Abstract
The t(2; 5)(p23;q35) translocation, associated with anaplastic large-cell lymphoma (ALCL), results in the production of the nucleolar protein nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) protein. This report describes an immunocytochemical study of the distribution of ALK and NPM-ALK proteins using a new monoclonal antibody, ALK1, that recognizes a formalin resistant epitope in both the 80-kD NPM-ALK chimeric and the 200-kD normal human ALK proteins. Cytoplasmic and nuclear labeling was seen in the t(2; 5)+ SU-DHL-1 and Karpas 299 cell lines. Normal ALK protein expression was restricted to the central nervous system (in scattered neurons, glial cells, and endothelial cells). Two hundred and thirty-nine cases of lymphoma and 80 nonhematopoietic tumors were immunostained. Antibody ALK1 labeled 53.4% (39 of 73 cases) of CD30+ ALCL. A case of ALCL with a t(1; 2) translocation was ALK1+. Three cases of CD30− ALCL with prominent nucleoli showed a unique pattern of coarse granular cytoplasmic labeling. All other tumors, including Hodgkin's disease and lymphomatoid papulosis, were ALK1−. These results indicate that reliable immunostaining of routine biopsy material for NPM-ALK and ALK proteins is feasible. Such analysis is of diagnostic importance, especially because t(2; 5)+ ALCL cases have a good prognosis with appropriate treatment.
ANAPLASTIC LARGE-CELL lymphoma (ALCL) was first reported in 1985.1 This entity constitutes approximately 5% of all human non-Hodgkin's lymphomas1-3 but accounts for as many as 30% to 40% of pediatric large-cell lymphomas.4,5 ALCL may arise de novo as a primary tumor or appear as a secondary neoplasm (eg, by morphologic transformation of a T-cell lymphoma). This tumor is a high-grade malignancy in which sheets of anaplastic cells infiltrate and destroy the normal architecture of lymph nodes and may involve soft tissues, gastrointestinal tract, skin, and bone marrow. Typically, the neoplastic cells have abundant cytoplasm, large irregular nuclei, and prominent, sometimes rod-shaped, nucleoli. These cells express the CD30 antigen.6 Despite their aggressive appearance, ALCL often respond well to treatment.2,5 7-9
In the late 1980s, it came to be realized that the t(2; 5)(p23;q35) chromosomal abnormality is associated with the disease,10-13 and the breakpoints involved were cloned in 1994. The gene at 5q35 encodes the cell cycle-regulated nucleolar protein nucleophosmin (NPM), whereas the gene at 2p23 encodes a novel receptor tyrosine kinase anaplastic lymphoma kinase (ALK), belonging to the insulin receptor family of tyrosine kinases.14
The 2; 5 translocation results in the expression of a chimeric 80-kD protein, NPM-ALK or p80, consisting of the N-terminal portion of NPM (comprising 40% of the molecule) linked to the entire intracellular portion of ALK.14 Other members of the receptor tyrosine kinase family, eg, TRK, RET, and MET,15-17 can function as oncogenes via dysregulation of their tyrosine kinase regions.18 It is, therefore, probable that aberrant phosphorylation of cellular substrates by the NPM-ALK hybrid protein contributes directly to the pathogenesis of ALCL.
There is little information on the presence of ALK in normal tissues.14,19,20 Although other members of the receptor tyrosine kinase family are known to be involved in the regulation of cell growth and differentiation,21 22 no function has yet been ascribed to ALK. An immunocytochemical study of the distribution of ALK in normal tissues might provide clues to its functional roles. The pattern of ALK protein expression in nonhematopoietic tumors is also unknown, and it remains to be established, in a large series of ALCL cases, how closely the 2; 5 translocation correlates with both the presence of NPM-ALK transcripts and expression of NPM-ALK protein.
There has been a report from Orscheschek et al23 that NPM-ALK mRNA is detectable in Hodgkin's disease, but this has not been confirmed in other studies.24-29 A definitive analysis of the expression of ALK protein in Reed-Sternberg cells is required. Because there have been suggestions that a subtype of Hodgkin's-like ALCL exists,6,27 30 further studies of the expression of NPM-ALK protein in these cases would throw light on the validity of this entity.
To answer these questions, it would be of value to have reagents that recognize both the NPM-ALK fusion protein and also wild-type ALK protein reliably in paraffin-embedded tissues. In 1994, Shiota et al20 described a polyclonal anti-ALK reagent that could be used for immunocytochemical studies. Work in several laboratories (using samples of this antiserum) suggested that expression of the NPM-ALK protein in ALCL correlates with the 2; 5 translocation, as inferred from the presence of NPM-ALK transcripts.20,27,29 31 However, further studies have been limited by the restricted quantity of the original antiserum.
In this report, we have used a new monoclonal antibody (MoAb) that detects ALK specifically (in fresh tissues and cell samples as well as in routinely processed biopsy material) to document the pattern of ALK expression in normal tissues and in a wide range of hematopoietic and nonhematopoietic neoplasms. We have also examined Reed-Sternberg cells for the presence of NPM-ALK protein and obtained more evidence concerning the nature of Hodgkin's-like ALCL.
MATERIALS AND METHODS
Generation of Recombinant ALK Protein
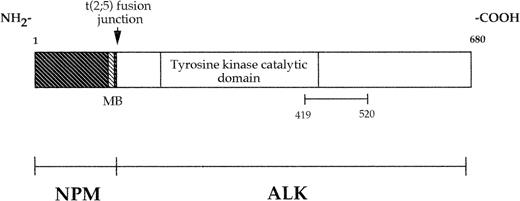
The fusion protein DHFR-ALK was prepared by ligating a 303-bp Acc I/Pst I human ALK cDNA fragment that encodes amino acids 1359-1460 of the full-length ALK receptor protein, corresponding to amino acids 419-520 of the chimeric NPM-ALK protein (Fig 1), into the pQE-41 bacterial expression vector (QIAexpress Expression System; Qiagen, Chatsworth, CA). This construct resulted in the production of an ALK polypeptide associated with an amino-terminal tag of six consecutive histidine residues for protein purification and the murine dihydrofolate reductase (DHFR) for added stability and enhanced antigenicity. This protein was expressed in the M15(pREP4) Escherichia coli strain and purified by metal chelate affinity chromatography following the manufacturer's instructions before being used for immunization.
Schematic diagram of the NPM-ALK fusion protein. As a result of the 2; 5 translocation, the amino-terminal portion of NPM is fused to the intracellular region (including the tyrosine kinase domain) of ALK. The recombinant protein used to raise the MoAb ALK1 consists of the portion of ALK between amino acids 419 and 520, inclusive. MB indicates putative metal binding site.
Schematic diagram of the NPM-ALK fusion protein. As a result of the 2; 5 translocation, the amino-terminal portion of NPM is fused to the intracellular region (including the tyrosine kinase domain) of ALK. The recombinant protein used to raise the MoAb ALK1 consists of the portion of ALK between amino acids 419 and 520, inclusive. MB indicates putative metal binding site.
Production of Antibodies
Balb/c mice were injected subcutaneously with 50 μg of the DHFR-ALK recombinant protein emulsified in Freund's adjuvant. Two further immunizations of 50 μg recombinant protein in phosphate-buffered saline (PBS) were administered at 8-day intervals. Three days after a fourth injection, hyperimmune spleen cells were fused with the NS1 myeloma cell line, as described by Pulford et al.32 Initial screening was performed using an immunoperoxidase technique on cytocentrifuge preparations of the t(2; 5)+ SU-DHL-1 cell line. Further screening was performed using the enzyme-linked immunosorbent assay (ELISA) technique against recombinant DHFR-ALK protein and against DHFR alone (see below). Additional screening was performed on cryostat sections of normal tonsil and on transfected cells.
ELISA
Fifty microliters of a 5 μg/mL solution of either DHFR-ALK or DHFR was added to each well of a Maxistrip microtiter plate (GIBCO Biocult Ltd, Paisley, UK). After 2 hours at room temperature, the plates were washed in PBS containing 0.1% bovine serum albumin (BSA) and 0.1% Tween 20 (Sigma Chemical Co, St Louis, MO). Free protein binding sites on the plates were blocked with PBS containing 0.5% BSA and 0.1% Tween 20 for a further 2 hours at 37°C. Fifty microliters of hybridoma supernatant was then added to each well for 30 minutes. After a wash in PBS containing 0.1% BSA and 0.1% Tween 20, 50 μL of horseradish peroxidase (HRP)-conjugated goat antimouse Ig (diluted 1:500; DAKO a/s, Glostrup, Denmark) was added. After 30 minutes and a final wash in distilled water, the reaction was developed using the soluble substrate 2,2′-azino-di-(3-ethylbenzlthiazoline sulfonic acid) (Sigma Chemical Co). Supernatants that reacted with the recombinant fusion protein but not with DHFR were selected for further study.
Tissue Samples
Normal cells and tissues.Normal peripheral blood mononuclear cells were separated on Lymphoprep (Nyegaard, Oslo), and cytocentrifuge preparations were made. Normal tonsil was obtained from the E.N.T. Department of the Radcliffe Infirmary (Oxford, UK). Samples of fresh and paraffin-embedded normal tissues were obtained from the Departments of Histopathology and Paediatric Pathology of the John Radcliffe Hospital (Oxford, UK) and Hospital/University Centre, Purpan (Toulouse, France).
Patients.Seventy-seven cases of ALCL were investigated. The age of the patients ranged from 4 to 92 years (median, 34 years). Most of these cases were from the files of the Lymphoma Study Group at Hospital/University Centre, Purpan (Toulouse, France) and were included in a recent study on the detection of the 2; 5 translocation by karyotypic analysis, reverse transcriptase-polymerase chain reaction (RT-PCR), and immunocytochemistry (using the polyclonal anti-ALK reagent p80NPM-ALK).29 The diagnosis of ALCL was based on immunomorphologic criteria.33-36 In particular, the majority of ALCL cases coexpressed the CD30 and EMA antigens and were also labeled by antibodies CBF.78 and BNH.9. In addition, in keeping with the REAL classification,30 only tumors of T or null phenotype were classified as ALCL. In the present study, 20 cases of ALCL were also investigated by both RT-PCR and immunostaining with antibody ALK1 (see Table 4). In the 53 remaining cases, only immunostaining with antibody ALK1 was performed. In accordance with the REAL classification, cases of B phenotype expressing CD30 were included in the large B-cell lymphoma category.
Correlation Between the 2; 5 Translocation and Immunocytochemical Labeling for NPM/ALK and ALK Protein Expression in ALCL
| Case No. . | Karyotype . | Phenotype . | RT-PCR4-150 . | Immunostaining . | ||
|---|---|---|---|---|---|---|
| . | . | CD304-150 . | T/Null4-150 . | . | ALK1 . | p804-150 . |
| 1 | t(2; 5) | + | T | + | + | + |
| 2 | t(2; 5) | + | NT | + | + | + |
| 3 | t(2; 5) | + | T | + | + | + |
| 4 | t(2; 5) | + | T | + | + | + |
| 5 | t(2; 5) | + | T | + | + | + |
| 6 | t(2; 5; 6) | + | T | + | + | + |
| 7 | t(2; 5) | + | T | NT | + | + |
| 8 | t(2; 5) | + | T | NT | + | + |
| 9 | t(2; 5) | + | NT | − | − | − |
| 10 | t(2; 5) | + | T | NT | + | NT |
| 11 | Normal | + | T | + | + | + |
| 12 | Normal | + | T | − | + | − |
| 13 | t(1; 2) | + | T | NT | + | + |
| 14 | NT | + | T | + | + | + |
| 15 | NT | + | T | + | + | + |
| 16 | NT | + | T | + | + | + |
| 17 | NT | + | Null | − | − | − |
| 18 | NT | + | T | − | − | (+) |
| 19 | NT | + | Null | − | + | (+) |
| 20 | NT | + | T | − | + | − |
| 21 | NT | + | NT | + | + | NT |
| 22 | NT | + | T | + | + | NT |
| 23 | NT | + | T | + | + | NT |
| 24 | NT | + | T | − | − | NT |
| Case No. . | Karyotype . | Phenotype . | RT-PCR4-150 . | Immunostaining . | ||
|---|---|---|---|---|---|---|
| . | . | CD304-150 . | T/Null4-150 . | . | ALK1 . | p804-150 . |
| 1 | t(2; 5) | + | T | + | + | + |
| 2 | t(2; 5) | + | NT | + | + | + |
| 3 | t(2; 5) | + | T | + | + | + |
| 4 | t(2; 5) | + | T | + | + | + |
| 5 | t(2; 5) | + | T | + | + | + |
| 6 | t(2; 5; 6) | + | T | + | + | + |
| 7 | t(2; 5) | + | T | NT | + | + |
| 8 | t(2; 5) | + | T | NT | + | + |
| 9 | t(2; 5) | + | NT | − | − | − |
| 10 | t(2; 5) | + | T | NT | + | NT |
| 11 | Normal | + | T | + | + | + |
| 12 | Normal | + | T | − | + | − |
| 13 | t(1; 2) | + | T | NT | + | + |
| 14 | NT | + | T | + | + | + |
| 15 | NT | + | T | + | + | + |
| 16 | NT | + | T | + | + | + |
| 17 | NT | + | Null | − | − | − |
| 18 | NT | + | T | − | − | (+) |
| 19 | NT | + | Null | − | + | (+) |
| 20 | NT | + | T | − | + | − |
| 21 | NT | + | NT | + | + | NT |
| 22 | NT | + | T | + | + | NT |
| 23 | NT | + | T | + | + | NT |
| 24 | NT | + | T | − | − | NT |
Abbreviations: (+), weak staining; NT, not tested.
Results obtained from present study and the study by Lamant et al.29
Fifty cases of Hodgkin's disease were studied; in 23 of these cases, both RT-PCR and labeling with antibody ALK1 were performed. Reed-Sternberg cells in all cases were positive for CD15 and CD30 but were negative for EMA and BNH.9.
One hundred four cases of non-Hodgkin's lymphoma of nonanaplastic type (B-cell phenotype, 82 cases; T-cell phenotype, 22 cases) and 80 cases of nonhematopoietic tumors were also immunostained with antibody ALK1.
Cell Lines
The following cell lines were obtained from the Sir William Dunn School of Pathology (Oxford, UK): K562 (erythroleukemia), U937 (malignant histiocytosis), HL60 (promyelocytic leukemia), HeLa (cervical epithelial carcinoma), Daudi and Raji (Burkitt's lymphoma), Nalm-1 (pre-B cell), Molt-4 and Jurkat (T cell), and RVH421 (melanoma). The cell lines SU-DHL-1 and Karpas 299 (ALCL of T-cell phenotype carrying the 2; 5 translocation) were obtained from Dr M.L. Cleary (Stanford, CA) and Dr A. Karpas (Cambridge, UK), respectively. The T-cell lines RPMI 8402, Molt-13, PEER, and CCRF-CEM were obtained from Dr E. Macintyre (Paris, France). The Hodgkin cell lines L428 and L540 were provided by Prof H. Stein (Berlin, Germany), whereas the KM-H2 and HDLM-2 Hodgkin cell lines were obtained from the German Collection of Micro-organisms and Cell Culture (Braunschweig, Germany). Rh30, a rhabdomyosarcoma cell line, and the human embryonic kidney epithelial cell line, 293T, were obtained from St Jude Children's Research Hospital (Memphis, TN) and the Institute of Cancer Research (London, UK), respectively.
All cells were maintained in culture in RPMI 1640 medium containing 10% foetal calf serum (GIBCO Biocult Ltd) at 37°C in 5% CO2 .
Preparation of Cells and Tissue Samples
Cytocentrifuge cell preparations were fixed and stored, as previously described.37 Fresh tissues were snap-frozen in liquid nitrogen and cryostat sections (6 μm) were cut, fixed, and stored, as previously described.38
Paraffin-embedded tissues were fixed mainly in Bouin's fluid or Duboscq-Brasil. A few samples were fixed in 10% formol saline or in alcohol:formalin:acetic acid. Reactivity with nonhematopoietic neoplasms was assessed using the sausage tissue block method described by Battifora.39
Expression of ALK in Transfected Cells
To produce the ALK expression plasmid, the complete full-length 6,226-bp Sal I/Not I ALK cDNA insert from pRMS17-214 was subcloned into the EcoRV/Not I-digested CMV promoter-based mammalian expression vector pcDNA3 (InVitrogen, San Diego, CA) following Klenow polymerase fill-in of the Sal I overhang. This plasmid was then grown up in E coli (strain TOP10F ).
Transient expression of either the pcDNA3-ALK plasmid or just the pcDNA3 expression vector was performed in the 293T cell line using the calcium phosphate method (Promega Corp, Southampton, UK) following the manufacturer's instructions. The cells were harvested after 72 hours of culture and cytocentrifuge preparations were made. The remaining cells were pelleted and used for Western blotting studies.
Source of Antibodies
MoAbs.Immunocytochemical studies were performed with the following antibodies that are reactive on paraffin sections: Dako-CD3, Dako-L26/CD20, Dako-UCHL1/CD45RO, Dako-Ber-H2/CD30, Dako-E29/EMA (DAKO a/s), and two antibodies from one of the investigators' laboratories, BNH935 and CBF.78,36 which are known to react with ALCL. The monoclonal antiphosphotyrosine antibody, 4G10, was obtained from Upstate Biotechnology Inc (Lake Placid, NY).
Polyclonal antibodies.The anti-ALK antibody p80NPM-ALK was kindly donated by Dr M. Shiota and the Diagnostics Division, Nicherei Corporation Ltd (Tokyo, Japan). HRP-conjugated goat antimouse Igs (P0447) and the StreptABComplex/HRP Duet (Mouse/ Rabbit) kit (DAKO; code no. K492) were obtained from DAKO a/s.
Immunocytochemical Labeling
The immunoperoxidase technique was performed using either a two-stage or a streptavidin-biotin-peroxidase three-stage technique. For the two-stage procedure, slides were incubated with an MoAb for 30 minutes, washed in TBS (0.5 mol/L Tris HCl, pH 7.6, diluted 1:10 with 1.5 mol/L saline) and then incubated with HRP-conjugated goat antimouse Ig (1:50 dilution). The streptavidin-biotin-peroxidase complex (ABC) method, using the StreptABComplex/HRP Duet (Mouse/Rabbit) kit, was used for the three-stage staining process. In this procedure, slides were incubated with either MoAb or polyclonal antibody for 30 minutes. They were then washed and incubated with biotinylated goat antimouse/antirabbit Ig before being washed and incubated for a further 30 minutes with streptavidin/HRP. The peroxidase reaction was developed using diaminobenzidine tetrahydrochloride (DAB; Sigma Chemical Co) and hydrogen peroxide (0.01% vol/vol). The slides were counterstained with hematoxylin before mounting.
Immunostaining on paraffin sections was performed using the method described by Shi et al,40 with some modifications.29 Briefly, paraffin sections were mounted on glass slides coated with silane (Sigma Chemical Co). Sections were deparaffinized, placed in 10 mmol/L Na-citrate buffer (pH 6), and heated in a microwave oven (Whirlpool model; Phillips, Eindhoven, Holland) at 900 W for cycles of 20 minutes and 10 minutes. The slides were removed from the oven and allowed to cool for 30 minutes at room temperature. After washing in water, endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol for 30 minutes. The slides were then rinsed in PBS before staining, using the three-stage immunoperoxidase technique.
RT-PCR Studies
RT-PCR studies were performed, as previously described,29 using the 5′NPM and 3′ALK primers and the NPM-ALK junction oligonucleotide described by Morris et al.14 These primers were as follows: 5′NPM, 5′-TCCCTTGGGGGCTTTGAAATAACACC-3′; 3′ALK, 5′-CGAGGTGCGGAGCTTGCTCAGC-3′; and the NPM-ALK junction oligonucleotide, 5′-AGCACTTAGTAGTGTACCGCCGGA-3′.
Western Blotting Studies
Cell pellets containing 2 × 107 cells were suspended in 1 mL lysis buffer containing 20 mmol/L sodium phosphate, pH 7.5, 50 mmol/L sodium fluoride, 5 mmol/L tetra sodium pyrophosphate, 10 mmol/L β-glycerophosphate, 0.5% NP-40, and protease inhibitors (1 mmol/L leupeptin, 1 mmol/L pepstatin, 1 mmol/L pefablock, and 20 mmol/L tosyl-L-phenylanine chloromethyl ketone; Sigma Chemical Co). After 10 minutes on ice, the lysates were centifuged at 6,000 rpm for 3 minutes at 4°C in a microfuge and the nuclear pellets were discarded. Cytoplasmic extracts containing 50 μg protein, determined by the Bradford's assay,41 were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)42 in a 7.5% gel under reducing conditions. Proteins were transferred to Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore UL Ltd, Watford, UK) by semidry electroblotting. Spare protein binding sites on the membrane were then blocked by incubating in blocking buffer (TBS containing 5% nonfat milk and 0.1% Tween 20) for 1 hour at 37°C. The membranes were then incubated with antibody ALK1 (neat tissue culture supernatant) and washed in TBS containing 0.1% Tween 20. The immobilized antigen-antibody conjugates were detected by incubation with HRP-conjugated goat antimouse (diluted 1/1,000) followed by chemiluminescent detection with the Amersham ECL reagents (Amersham, Amersham, UK).
Immunoprecipitations
Lysates from SU-DHL-1 and Daudi cells were prepared using a buffer containing 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 0.5% NP-40, and the protease inhibitors, as described above. One-milliliter aliquots of cell lysate were precleared twice with 50 μL protein G (Pharmacia, Uppsala, Sweden). This precleared lysate was added to 25 μL of protein G preloaded with the monoclonal antiphosphotyrosine antibody 4G10 and incubated on ice for 1 hour. Immunoprecipitates were washed in lysis buffer and analyzed by SDS-PAGE and Western blotting as above.
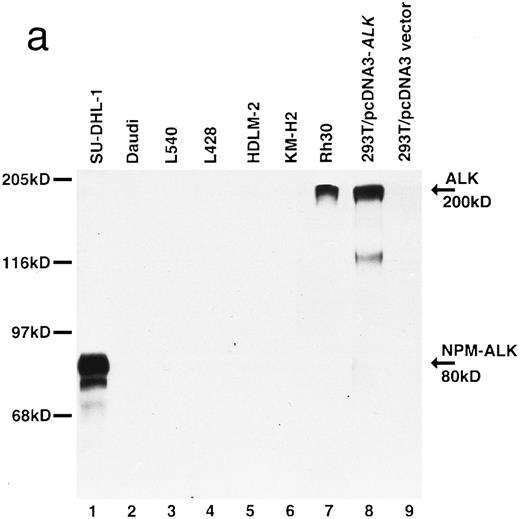
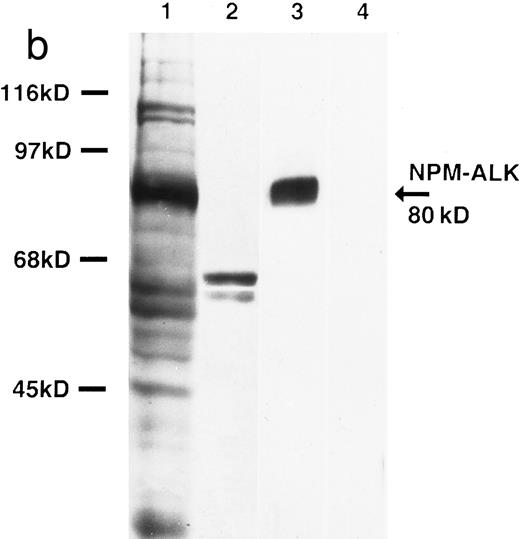
Biochemical characterization of antibody ALK1. (a) Cell lysates were separated by SDS-PAGE and then subjected to Western blotting. Antibody ALK1 recognizes a protein of 80 kD in the t(2; 5)+ SU-DHL-1 cell line (lane 1). No protein bands are detected in the Daudi B-cell line or the Hodgkin's-derived cell lines L540, L428, HDLM-2, and KM-H2 (lanes 2 through 6). However, antibody ALK1 did detect a band of 200 kD in lysates of the human rhabdomyosarcoma Rh30 cell line (lane 7) that expresses the full-length ALK receptor protein19 and in 293T (human embryonic kidney epithelial) cells that had been transfected with a cDNA encoding the full-length ALK protein (lane 8). No bands were seen in 293T cells that had been tranfected with pcDNA3 vector only (lane 9). The smaller bands seen in the lysates from the SU-DHL-1 and 293T cells transfected with pcDNA3-ALK have been observed using polyclonal anti-ALK sera ( S.W. Morris, personal communication, March 1996) and probably represent proteolytic breakdown products of the NPM-ALK and ALK proteins, respectively. (b) Immunoprecipitates prepared from SU-DHL-1 and Daudi cell lysates using the antiphosphotyrosine antibody 4G10 were subjected to SDS-PAGE and then electroblotted. The blots were then stained with either the antiphosphotyrosine antibody 4G10 (lanes 1 and 2) or with antibody ALK1 (lanes 3 and 4). The t(2; 5)+ SU-DHL-1 cell line contained many phosphorylated proteins (lane 1) in contrast to only two found in the Daudi cell line (lane 2). Antibody ALK1 labeled only the prominent 80-kD phosphorylated NPM-ALK protein present in the SU-DHL-1 cells (lane 3). No bands were detected by antibody ALK1 in the Daudi cell immunoprecipitate (lane 4). The position of molecular weight standards (myosin, 205 kD; galactosidase, 116 kD; phosphorylase, 97 kD; bovine serum albumin, 68 kD; and ovalbumin, 45 kD) are indicated.
Biochemical characterization of antibody ALK1. (a) Cell lysates were separated by SDS-PAGE and then subjected to Western blotting. Antibody ALK1 recognizes a protein of 80 kD in the t(2; 5)+ SU-DHL-1 cell line (lane 1). No protein bands are detected in the Daudi B-cell line or the Hodgkin's-derived cell lines L540, L428, HDLM-2, and KM-H2 (lanes 2 through 6). However, antibody ALK1 did detect a band of 200 kD in lysates of the human rhabdomyosarcoma Rh30 cell line (lane 7) that expresses the full-length ALK receptor protein19 and in 293T (human embryonic kidney epithelial) cells that had been transfected with a cDNA encoding the full-length ALK protein (lane 8). No bands were seen in 293T cells that had been tranfected with pcDNA3 vector only (lane 9). The smaller bands seen in the lysates from the SU-DHL-1 and 293T cells transfected with pcDNA3-ALK have been observed using polyclonal anti-ALK sera ( S.W. Morris, personal communication, March 1996) and probably represent proteolytic breakdown products of the NPM-ALK and ALK proteins, respectively. (b) Immunoprecipitates prepared from SU-DHL-1 and Daudi cell lysates using the antiphosphotyrosine antibody 4G10 were subjected to SDS-PAGE and then electroblotted. The blots were then stained with either the antiphosphotyrosine antibody 4G10 (lanes 1 and 2) or with antibody ALK1 (lanes 3 and 4). The t(2; 5)+ SU-DHL-1 cell line contained many phosphorylated proteins (lane 1) in contrast to only two found in the Daudi cell line (lane 2). Antibody ALK1 labeled only the prominent 80-kD phosphorylated NPM-ALK protein present in the SU-DHL-1 cells (lane 3). No bands were detected by antibody ALK1 in the Daudi cell immunoprecipitate (lane 4). The position of molecular weight standards (myosin, 205 kD; galactosidase, 116 kD; phosphorylase, 97 kD; bovine serum albumin, 68 kD; and ovalbumin, 45 kD) are indicated.
RESULTS
Production and Characterization of Antibody ALK1
Of the initial 19 hybridoma supernatants that labeled the t(2; 5)-bearing cell line SU-DHL-1, only 3 reacted with recombinant DHFR-ALK protein (but not with DHFR) and only 1 of these supernatants was unreactive on cryostat sections of tonsil (a tissue known to contain no ALK mRNA). The cells producing this supernatant were cloned to yield the MoAb ALK1 (IgG3 isotype).
This antibody detected a strong band of 80 kD molecular weight in SU-DHL-1 cell lysates by Western blotting (Fig 2a, lane 1). No bands were detected in lysates of Daudi or Hodgkin's cell lines (Fig 2a, lanes 2 through 6). The human rhabdomyosarcoma cell line, Rh30, is known to express the full-length 200-kD ALK protein,19 and antibody ALK1 detected a band of this size in lysates of Rh30 cells (Fig 2a, lane 7). Antibody ALK1 also recognized a protein of 200 kD in 293T cells transfected with the pcDNA3-ALK plasmid encoding the full-length ALK protein (Fig 2a, lane 8) but not when this cell line was transfected with pcDNA3 vector alone (Fig 2a, lane 9).
Immunoprecipitation studies using the antiphosphotyrosine antibody 4G10 showed the presence of phosphorylated proteins in lysates of the SU-DHL-1 and Daudi cell lines (Fig 2b, lanes 1 and 2). After Western blotting, antibody ALK1 labeled the phosphorylated 80-kD NPM-ALK protein in immunoprecipitates prepared from the SU-DHL-1 cells (Fig 2b, lane 3) but not in those from Daudi cells (Fig 2b, lane 4).
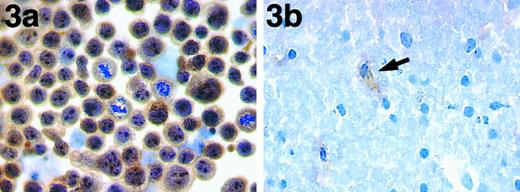
Immunocytochemical studies on cell lines (summarized in Table 1) confirmed the results obtained from the biochemical studies. Only the two hematopoietic cell lines, SU-DHL-1 and Karpas 299 (both known to have the 2; 5 translocation and to express NPM-ALK mRNA), were labeled. Both the cytoplasm and the nuclei in these two cell lines were stained and there was intense labeling of nucleoli (Fig 3a). Cytoplasmic labeling was seen in mitotic cells. All other hematopoietic cell lines tested, including lines derived from Hodgkin's disease, were ALK−. The only other cell line stained by antibody ALK1 was the rhabdomyosarcoma line Rh30. These cells showed only weak cytoplasmic labeling.
Expression of NPM-ALK or ALK Proteins in Cell Lines
| Cell Line . | Reactivity* . |
|---|---|
| Erythroid | |
| K562 | − |
| Lymphoid | |
| t(2; 5)+ | |
| SU-DHL-1 | +++ |
| Karpas 299 | +++ |
| T cell | |
| Molt-4 | − |
| Molt-13 | − |
| PEER | − |
| Jurkat | − |
| CCRF-CEM | − |
| RPMI 8402 | − |
| B cell | |
| Nalm-1 | − |
| Daudi | − |
| Raji | − |
| Hodgkin's cell lines | |
| L428 | − |
| KM-H2 | − |
| HDLM-2 | − |
| L540 | − |
| Myeloid | |
| U937 | − |
| HL60 | − |
| Rhabdomyosarcoma | |
| Rh30 | + |
| Carcinoma | |
| RVH421 | − |
| HeLa | − |
| Cell Line . | Reactivity* . |
|---|---|
| Erythroid | |
| K562 | − |
| Lymphoid | |
| t(2; 5)+ | |
| SU-DHL-1 | +++ |
| Karpas 299 | +++ |
| T cell | |
| Molt-4 | − |
| Molt-13 | − |
| PEER | − |
| Jurkat | − |
| CCRF-CEM | − |
| RPMI 8402 | − |
| B cell | |
| Nalm-1 | − |
| Daudi | − |
| Raji | − |
| Hodgkin's cell lines | |
| L428 | − |
| KM-H2 | − |
| HDLM-2 | − |
| L540 | − |
| Myeloid | |
| U937 | − |
| HL60 | − |
| Rhabdomyosarcoma | |
| Rh30 | + |
| Carcinoma | |
| RVH421 | − |
| HeLa | − |
All cell lines were stained on cytocentrifuge preparations with MoAb ALK1 as described in the text. The intensity of labeling was scored from + to +++.
(a) Immunoperoxidase labeling of the t(2; 5)+ cell line with antibody ALK1. The nuclei, cytoplasm, and nucleoli are strongly labeled by antibody ALK1. During mitosis, the staining of these cells becomes cytoplasmic. (b) Immunoperoxidase labeling of a cryostat section of basal ganglia showing weak labeling of a glial cell (arrow).
(a) Immunoperoxidase labeling of the t(2; 5)+ cell line with antibody ALK1. The nuclei, cytoplasm, and nucleoli are strongly labeled by antibody ALK1. During mitosis, the staining of these cells becomes cytoplasmic. (b) Immunoperoxidase labeling of a cryostat section of basal ganglia showing weak labeling of a glial cell (arrow).
Antibody ALK1 labeled the cytoplasm of 293T cells that had been transfected with pcDNA3-ALK. In contrast, 293T cells transfected with pcDNA3 vector alone remained unstained.
Immunocytochemical Studies on Normal Human Tissues
Fresh frozen samples of normal human tissues were analyzed (Table 2) and no labeling of any normal cells was seen outside the central nervous sytem. Weak labeling of some endothelial cells, pericytes, and scattered glial cells was seen in the hypothalamus, basal ganglia (Fig 3b), cerebral cortex, and cerebellum. Antibody ALK1 also weakly labeled scattered neuronal cells in the hypothalamus, thalamic nuclei, and basal ganglia.
Distribution of ALK Protein in Normal Tissues
| Tissue . | Reactivity* . |
|---|---|
| Hematopoietic | |
| Fetal liver (16 wk) | − |
| Bone marrow | − |
| Peripheral blood | − |
| Lymphoid | |
| Tonsil | − |
| Spleen | − |
| Lymph node | − |
| Thymus | − |
| Gastrointestinal tract | |
| Liver | − |
| Duodenum | − |
| Stomach | − |
| Genito-urinary system | |
| Kidney | − |
| Prostate | − |
| Testis | − |
| Placenta | − |
| Nervous system | |
| Cerebral cortex, hypothalamus, and cerebellum | +† |
| Basal ganglia, hypothalamus, and thalamic nuclei | +‡ |
| Tissue . | Reactivity* . |
|---|---|
| Hematopoietic | |
| Fetal liver (16 wk) | − |
| Bone marrow | − |
| Peripheral blood | − |
| Lymphoid | |
| Tonsil | − |
| Spleen | − |
| Lymph node | − |
| Thymus | − |
| Gastrointestinal tract | |
| Liver | − |
| Duodenum | − |
| Stomach | − |
| Genito-urinary system | |
| Kidney | − |
| Prostate | − |
| Testis | − |
| Placenta | − |
| Nervous system | |
| Cerebral cortex, hypothalamus, and cerebellum | +† |
| Basal ganglia, hypothalamus, and thalamic nuclei | +‡ |
All tissues were stained as cryostat sections with MoAb ALK1, as described in the text.
Some glial cells, a few endothelial cells, and pericytes.
Some glial cells, a few endothelial cells, pericytes, and scattered neuronal cells.
Immunohistochemical Study of Lymphomas for ALK Expression
A total of 239 cases of lymphoma were analyzed for expression of ALK protein by immunocytochemical staining (Table 3). Overall, 53.4% (39/73) cases of CD30+ ALCL were labeled. More cases of ALCL in children (23/26 cases [88.5%]) were positive than in adults (16/47 cases [34.0%]). With only a few exceptions, all malignant cells in any positive sample were strongly labeled (Fig 4a). The absence of background staining of normal cells allowed scattered malignant cells, eg, in lymphatic sinuses (Fig 4b) or admixed with non-neoplastic lymphocytes (Fig 4c) to be easily detected. In addition to cytoplasmic labeling, nuclear staining was noted in most cases (Fig 4d). All ALK+ ALCL were also positive for EMA (39/39) and were stained by antibody BNH.9 (33/33 cases).
Immunocytochemical Reactivity of Lymphomas for ALK and NPM-ALK Proteins
| Histologic Type3-150 . | No. Positive/Tested . | Total . |
|---|---|---|
| ALCL | 42/77 | |
| CD30+ | 38/56 | |
| CD30+ Hodgkin's-like | 1/17 | |
| CD30− (EMA+) | 3/4 | |
| Hodgkin's disease3-151 | 0/50 | |
| Lymphomatoid papulosis | 0/8 | |
| T-cell lymphomas | 0/22 | |
| Precursor T-lymphoblastic lymphomas | 0/3 | |
| T-cell chronic lymphocytic leukemia | 0/1 | |
| Mycosis fungoides/Sezary syndrome | 0/2 | |
| Peripheral T-cell lymphoma, unspecified | 0/16 | |
| B-cell lymphomas | 0/82 | |
| Precursor B-lymphoblastic lymphomas | 0/1 | |
| B-cell chronic lymphocytic leukemia | 0/10 | |
| Immunocytoma/lymphoplasmacytic lymphoma | 0/3 | |
| Mantle cell lymphoma | 0/4 | |
| Follicular center lymphoma | 0/12 | |
| Nodal monocytoid B-cell lymphoma | 0/1 | |
| Hairy cell leukemia | 0/4 | |
| Plasmacytoma/myeloma | 0/2 | |
| Diffuse large B-cell lymphoma | 0/36 | |
| Unclassified high grade | 0/6 | |
| Unclassified low grade | 0/3 | |
| Total no. of cases tested | 42/239 |
| Histologic Type3-150 . | No. Positive/Tested . | Total . |
|---|---|---|
| ALCL | 42/77 | |
| CD30+ | 38/56 | |
| CD30+ Hodgkin's-like | 1/17 | |
| CD30− (EMA+) | 3/4 | |
| Hodgkin's disease3-151 | 0/50 | |
| Lymphomatoid papulosis | 0/8 | |
| T-cell lymphomas | 0/22 | |
| Precursor T-lymphoblastic lymphomas | 0/3 | |
| T-cell chronic lymphocytic leukemia | 0/1 | |
| Mycosis fungoides/Sezary syndrome | 0/2 | |
| Peripheral T-cell lymphoma, unspecified | 0/16 | |
| B-cell lymphomas | 0/82 | |
| Precursor B-lymphoblastic lymphomas | 0/1 | |
| B-cell chronic lymphocytic leukemia | 0/10 | |
| Immunocytoma/lymphoplasmacytic lymphoma | 0/3 | |
| Mantle cell lymphoma | 0/4 | |
| Follicular center lymphoma | 0/12 | |
| Nodal monocytoid B-cell lymphoma | 0/1 | |
| Hairy cell leukemia | 0/4 | |
| Plasmacytoma/myeloma | 0/2 | |
| Diffuse large B-cell lymphoma | 0/36 | |
| Unclassified high grade | 0/6 | |
| Unclassified low grade | 0/3 | |
| Total no. of cases tested | 42/239 |
All cases were tested as paraffin sections with MoAb, AKL1 as described in the text.
Neoplasms were categorized according to the REAL30 classification of lymphoid malignancy.
Subtypes of Hodgkin's disease studied comprised 4 cases of lymphocyte predominance, 22 cases of nodular sclerosis, and 24 cases of mixed cellularity.
Immunoperoxidase labeling of a paraffin-embedded lymph node section from a case of ALCL. (a) Low-power view showing areas of tumor infiltration. LF, a residual lymphoid follicle surrounded by malignant cells. The lack of staining of normal cells allows scattered malignant cells to be easily detected in (b) lymphatic sinuses or as (c) isolated cells. (d) At higher power, antibody ALK1 labels both the cytoplasm and, more variably, the nuclei of the malignant cells.
Immunoperoxidase labeling of a paraffin-embedded lymph node section from a case of ALCL. (a) Low-power view showing areas of tumor infiltration. LF, a residual lymphoid follicle surrounded by malignant cells. The lack of staining of normal cells allows scattered malignant cells to be easily detected in (b) lymphatic sinuses or as (c) isolated cells. (d) At higher power, antibody ALK1 labels both the cytoplasm and, more variably, the nuclei of the malignant cells.
Only 1 of the 17 cases of “Hodgkin's-like ALCL” studied was positive for ALK. All 50 cases of classical Hodgkin's disease were unlabeled. The 8 cases of lymphomatoid papulosis studied were ALK−, as were all other lymphomas and leukemias analyzed (Table 3).
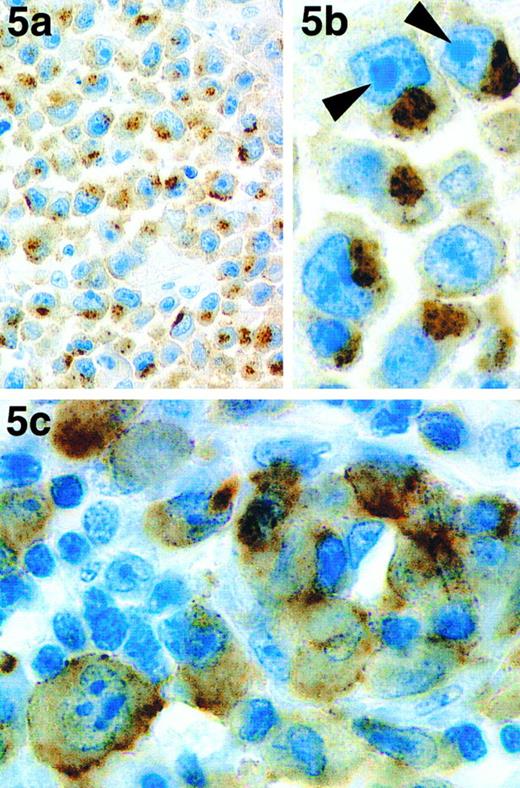
Three of the four cases that, although expressing EMA, were atypical because they lacked CD30, were found to be stained by antibody ALK1 (Table 3). However, labeling was restricted to discrete areas and coarse granules in the cytoplasm, being most prominent in the paranuclear area (Fig 5a and b). Two of these cases (both of T phenotype) were investigated using RT-PCR and both were found to be negative for NPM-ALK transcripts.
Immunoperoxidase labeling of paraffin-embedded sections of 2 atypical cases of ALCL. One of the three cases of CD30− ALCL that were labeled by antibody ALK1 is shown at low power (a) and high (b) magnification. Staining is in the form of coarse granules in the cytoplasm that tend to be localized to the paranuclear area. The prominent nucleoli (characteristic of these 3 cases) are indicated with arrows. (c) The neoplastic cells in a case of ALCL carrying a 1; 2 translocation are clearly labeled by antibody ALK1.
Immunoperoxidase labeling of paraffin-embedded sections of 2 atypical cases of ALCL. One of the three cases of CD30− ALCL that were labeled by antibody ALK1 is shown at low power (a) and high (b) magnification. Staining is in the form of coarse granules in the cytoplasm that tend to be localized to the paranuclear area. The prominent nucleoli (characteristic of these 3 cases) are indicated with arrows. (c) The neoplastic cells in a case of ALCL carrying a 1; 2 translocation are clearly labeled by antibody ALK1.
Correlation Between Cytogenetics, RT-PCR and Immunostaining for ALK Protein Using ALK1 Antibody and the Polyclonal Anti-p80 NPM-ALK
Table 4 summarizes the results obtained in 24 cases of ALCL investigated using cytogenetic analysis, RT-PCR, and anti-ALK reagents. There was correlation between the presence of the 2; 5 translocation and/or NPM-ALK mRNA and the detection of the chimeric NPM-ALK protein by antibody ALK1 in 18 cases (cases no. 1 through 8, 10, 14 through 18, and 21 through 24). Evidence of the 2; 5 translocation and NPM-ALK protein expression was found in 83.3% (15/18) of these cases.
Apart from 3 cases (no. 12, 18, and 20), the results obtained with antibody ALK1 were in agreement with those obtained using the anti-p80NPM-ALK. In general, the labeling by the ALK1 antibody was stronger than that given by the polyclonal reagent, and it was easier to distinguish between positive and negative cases. Staining with antibody ALK1 was limited to the malignant cells, whereas anti-p80NPM-ALK also labeled some fibroblast-like cells and endothelial cells.
Discrepancies between the results of immunostaining, cytogenetic analyses, and/or RT-PCR were noted in 6 cases. In case no. 9, the t(2; 5) translocation was detected, but there was no NPM-ALK mRNA or ALK1 immunoreactivity in the tumor cells. Case no. 11 showed the reverse pattern: a normal karyotype but NPM-ALK mRNA and ALK1 immunostaining were both detected. In case no. 12, there was no evidence of the t(2; 5) translocation or of NPM-ALK mRNA, but the tumor cells were clearly labeled by antibody ALK1. Anti-p80NPM-ALK was, however, negative in this case. Cases no. 19 and 20 showed labeling of the tumor cells by antibody ALK1 (and also by anti-p80NPM-ALK in case no. 19) but were negative for NPM-ALK transcripts. In case no. 13, which was morphologically and phenotypically identical to the other cases of ALCL (ie, CD30+, EMA+, and BNH.9+), the malignant cells showed a novel (1; 2)(q25;p23) translocation. These cells were stained by both antibody ALK1 (Fig 5c) and by anti-p80NPM-ALK.
None of the 23 cases of Hodgkin's disease investigated were labeled by antibody ALK1. This finding was in agreement with the lack of expression of NPM-ALK mRNA in these tumors.
Nonhematopoietic Tumors
Tumors arising from a wide variety of cell types (Table 5) were immunostained for ALK protein. These were all found to be negative.
Immunocytochemical Reactivity of Nonhematopoietic Tumors for ALK Protein
| Tumor . | No. Positive/Tested . |
|---|---|
| Carcinoma | |
| Lung | 0/11 |
| GI tract | 0/12 |
| Breast | 0/5 |
| Thyroid | 0/6 |
| Pancreas | 0/2 |
| Kidney | 0/2 |
| Bladder | 0/1 |
| Prostate | 0/2 |
| Liver | 0/3 |
| Uterus | 0/1 |
| Sarcoma | |
| Liposarcoma | 0/1 |
| Synovial sarcoma | 0/1 |
| Leiomyosarcoma | 0/1 |
| Rhabdomyosarcoma | 0/2 |
| Others | |
| Carcinoid | 0/7 |
| Pheochromocytoma | 0/4 |
| Adrenocortical carcinoma | 0/1 |
| Nephroblastoma | 0/3 |
| Thymoma | 0/3 |
| Neuroblastoma | 0/2 |
| Ganglioneuroblastoma | 0/2 |
| Melanoma | 0/4 |
| Leiomyoma | 0/1 |
| Oncocytoma | 0/2 |
| Adenoma | 0/1 |
| Total | 0/80 |
| Tumor . | No. Positive/Tested . |
|---|---|
| Carcinoma | |
| Lung | 0/11 |
| GI tract | 0/12 |
| Breast | 0/5 |
| Thyroid | 0/6 |
| Pancreas | 0/2 |
| Kidney | 0/2 |
| Bladder | 0/1 |
| Prostate | 0/2 |
| Liver | 0/3 |
| Uterus | 0/1 |
| Sarcoma | |
| Liposarcoma | 0/1 |
| Synovial sarcoma | 0/1 |
| Leiomyosarcoma | 0/1 |
| Rhabdomyosarcoma | 0/2 |
| Others | |
| Carcinoid | 0/7 |
| Pheochromocytoma | 0/4 |
| Adrenocortical carcinoma | 0/1 |
| Nephroblastoma | 0/3 |
| Thymoma | 0/3 |
| Neuroblastoma | 0/2 |
| Ganglioneuroblastoma | 0/2 |
| Melanoma | 0/4 |
| Leiomyoma | 0/1 |
| Oncocytoma | 0/2 |
| Adenoma | 0/1 |
| Total | 0/80 |
All tissues were stained as cryostat sections with MoAb ALK1, as described in the text.
DISCUSSION
The validity of the immunocytochemical results reported here are dependent on the specificity of the ALK1 MoAb. The NPM-ALK protein was predicted by Morris et al14 to be a protein tyrosine kinase of approximately 75 kD molecular weight and this has been confirmed using polyclonal anti-ALK reagents.14,19,20 Morris et al14 19 also reported that the full-length 200-kD ALK protein is expressed in a rhabdomyosarcoma cell line (Rh30). Antibody ALK1 recognized a phosphorylated protein of the correct molecular weight in a cell line carrying the (2; 5) translocation. This antibody also recognized a 200-kD protein in Rh30 cells. These were the only cell lines to be stained by antibody ALK1. Furthermore, antibody ALK1 recognized a 200-kD protein in cells transfected with a cDNA encoding full-length ALK protein and clear immunocytochemical labeling was observed in these cells. These results confirm that the antibody ALK1 specifically recognizes an epitope that is present in both the NPM-ALK chimeric protein as well as in the full-length ALK protein. Antibody ALK1 should also recognize any proteins produced as a result of variant translocations involving the ALK gene, providing that they include the cytoplasmic portion of the ALK protein.
In normal tissues, the only cells to be labeled (and the reactions were weak) were found in the brain. This correlates with a recent study of embryonic and adult mice by Morris et al,19 who found ALK mRNA only in neural tissues. In an earlier study of human tissues by Morris et al,14ALK mRNA was also found in the gut, prostate, testis, and fetal liver, but there was no evidence of ALK protein expression at these sites in the present study. It may be added that this restricted distribution of the ALK protein was supported by the absence of labeling found in a range of nonhematopoietic tumors.
In contrast to our results obtained with the ALK1 antibody, Shiota et al,20 using the polyclonal antibody p80NPM-ALK, found staining of follicular dendritic reticulum cells. Labeling of smooth muscle in endothelium and fibroblast-like sinusoidal cells was also found by Lamant et al29 and in the present study. These results may represent cross-reactivity of the p80NPM-ALK reagent with molecules other than ALK.
The reactivity of antibody ALK1 was compared directly with the polyclonal anti-p80NPM-ALK in 19 cases of ALCL in the present study. Correlation was found in 16 cases. The strong specific labeling by antibody ALK1 of the tumor cells meant that it was easier, in some cases, to interpret the staining obtained with the MoAb compared with that obtained with anti-p80NPM-ALK. Indeed, in both the present study and in that of Lamant et al,29 the labeling obtained using anti-p80NPM-ALK was often weak and therefore not always easy to differentiate from background staining. The 3 discordant cases (2 of which were positive with the monoclonal and not with the polyclonal and 1 of which gave the opposite pattern) reported here could, therefore, reflect difficulties in interpreting equivocal reactions obtained with the polyclonal antibody.
Good correlation was obtained in 18 of 24 cases [15 positive for NPM-ALK mRNA and/or the t(2; 5) and ALK proteins, whereas 3 lacked all of these markers] when labeling with antibody ALK1 was compared with RT-PCR analysis and/or the presence of the 2; 5 translocation. However, the positive labeling with antibody ALK1 seen in 1 case of ALCL with a 1; 2 translocation indicated that the presence of ALK protein does not equate solely with the presence of the 2; 5 translocation. Thus, in cases no. 12, 19, and 20, the absence of NPM-ALK mRNA, together with positive ALK1 staining, may simply indicate the presence of a chromosomal abnormality involving the ALK gene on 2p23 but with no involvement of the NPM gene at 5q35. In support of this, Yee et al43 reported weak labeling with anti-p80NPM-ALK in a case of ALCL that showed rearrangement of the ALK gene but no NPM-ALK mRNA. Furthermore, there is ample precedent for genes involving chromosomal translocations to have more than one fusion partner. The NPM gene is such an example and is involved in the formation of the fusion proteins NPM-myelodysplasia/myeloid leukemic factor-1 (NPM-MLF-1)44 and NPM-retinoic acid receptor α (NPM-RAR)45 as a result of the t(3; 5)(q25.1;q34) and t(5; 17)(q32;q12), respectively. The 2; 5 translocation was present in case no. 9, but no NPM-ALK mRNA or ALK proteins were detected using either antibody ALK1 or anti-p80NPM-ALK. This case has been described previously by Lamant et al.29
Immunocytochemical evidence for NPM-ALK or ALK protein expression was found in just over half of our cases of ALCL. This percentage is higher than has been found by immunocytochemistry and/or RT-PCR in some other series, probably because of our stricter criteria for the diagnosis of ALCL, namely inclusion only of cases with a T- or null-cell phenotype.30 Our results show that ALK1+ cases were more common in children than in adults, confirming the results of previous studies.9,27,29,46 Furthermore, Herbst et al27 found that the presence of NPM-ALK products was limited to primary ALCL and seen mainly in young patients, whereas all cases of secondary ALCL lacked either NPM-ALK transcripts or NPM-ALK protein. These results, together with those obtained in the present study, indicate that ALCL occurring in adults, especially arising as secondary tumors, may constitute a different and possibly more heterogeneous group of tumors than the primary ALCL cases seen in children. Indeed, previous studies have found that ALCL exhibiting evidence of NPM-ALK mRNA or labeling by anti-p80NPM-ALK have a better prognosis than those with no evidence of mRNA or protein.5,7,9 47 The immunocytochemical detection of ALK could therefore be of clinical value in the prognostic evaluation of ALCL.
Our immunocytochemical labeling studies emphasize the rarity of cases of lymphoma, other than ALCL exhibiting the 2; 5 translocation, in which ALK is expressed. One of these exceptions was of interest because, although it was a typical case of ALCL in terms of clinical and histopathologic features, a variant t(1; 2)(q25;p23) translocation was present. This anomaly presumably links part of the ALK gene to an unidentified gene on chromosome 1, which may supply an active promoter, resulting in ALK protein expression.
In a further 3 cases, the lymphoma cells had an anaplastic morphology similar to that of classical ALCL, but on closer inspection, their appearance was subtly different. They were also atypical in that they did not express the CD30 antigen, a hallmark of ALCL.1,34 35 The fact that antibody ALK1 labeled the cytoplasm of these malignant cells in an unusual granular pattern provides further evidence that a novel mechanism(s) may be responsible for the switch on of the ALK gene. It will be of interest to see if similar cases will be found in other centres and whether such CD30− lymphomas expressing ALK in this unusual pattern represent a rare novel subtype of lymphoma.
Immunocytochemical analysis is also of potential value for distinguishing ALCL from Hodgkin's disease. In keeping with previous studies, which have found no evidence of the 2; 5 translocation or of NPM-ALK gene products in Hodgkin's disease,24-29,31,48-50 we found Reed-Sternberg cells (and cell lines derived from Hodgkin's disease) to be consistently negative for NPM-ALK. The controversial reports by Orscheschek et al23 of NPM-ALK transcripts in 11 of 13 cases of Hodgkin's disease and of the labeling by the p80NPM-ALK antiserum of Reed-Sternberg cells51 are, therefore, difficult to explain, especially because this reagent did not stain any Hodgkin's disease cases in the study by Lamant et al.29
The absence of labeling by antibody ALK1 in cases of Hodgkin's disease shows that this reagent can be used to clarify problem cases. The rarity of labeling of so-called Hodgkin's-like ALCL (only 1 case of 17 was ALK1+) means that the majority of such cases are probably atypical Hodgkin's disease that are rich in neoplastic cells.27,30 It has also been suggested that lymphomatoid papulosis is related to ALCL, because the cells share many morphologic characteristics and express CD30.52-56 Indeed, a study by Davis et al53 reported evidence for a common cell of origin in these three diseases. However, the absence of the 2; 5 translocation in lymphomatoid papulosis found by DeCoteau et al57 and the lack of staining for ALK or NPM-ALK found in the present study weakens the argument for a link (at least in most cases) between ALCL and lymphomatoid papulosis.
In conclusion, this study has shown the very restricted distribution of ALK in normal tissues and also established that antibody ALK1 is a reliable reagent for the diagnosis of cases of ALCL expressing NPM-ALK or other ALK proteins. The diagnosis of cases of ALCL carrying the 2; 5 translocation is of importance because of their apparent improved prognosis compared with ALCL cases showing no involvement of the ALK gene. Immunocytochemistry also provides a means of detecting malignant ALCL cells in technically poor biopsies or in those in which only rare tumor cells are present. In addition, the use of antibody ALK1 has permitted a clear distinction to be made between ALCL and Hodgkin's disease. The presence of ALK protein in occasional cases of ALCL that did not exhibit the 2; 5 translocation suggests that alternative mechanisms may promote the expression of ALK protein. A final implication of the present study is, therefore, that antibodies such as ALK1 should be of value in studying the function of the ALK gene, the mechanism(s) of its deregulation, and possibly in defining new lymphoma entities.
ACKNOWLEDGMENT
We thank Prof P. Deschelotte, Dr J. Dumont, Prof F. Fetissof, Dr K.C. Gatter, Prof P. Levillain, and Dr M.J. Terrier-Lacombe for providing cases included in the present study. We are also grateful to Dr K. Micklem for his help in producing the illustrations. The excellent technical assistance of M. Kirstein is also gratefully acknowledged.
Supported by grants from the Leukaemia Research Fund, by the Ligue Nationale Contre le Cancer, by the Délégation à la Recherche Clinique “Projet de Hospitalier de Recherche Clinique,” by National Cancer Research Institute Grants No. CA 01702 and CA 69129, by the American Lebanese Syrian Associated Charities, and by the Wellcome Trust.
Address reprint requests to Karen Pulford, PhD, LRF Immunodiagnostics Unit, University Department of Cellular Science, Room 5501, Level 5, John Radcliffe Hospital, Oxford, OX3 9DU, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal