with anti-CD3 monoclonal antibody (MoAb), OKT3, and cyclosporine A is the only such report published to date.8 In fact, not all of the manifestations of the disease are explicable solely in terms of CTL effects.
With the aim to develop an effective treatment for TA-GVHD, we established and characterized T-cell clones from peripheral blood lymphocytes (PBLs) of a TA-GVHD patient. We already reported the establishment of clones from PBLs of TA-GVHD patients and identified the target host HLAs in two cases.9-11 Here, we describe the establishment of three types of clones from TA-GVHD patient PBLs and our findings indicating that the targets of these clones are the patient's HLA B52 for type I, and DR15 for types II and III. Characterization of type I and II clones showed that they are CD8+ and CD4+ CTL clones, respectively, and that the Fas/Fas-L system12-14 is involved in their cytotoxicity. Moreover, the noncytotoxic CD4+ clones, type III, were found to produce and secrete tumor necrosis factor β (TNFβ)15 16 after antigen stimulation. Here again, we report findings indicating that not only direct effects of CTLs, but also indirect effects of humoral factors might play important roles in the development of TA-GVHD.
MATERIALS AND METHODS
Patient.A 69-year-old man underwent emergency surgery for ascending aorta replacement for an aortic aneurysm in September 1995. During surgery, he received 10 U of stored red blood cell concentrate, 2 U of fresh platelet concentrate derived from his son and daughter, and 4 U of fresh-frozen plasma. His postoperative course was stable until day 14 after surgery, when he developed a fever and a rash appeared on his chest bilaterally. Liver dysfunction occurred on the same day: serum AST and ALT concentrations were 309 and 484 IU/L, respectively. On postoperative day 17, the rash covered his entire body. Pancytopenia occurred on postoperative day 21: the white blood cell count and platelet count were 500/μL and 12,000/μL, respectively. After informed consent was obtained, blood samples were collected on postoperative day 21. Diarrhea appeared on postoperative day 22 and the patient died on postoperative day 23. Soon after his death, skin biopsy samples were collected from his forearm.
Confirmation of TA-GVHD diagnosis.Our diagnosis of TA-GVHD was confirmed by the results of analysis of microsatellite DNA polymorphisms as described previously.17 18 Briefly, high–molecular-weight DNA was extracted from PBLs and skin samples. Several microsatellite regions were amplified by polymerase chain reaction (PCR) and the products fractionated by electrophoresis in 5% polyacrylamide gel and stained with ethidium bromide. The differences in DNA types between the skin samples and PBLs confirmed the diagnosis of TA-GVHD. The responsible donor was identified as the patient's son from the results of the microsatellite DNA polymorphism analysis.
HLA typing.Patient HLA genotyping was performed using the same DNA as that used in the diagnostic test, according to the PCR–restriction fraction length polymorphism (RFLP) method described elsewhere.19 The HLAs of the responsible donor, the patient's son, were typed using a conventional serologic typing method.
Establishment of T-cell clones.PBLs were separated by Ficoll density-gradient centrifugation from the blood collected on postoperative day 21, and were then cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) in the presence of 20 U/mL of recombinant interleukin-2 (rIL-2; Genzyme, Boston, MA) and 5 μg/mL of phytohemagglutinin (PHA) for 7 days. The cell line thus established was subcloned by limiting dilution method (effector cell concentration of 3, 1, or 0.3 cells/well) in the presence of 20 U/mL of rIL-2 and 30 Gy irradiated allogeneic PBLs (5 × 104 cells/well) derived from healthy donors that shared at least one HLA locus with the patient.
The phenotypes of the established clones were analyzed on a FACScan (Cytoron; Ortho-Clinical Diagnostic KK, Tokyo, Japan), with a double staining method using fluorescein isothiocyanate (FITC)-conjugated anti-CD4 and phycoerythrin (PE)-conjugated anti-CD8 MoAbs (Becton Dickinson, Mountain View, CA).
Cytotoxicity assay.To assess the cytotoxicity of the clones against several targets, we performed a standard 4-hour 51Cr release assay as described elsewhere.20 The established clones were used as effector cells. As target cells, Epstein-Barr virus (EBV)-transformed B cells (B-LCLs) derived from PBLs of healthy donors were used. 51Cr-labeled target cells (1 × 104 cells/well) were mixed with effector cells (2 × 104 cells/well), and the mixture was incubated at 37°C for 4 hours (effector:target [E:T] ratio = 2:1), after which the amount of released radioactivity in each well was measured with a gamma-counter. The percents of specific lysis were determined as follows: 100% × [(experimental release cpm − spontaneous release cpm)/(maximum release cpm − spontaneous release cpm)]. For measurement of the spontaneous release, 51Cr-labeled target cells were incubated in the absence of effector cells. Maximum release was measured using 0.2% Triton X-100 instead of effector cells.
Proliferation assay.To determine the degrees of proliferation in response to stimulation with cells expressing specific HLAs, the established clones (5 × 104 cells) were mixed with 1 × 105 of 30 Gy irradiated allogeneic PBLs derived from healthy donors in 96-well microcloning plates in RPMI 1640 medium supplemented with 10% FCS in the absence of rIL-2. After incubation at 37°C for 24 hours, 1 μCi of 3H-thymidine was added periodically to each well while incubation was continued for another 18 hours at 37°C. The cells were harvested on a glass fiber filter and rates of uptake (cpm) of radioactivity into high–molecular-weight DNA were determined using a liquid scintillation counter.
Cytotoxicity assay of supernatants of cultures of the clones to L929 cells.To determine whether supernatants of cultures of the clones are cytotoxic to murine L929 cells, we performed conventional TNF assays using L929 cells.21 Briefly, serially diluted supernatants of 4-day cultures of the clones after stimulation with 30 Gy irradiated allogeneic PBLs having DRB1*1502 were added to monolayers of L929 cells in the presence of 1 μg/mL of actinomycin D in a flat-bottomed 96-well plate (6 × 104 L929 cells/well). After incubation at 37°C for 18 hours, the culture supernatants were discarded and the cells were fixed with 100% methanol for 2 minutes. The viable cells were stained with 0.05% methylene blue for 30 minutes. After five washes with distilled water, the dye was eluted with 0.2% HCl, and absorbance at 660 nm of each well was measured.
Blocking studies with MoAbs.To identify the target HLAs in the cytotoxicity assay, we performed blocking studies using MoAbs. 51Cr-labeled target cells were incubated at 37°C with saturating concentrations of anti-HLA class I, DR, DQ, and DP MoAbs (Becton Dickinson) for 60 minutes before effector cells were added. Noncytotoxic anti-Fas MoAb22 (clone ZB4; Medical & Biological Laboratories, Nagoya, Japan) was also preincubated at 37°C with 51Cr-labeled target cells at a final concentration of 500 ng/mL or 5 μg/mL for 60 minutes. Then effector cells were added and a 4-hour 51Cr release assay was performed as described earlier.
Neutralization of culture supernatants by anti-TNFβ MoAb. Supernatants of 4-day cultures of the clones after stimulation with 30 Gy irradiated allogeneic PBLs having DRB1*1502 in the presence of 20 U/mL of rIL-2 (initial cell concentration of 2 × 105 cells/mL) were incubated with saturating concentrations of anti-TNFβ MoAb (Boehringer Mannheim, Mannheim, Germany) for 60 minutes at 37°C. Along with nontreated supernatants, MoAb-treated supernatants were added with 1 μg/mL of actinomycin D to monolayers of L929 cells in 96-well flat-bottomed plates, which were then incubated at 37°C for 18 hours. Then the viable cells were stained with methylene blue as described earlier.
RESULTS
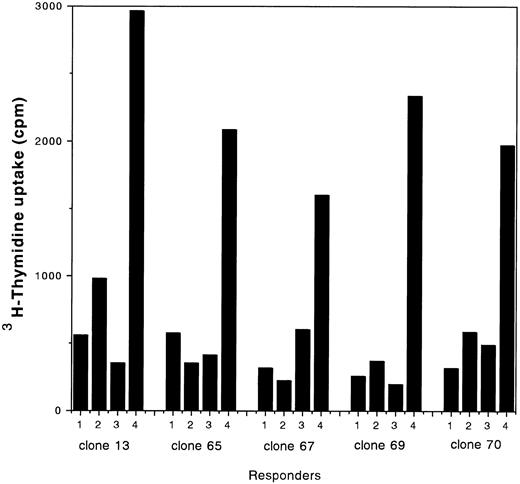
The HLA genotype of the patient was found to be A*2402, B*4002/*52011, DRB1*1502/0901 (A*2402 is serologically classified as A24, B*4002 is B61, B*52011 is B52, DRB1*1502 is DR15 and DRB1*0901 is DR9), and the donor was serologically typed as A24, B38/61, DR9. Through limiting-dilution subcloning of the cell line, several clones were obtained. From these, nine stable clones were established and were maintained for more than 6 months. From the results of microsatellite DNA polymorphism analysis, these clones were shown to be of the son type. Based on the specific reactivities determined in the cytotoxicity assays, these nine clones were classified into three types. Type I clones (clones 12, 28, 35, and 41), of which the surface phenotype was CD8+, specifically lysed cells that expressed HLA B52 (Table 1). HLA B52 is one of the patient HLAs that might have been recognized as alloantigens by the donor cells. Type II clones (clones 13, 69, and 70) were CD4+ and specifically lysed cells that carried DRB1*1502 or 1501; the products of both genes are serologically typed as DR15 (Table 1). HLA DR15 is also one of the patient HLAs that might have been recognized as alloantigens by the donor cells. Type III clones (clones 65 and 67) were CD4+, did not lyse any target cells (Table 1), and exhibited specific proliferation in response to stimulation with cells that carried DRB1*1502 (Fig 1). Type II clones also exhibited specific proliferation in response to stimulation with cells that carried DRB1*1502 (Fig 1).
Specific Lysis of Various Target Cells by 9 Clones
| Target . | HLA . | % Lysis by Clones . | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | A . | B . | DRB1* . | DQB1* . | CD8+ . | CD4+ . | . | . | . | . | . | . | . | |||||||
| . | . | . | . | . | 12 . | 28 . | 35 . | 41 . | 13 . | 65 . | 67 . | 69 . | 70 . | . | . | . | . | . | . | . |
| 1. | *0201, 2402 | *3501, 5101 | 1502, 08032 | 0601 | 0 | 0 | 0 | 0 | 23.0 | 0 | 0 | 30.3 | 24.0 | |||||||
| 2. | 11, 19 | 7, 40 | 1001, 1100 | 0501, 0301 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 3. | 24 | 52 | 2 | 3 | 45.8 | NT | NT | NT | 0 | 0 | 0 | 0 | 0 | |||||||
| 4. | *2402, 3303 | *4403, 5201 | 1502, 1406 | 0301, 0601 | 29.1 | 31.9 | 53.2 | 33.8 | 24.3 | 0 | 0 | 41.0 | 18.3 | |||||||
| 5. | 1 | 52 | 0101 | 0501 | 76.6 | 46.5 | 87.8 | 88.3 | 0 | 0 | 0 | 0 | 0 | |||||||
| 6. | *2402, 2602 | *5201, 4002 | 1405, 0901 | 03032, 05031 | NT | 26.2 | 44.8 | 15.7 | 0 | 0 | 0 | 0 | 0 | |||||||
| 7. | *2402, 1101 | *5201, 1501 | 0406, 1501 | 0302, 0602 | NT | 22.6 | 29.5 | 20.5 | 0 | 0 | 0 | 34.9 | 4.2 | |||||||
| 8. | *2402, 3303 | *5201, 4403 | 1302, 1501 | 0602, 0604 | NT | NT | NT | NT | 2.6 | 0 | 0 | 16.7 | 0 | |||||||
| Target . | HLA . | % Lysis by Clones . | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | A . | B . | DRB1* . | DQB1* . | CD8+ . | CD4+ . | . | . | . | . | . | . | . | |||||||
| . | . | . | . | . | 12 . | 28 . | 35 . | 41 . | 13 . | 65 . | 67 . | 69 . | 70 . | . | . | . | . | . | . | . |
| 1. | *0201, 2402 | *3501, 5101 | 1502, 08032 | 0601 | 0 | 0 | 0 | 0 | 23.0 | 0 | 0 | 30.3 | 24.0 | |||||||
| 2. | 11, 19 | 7, 40 | 1001, 1100 | 0501, 0301 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 3. | 24 | 52 | 2 | 3 | 45.8 | NT | NT | NT | 0 | 0 | 0 | 0 | 0 | |||||||
| 4. | *2402, 3303 | *4403, 5201 | 1502, 1406 | 0301, 0601 | 29.1 | 31.9 | 53.2 | 33.8 | 24.3 | 0 | 0 | 41.0 | 18.3 | |||||||
| 5. | 1 | 52 | 0101 | 0501 | 76.6 | 46.5 | 87.8 | 88.3 | 0 | 0 | 0 | 0 | 0 | |||||||
| 6. | *2402, 2602 | *5201, 4002 | 1405, 0901 | 03032, 05031 | NT | 26.2 | 44.8 | 15.7 | 0 | 0 | 0 | 0 | 0 | |||||||
| 7. | *2402, 1101 | *5201, 1501 | 0406, 1501 | 0302, 0602 | NT | 22.6 | 29.5 | 20.5 | 0 | 0 | 0 | 34.9 | 4.2 | |||||||
| 8. | *2402, 3303 | *5201, 4403 | 1302, 1501 | 0602, 0604 | NT | NT | NT | NT | 2.6 | 0 | 0 | 16.7 | 0 | |||||||
Specific lysis of various B-LCL targets by 9 clones was measured using a standard 4-hour 51Cr release assay at an E:T ratio of 2:1. HLAs of targets and the percentage of specific lysis by each clone are indicated. Greater than 10% specific lysis was scored as positive lysis and <10% specific lysis was scored as negative lysis. Donor HLA: A24, B38/61, DRB1*0901; patient HLA: A*2402, B*4002/52011, DRB1*1502/0901.
Abbreviation: NT, not tested.
Type II and III CD4+ clones were examined for their degrees of proliferation in response to stimulation with various allogeneic PBLs. Responder clones (5 × 104 cells) were cultured with 30 Gy irradiated allogeneic PBLs (1 × 105 cells). After 24 hours of culture, 1 μCi of 3H-thymidine was added periodically to each well while incubation was continued for another 18 hours at 37°C. The rate of uptake of 3H-thymidine (cpm) into high–molecular-weight DNA was determined using a liquid scintillation counter. Stimulators: (1) responder alone; (2) A*2402,3303, B*4403,5401, DRB1*0405,1302; (3) A*2402,2602, B*5201,4002, DRB1*1405,0901; (4) A*2402,3303, B*5201,4403, DRB1*1406,1502.
Type II and III CD4+ clones were examined for their degrees of proliferation in response to stimulation with various allogeneic PBLs. Responder clones (5 × 104 cells) were cultured with 30 Gy irradiated allogeneic PBLs (1 × 105 cells). After 24 hours of culture, 1 μCi of 3H-thymidine was added periodically to each well while incubation was continued for another 18 hours at 37°C. The rate of uptake of 3H-thymidine (cpm) into high–molecular-weight DNA was determined using a liquid scintillation counter. Stimulators: (1) responder alone; (2) A*2402,3303, B*4403,5401, DRB1*0405,1302; (3) A*2402,2602, B*5201,4002, DRB1*1405,0901; (4) A*2402,3303, B*5201,4403, DRB1*1406,1502.
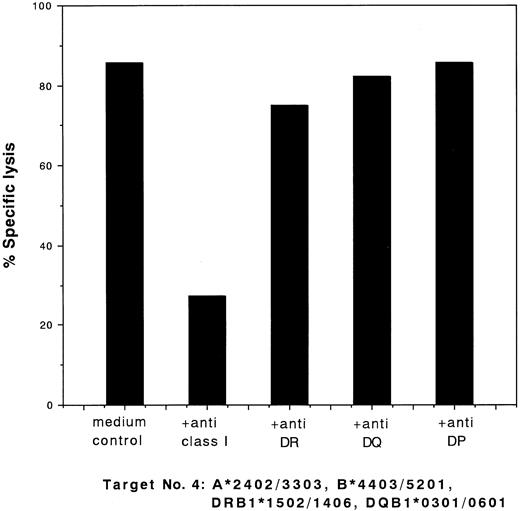
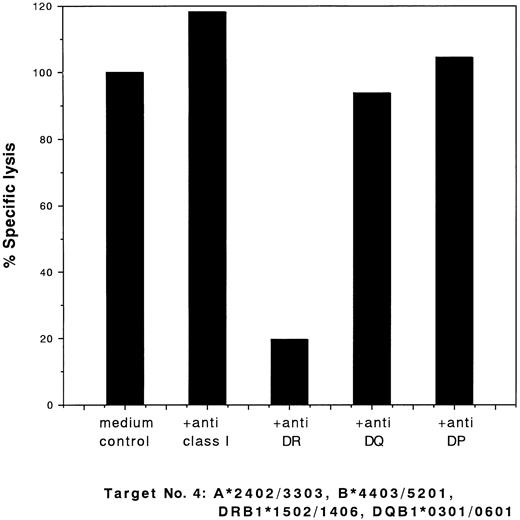
To identify the target HLAs, we performed studies on the ability of MoAbs to block the cytotoxic activities of the clones. The cytotoxic activity of the type I clones was blocked by anti–class I MoAb and that of the type II clones was blocked by anti-DR MoAb. Typical results of the blocking studies for these two types of clones (clones 28 and 13) are shown in Figs 2 and 3, respectively.
Blocking of cytotoxic activity of CD8+ clone 28 against target cells no. 4 (Table 1) by MoAbs. 51Cr-labeled target cells were incubated with a saturating concentration of MoAbs for 60 minutes at 37°C. Effector cells were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated.
Blocking of cytotoxic activity of CD8+ clone 28 against target cells no. 4 (Table 1) by MoAbs. 51Cr-labeled target cells were incubated with a saturating concentration of MoAbs for 60 minutes at 37°C. Effector cells were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated.
Blocking of cytotoxic activity of CD4+ clone 13 against target cells no. 4 (Table 1) by MoAbs. 51Cr-labeled target cells were incubated with a saturating concentration of MoAbs for 60 minutes at 37°C. Effector clones were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated.
Blocking of cytotoxic activity of CD4+ clone 13 against target cells no. 4 (Table 1) by MoAbs. 51Cr-labeled target cells were incubated with a saturating concentration of MoAbs for 60 minutes at 37°C. Effector clones were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated.
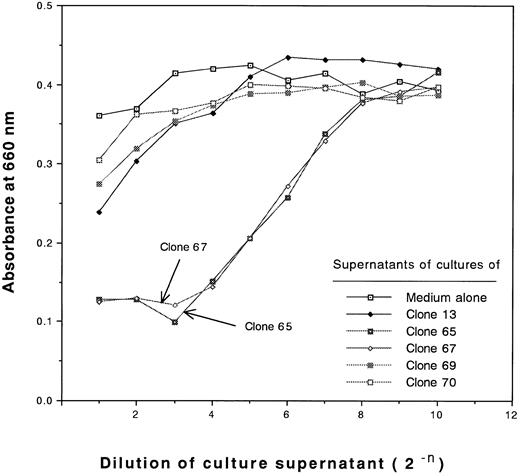
During the characterization of type III clones, we found that these clones produce and secrete large amounts of cytotoxic cytokines after antigen stimulation. The cytotoxic activities of the supernatants of cultures of type III clones against L929 cells are shown in Fig 4. No cytotoxic activity of supernatants of type II clones or type I clones against L929 cells was detected (Fig 4 and data not shown, respectively). The cytotoxic activities of the supernatants of cultures of clones 65 and 67 against L929 cells were not blocked by anti-TNFα MoAb, but were blocked by anti-TNFβ MoAb (Fig 5). Thus, the type III clones were demonstrated to be TNFβ-producing cells.
Measurement of cytotoxic activities of supernatants of cultures of type II and III clones against L929 cells. Serially diluted supernatants of 4-day cultures of 5 CD4+ clones after stimulation of 30 Gy irradiated allogeneic PBLs having DRB1*1502 in the presence of 20 U/mL rIL-2 (initial cell concentration of 2 × 105 cells/mL) or control medium containing 20 U/mL rIL-2 were incubated with monolayer L929 cells in the presence of 1 μg/mL actinomycin D for 18 hours, after which the viable cells were stained with methylene blue and the absorbance at 660 nm was measured.
Measurement of cytotoxic activities of supernatants of cultures of type II and III clones against L929 cells. Serially diluted supernatants of 4-day cultures of 5 CD4+ clones after stimulation of 30 Gy irradiated allogeneic PBLs having DRB1*1502 in the presence of 20 U/mL rIL-2 (initial cell concentration of 2 × 105 cells/mL) or control medium containing 20 U/mL rIL-2 were incubated with monolayer L929 cells in the presence of 1 μg/mL actinomycin D for 18 hours, after which the viable cells were stained with methylene blue and the absorbance at 660 nm was measured.
Neutralization of supernatants of cultures of clones 65 and 67 with anti-TNFβ MoAbs. Supernatants of 4-day cultures of clones 65 and 67 after stimulation of 30 Gy irradiated allogeneic PBLs having DRB1*1502 in the presence of 20 U/mL rIL-2 (initial cell concentration of 2 × 105 cells/mL), which exhibited cytotoxic activity against L929 cells as shown in Fig 4, were incubated with a saturating concentration of anti-TNFβ MoAb for 60 minutes at 37°C. The treated supernatants or nontreated controls were added to monolayers of L929 cells in the presence of 1 μg/mL actinomycin D. After an 18-hour incubation, the viable cells were stained with methylene blue and absorbance at 660 nm was measured.
Neutralization of supernatants of cultures of clones 65 and 67 with anti-TNFβ MoAbs. Supernatants of 4-day cultures of clones 65 and 67 after stimulation of 30 Gy irradiated allogeneic PBLs having DRB1*1502 in the presence of 20 U/mL rIL-2 (initial cell concentration of 2 × 105 cells/mL), which exhibited cytotoxic activity against L929 cells as shown in Fig 4, were incubated with a saturating concentration of anti-TNFβ MoAb for 60 minutes at 37°C. The treated supernatants or nontreated controls were added to monolayers of L929 cells in the presence of 1 μg/mL actinomycin D. After an 18-hour incubation, the viable cells were stained with methylene blue and absorbance at 660 nm was measured.
Further investigation of type I and type II clones showed involvement of the Fas/Fas-L system in their cytotoxic activities. Target-specific lysis by both types of clones was partially inhibited by pretreatment of the target cells with anti-Fas MoAbs, although the degrees of the blocking differed among the clones (Fig 6).
Blocking of cytotoxic activities of type I and type II clones by anti-Fas MoAb. 51Cr-labeled target cells no. 4 (Table 1) were incubated with 500 ng/mL or 5 μg/mL of anti-Fas MoAb or medium alone for 60 minutes at 37°C. Then effector cells were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated. (▪) Control; (▨) anti-Fas MoAb (500 ng/mL); (▧) anti-Fas MoAb (5 μg/mL). Target: A*2402/3303, B*4403/5201, DRB1*1406/1502, DQB1*0301/06.
Blocking of cytotoxic activities of type I and type II clones by anti-Fas MoAb. 51Cr-labeled target cells no. 4 (Table 1) were incubated with 500 ng/mL or 5 μg/mL of anti-Fas MoAb or medium alone for 60 minutes at 37°C. Then effector cells were added at an E:T ratio of 2:1 and a standard 4-hour 51Cr release assay was performed. The percentage of specific lysis is indicated. (▪) Control; (▨) anti-Fas MoAb (500 ng/mL); (▧) anti-Fas MoAb (5 μg/mL). Target: A*2402/3303, B*4403/5201, DRB1*1406/1502, DQB1*0301/06.
DISCUSSION
In the present study, we established three types of clones, CD8+ CTL type I clones, CD4+ CTL type II clones, and noncytotoxic CD4+ type III clones, from TA-GVHD patient PBLs. In the cytotoxicity assay, the target antigen of the type I clones was identified as HLA B52 and that of the type II clones was identified as HLA DR15. The type III clones exhibited specific proliferation in response to stimulation with cells that carried DRB1*1502. Type II clones also proliferated in response to stimulation with cells that carried DRB1*1502. These findings indicate that the target antigens that were recognized as alloantigens by the donor cells were not always restricted to one HLA. The present findings suggest that each type of effector cell potentially involved in the pathogenesis of GVHD has a unique mechanism of action. CTLs might directly attack host cells, recognizing the HLA B52 or DR15 expressed by them. On the other hand, noncytotoxic T cells, which produce and secrete cytolytic cytokines after antigen stimulation that cause nonspecific injury to the host organs and tissues, might proliferate in response to stimulation with cells that express DR15.
We demonstrated that the Fas/Fas-L system is involved in both CD8+ and the CD4+ CTL cytotoxicity. Recently, evidence has gradually accumulated that the Fas/Fas-L system plays various roles in the human immune system. In many reports, evidence that the Fas/Fas-L system greatly contributes to immune homeostasis through depletion of autoreactive lymphocytes, maintenance of peripheral tolerance, and/or target-cell lysis by CTLs or natural killer (NK) cells was described.12-14 Abnormal activation of the Fas/Fas-L system was suggested to lead to acute liver dysfunction or GVHD. Braun et al.23 reported that in mice, development of acute lethal GVHD involved both perforin and Fas-L –mediated pathways. The Fas/Fas-L system is also said to be involved in the elimination of activated lymphocytes from the peripheral blood in mice.24 In this study, we obtained evidence that CTLs isolated from a TA-GVHD patient can kill targets via the Fas/Fas-L system, which suggests that such a system may be involved in tissue damage of GVHD in humans. This is consistent with the report that injection of cytotoxic anti-Fas MoAb into adult mice caused rapid death due to liver failure.25
Both TNF and IL-1 are thought to be important trigger cytokines for activation of humoral mediator networks.26-28 TNF and IL-1 stimulate monocytes or macrophages to produce other cytokines. These cytokines induce expression of adhesion molecules and stimulate production of platelet activation factors, eicosanoids, and nitric oxidase. Adhesion molecules enhance neutrophil accumulation in various organs. Neutrophil production of free radicals or esterases leads to cell injury throughout the body.
In the present study, we established TNFβ-producing CD4+ clones that proliferated in response to stimulation with patient allogeneic HLA DR. The secreted TNFβ might have contributed to the development of TA-GVHD both locally and throughout the patient's body.
Recently, lymphotoxin-β (LT-β), a type II transmembrane protein that is another member of the TNF ligand family, has gradually been characterized. TNFβ (also called LT-α) forms a heteromeric complex with LT-β on the cell surface.29,30 CTLs and IL-2–stimulated NK cells are known to express the LT-α–LT-β complex on their surface at high levels.15,16,31 Thus, it is suggested that these ligands might function as positive or negative regulators in inflammatory and immune responses. The suggestion by Mallett and Barclay32 that the LT-α–LT-β complex might interact with Fas or other receptors might point to a fundamental role for these ligands in immune regulation.
Tracy et al33 reported that anti-TNF antibodies prevent septic shock during lethal bacteremia and Mohler et al34 reported that a metalloprotease inhibitor inhibits lipopolysaccharide-induced endotoxin shock. Protease inhibitors apparently inhibit TNF secretion by monocytes. Collagenase inhibitors were also demonstrated to inhibit TNF secretion.35 36 These inhibitors, along with anti-TNF antibody and antibodies to Fas and/or Fas-L that might partially inhibit cytotoxic activities of CTLs, seem to be the good candidates for further study in attempts to develop effective treatments for TA-GVHD. We expect that the clones described here will be useful in such studies.
Address reprint requests to Motoko Nishimura, PhD, Department of Research, The Japanese Red Cross, Central Blood Center, 4-1-31 Hiroo, Shibuya-ku, Tokyo 150, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal