Abstract
Direct killing of CD4+ lymphocytes by human immunodeficiency virus-1 (HIV-1) probably cannot account for the magnitude of the loss of these cells during the course of HIV-1 infection. Experimental evidence supports a pathophysiologic role of the apoptotic process in depletion of CD4 cells in acquired immunodeficiency syndrome (AIDS). The Fas-receptor/Fas-ligand (Fas-R/Fas-L) system mediates signals for apoptosis of susceptible lymphocytes and lympoblastoid cell lines. A number of investigators have recently reported increased expression of the Fas receptor in individuals with HIV infection, along with increased sensitivity of their lymphocytes to anti-Fas antibody mimicking Fas ligand. We attempted to determine the role of Fas-mediated apoptosis in disease progression and viral replication. Increased Fas-receptor (CD95) expression on CD4+ and CD8+ lymphocytes was found in a large group of HIV-1–infected patients compared with normal controls; individuals with a diagnosis of AIDS and a history of opportunistic infection had significantly more Fas receptor expression than did asymptomatic HIV-infected persons and normal blood donor controls (P < .01). Triggering of the Fas-R by agonistic anti-Fas monoclonal antibody, CH11, was preferentially associated with apoptosis in the CD4+ cells; this effect was more pronounced in lymphocytes derived from HIV+ individuals. Soluble and membrane-bound forms of Fas-L were produced in greater amounts in peripheral blood mononuclear cells (PBMC) cultures and in plasma obtained from HIV-1–infected persons than from normal controls. Furthermore, triggering of lymphocytes from HIV-infected persons by CH11 increased levels of interleukin-1β converting enzyme (ICE), a protein associated with apoptosis. When PBMC were cultured in the presence of CH11, p24 production per number of viable cells was decreased as compared with the same PBMC without CH11 (P < .01). These findings suggest that multiple mechanisms, including increased production of Fas-L by infected PBMC, increased Fas-R expression, and induction of a protease of ICE family, may play roles in the apoptotic depletion of CD4+ cells in HIV infection.
A NUMBER OF pathophysiologic mechanisms appear to contribute to the dramatic depletion of CD4+ cells seen in the acquired immunodeficiency syndrome (AIDS). Although the magnitude of the viral burden increases with disease progression,1 progressive CD4 lymphocytopenia and immunologic dysfunction cannot be explained by human immunodeficiency virus-1 (HIV-1)–mediated cytolysis. Spontaneous apoptosis of HIV-1–infected and uninfected lymphocytes, as well as enhanced activation-induced programmed cell death, have been described in HIV-1 infection, suggesting that nonselective apoptosis of CD4+ cells may be responsible for their depletion in AIDS.2-5
The process of apoptosis involves activation of metabolic pathways within the cytoplasm and nucleus, leading to the final degradation of genomic DNA into monomeric and multimeric nucleosomal fragments.6,7 Physiologically, apoptosis is required for termination of the immune response, deletion of self-reactive T-cell clones, and/or elimination of malignant and virus-infected cells.8,9 The Fas receptor/Fas ligand (Fas-R/Fas-L) system plays an important role in these processes, because triggering of the Fas receptor leads to apoptosis in various primary cells and cell lines.10,11 A member of the tumor necrosis factor (TNF ) receptor superfamily, Fas-R, is expressed within the lymphatic system on activated mature lymphocytes, but is absent on resting, naive T cells.12 Defective Fas-R expression in mutant mouse models (lpr and gld mice) results in a syndrome of autoimmune disease, lymphocytosis, and hypergammaglobulinemia.13,14 Fas-R expression can be increased by cytokines such as TNF-α and interferon-γ.15 Induction of Fas-R expression on virus-infected cells is believed to play a role in control of infection16 by eliminating infected cells.
Previous studies have reported upregulation of Fas-R expression on both CD4+ and CD8+ cells in patients with HIV infection17-21 as well as increased sensitivity of lymphocytes to Fas-R triggering anti-Fas monoclonal antibody (MoAb; CH11). These observations support the hypothesis that the Fas-R/Fas-L system plays an important part in the pathophysiology of CD4+ lymphocyte depletion. We have studied a large cohort of HIV-infected patients, as well as examined other factors involved in Fas-mediated apoptosis, in an effort to better define the role and contribution of apoptosis to CD4 lymphopenia. We observed that the Fas ligand and interleukin-1β converting enzyme (ICE) are also modulated by HIV infection and that there is a correlation between Fas-R expression, disease progression, and viral production.
MATERIALS AND METHODS
Patients.Peripheral blood samples were derived from patients with AIDS and normal volunteers after obtaining informed consent according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (Bethesda, MD) and the Georgetown University Medical Center (Washington, DC). Blood samples were obtained from 116 patients with HIV-1 infection. In the 116 HIV-1–infected patients, 80 had CD4 counts of less than 200, 71 had a history of at least one opportunistic infection, and 100 had been treated with antiviral agents, including dideoxycytosine (ddC), zidovudine (AZT), or a combination. A group of 40 normal uninfected blood donors were used as controls.
Cell separation and culture.Cells were separated using density gradient centrifugation with lymphocyte separation media (Organon, Durham, NC). Thereafter, cells were washed twice with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 supplemented with fetal calf serum (both from Life Technologies, Gaithersburg, MD). Cultures were performed at a cell density of 0.5 × 106 cells/mL. When appropriate, natural lymphocyte-derived interleukin-2 (IL-2; Boehringer Mannheim, Indianapolis, IN) or phytohemagglutinin (PHA; Boehringer Mannheim) were used for stimulation at concentrations of 10 U/mL or 5 μg/mL, respectively. Anti-Fas MoAb CH11 (Kamyia, San Francisco, CA),22 an antibody that mimics the Fas-L by triggering the Fas receptor or ZB4, a Fas blocking antibody (AMAC, Westbrook, ME),23 were used at 1 μg/mL.
Cell viability.Cell viability was measured using a standard Trypan blue (Life Technologies) exclusion test or an automated colorimetric assay using tetrazolium bromide (MTT) reduction reaction. The blue formazan product was assayed spectrophotometrically based on a standard curve and correlated to trypan blue exclusion results.
Flow cytometry.For the measurement of Fas expression on CD4+ and CD8+ lymphocytes by flow cytometry, a whole blood test was used. Briefly, 100 μL of blood was incubated with 20 μL phycoerythrin (PE)-conjugated anti-CD4 or -CD8 MoAb (Becton Dickinson, Mountain View, CA) combined with 20 μL of FITC-conjugated anti-CD95 MoAb (UB2; Pharminogen, San Diego, CA). After 30 minutes of incubation, erythrocytes were lysed with 0.12% formic acid and the remaining cells were washed and fixed in 0.2% paraformaldehyde using the Q-prep reagent system (Coulter, Hialeah, FL). Samples were analyzed using an Epics ELITE flow cytometer (Coulter). In some experiments, density gradient purified cells or cultured lymphocytes were analyzed. Cells were incubated in a volume of 100 μL with the appropriate MoAb, washed twice with PBS, and fixed with paraformaldhyde. For sorting, mononuclear cells were stained with either CD4 or CD8 MoAb, washed, and separated by the flow cytometer. The purity of sorted cells was routinely 95% to 98%.
Cell cycle analysis and apoptosis assay.DNA analysis of cell cycle status was performed using flow cytometry. After culture, cells were harvested, washed with PBS containing 2% fetal calf serum, fixed with ice-cold 70% ethanol, rewashed, and resuspended with 10 μg/mL propidium iodide solution (Boehringer Mannheim) containing 10 μg/mL bovine RNAse (Boehringer Mannheim). After 30 minutes, DNA content was measured by flow cytometry. Collected data were analyzed using MCycle software version 2.5 (Phoenix Flow Systems, Phoenix, AR). This method allows determination of cell cycle status and also quantitation of apoptotic cells by measurement of the hypodiploid peak.
An in situ terminal deoxynucleotidyl transferase (TdT) assay (Apotag; Oncor, Gaithersburg, MD) also was used to quantitate the number of cells undergoing apoptosis. Cells were harvested, fixed with 4% neutral buffered formalin, and centrifuged. Endogenous peroxidase was quenched with 0.5% hydrogen peroxide and the cells were permeabilized using company-supplied equilibration buffer. The 3′-OH ends of degraded DNA were reacted with TdT and digoxygenin-labeled adenosine triphosphate for 30 minutes. After washing with PBS, cells were reacted with antidigoxygenin MoAb conjugated to peroxidase, washed, and exposed to 3,3′ diaminobenzidine tetrahydrochloride (Pierce, Rockford, IL), and the percentage of fluorescence was quantitated by flow cytometry. In some experiments, apoptosis was also shown by ethidium bromide agarose gel electrophoresis. Low molecular weight (LMW) DNA was extracted from constant numbers of cells using a modified Hirt extraction method.24 After precipitation, DNA was resuspended in TE buffer and run in 1.4% agarose gel. An apoptotic DNA ladder was visualized using ethidium bromide and UV light.
p24 enzyme-linked immunosorbent assay (ELISA).Viral replication was determined by p24 production. Tissue culture supernatants were harvested and stored at −70°C until analysis. p24 concentrations in tissue culture supernatants were determined in duplicate using a commercially available ELISA (Coulter) according to the manufacturer's recommendations. To account for cell death, p24 production was expressed per 1 × 106 viable cells.
Western blot analysis.For immunoblotting, similar numbers of peripheral blood mononuclear cells (PBMC) obtained from HIV-1–infected persons and normal controls were cultured without antibodies as previously described for 72 hours. Cells were subsequently pelleted and the supernatant applied to a 100-kD filter to remove fragments. For analysis of Fas-L, plasma samples were also concentrated using a 3-kD filter before analysis. For analysis of ICE, cells were solubilized in 3% sodium dodecyl sulfate (SDS). Supernatants and cells were electrophoresed in 12% of SDS-polyacrylamide gels and were equilibrated in transfer buffer (125 mmol/L TRIS-base, 960 mmol/L glycine, 20% methanol), and separated proteins were electrophoretically transferred on PVDF (Immobilon-P) membranes (Millipore, Bedford, MA). Membranes were blocked in TBST-milk (10 mmol/L TRIS-HCl [pH 8.0], 150 mmol/L NaCl, 0.5% Tween-20, 1% nonfat dry milk, 1% bovine serum albumin [Cohn fraction V; Miles, Kankakee, IL]) and treated with rabbit anti–Fas-L (an antibody to the N-terminal of Fas-ligand; Santa Cruz Biotechnology, Santa Cruz, CA) or a pan-ICE antibody at room temperature (Amersham, Arlington Heights, IL).25 After washing three times in TBST-milk, the membranes were incubated with alkaline phosphatase-labeled goat antirabbit IgG (1:2,000 dilution) at room temperature and subsequently with mouse anti-alkaline phosphatase MoAb (Dako, Carpinteria, CA). Specific bands were detected using nitro blue tetrazolium (NBT)/5-bromo-4 chloro-3-indolyl phosphate (BCIP) substrate (Pierce).
Statistical analysis.The percentage of Fas expressing cells was calculated by dividing the number of CD4+/CD8+ cells binding MoAb CD95 by the total number of CD4+/CD8+ cells. Statistical significance was calculated using a nonparametric Wilcoxon two-sample or one-sample test.
RESULTS
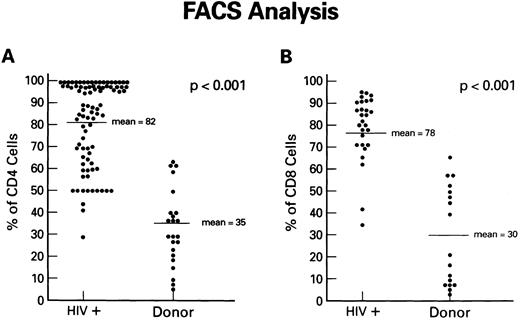
Expression of Fas antigen on fresh and cultured CD4+ and CD8+ cells from patients with HIV-1 infection.To determine if the Fas-R/Fas-L system plays a role in the depletion of CD4+ lymphocytes in AIDS, we first analyzed Fas-R expression in PBMC from HIV-1–infected individuals and normal volunteers. The expression of Fas (CD95) on CD8+ and CD4+ cells was determined using dual-color flow cytometry. Although significant percentages of CD4+ cells from normal uninfected controls expressed Fas-R (Fig 1A), a pronounced increase in CD95+CD4+ lymphocytes was found in the PB of HIV-1–infected patients. Increased Fas-R was not restricted to CD4+ cells, as similar increases were seen on CD8+ lymphocytes (Fig 1B). To determine whether increased Fas-R expression correlated with the severity of HIV-1 disease, we stratified patients by history of opportunistic infection and CD4 count. Significantly higher percentages of CD4+ and CD8+ cells expressing Fas-R were observed among cells from patients with a history of opportunistic infection (P < .01) or a CD4 count less than 200 cells/mL (P < .01).
Expression of Fas-R (CD95) in HIV-1 infection. Samples of blood were obtained from a group of 86 HIV-1–infected patients and a group of normal blood donors and stained with FITC-conjugated CD95 MoAb and PE-conjugated CD4 or CD8 MoAb. The percentage of CD4+ cells (A) or CD8+ cells (B) reacting with CD95 MoAb is shown. Statistical analysis showed the following relationships for differences in Fas-R expression: CD4+ cells: control v HIV-1+ (P < .01), CD8+ cells normal control v HIV-1+ (P < .01; Wilcoxon two-sample test).
Expression of Fas-R (CD95) in HIV-1 infection. Samples of blood were obtained from a group of 86 HIV-1–infected patients and a group of normal blood donors and stained with FITC-conjugated CD95 MoAb and PE-conjugated CD4 or CD8 MoAb. The percentage of CD4+ cells (A) or CD8+ cells (B) reacting with CD95 MoAb is shown. Statistical analysis showed the following relationships for differences in Fas-R expression: CD4+ cells: control v HIV-1+ (P < .01), CD8+ cells normal control v HIV-1+ (P < .01; Wilcoxon two-sample test).
Effects of Fas receptor triggering on apoptosis and viability of lymphocytes from HIV-1–infected persons.Because increased susceptibility for cell death was described in lymphocytes derived from patients with HIV-1 infection,20 we investigated the involvement of Fas-R/Fas-L system in spontaneous and activation-induced cell death in lymphocytes derived from HIV-1–infected patients. Normal or patient PBMCs were cultured in the presence of anti-Fas MoAb, CH11, to mimic Fas-L, in medium with or without PHA for polyclonal activation. After 3 days, cultures of PBMC from HIV-1–infected patients, stimulated with Fas-R agonist, showed significantly increased apoptosis (P < .01; Figs 2 and 3) and decreased viability in cultures (P < .01; data not shown). The addition of Fas agonist CH11 also significantly decreased the viability of normal PBMCs in cultures.
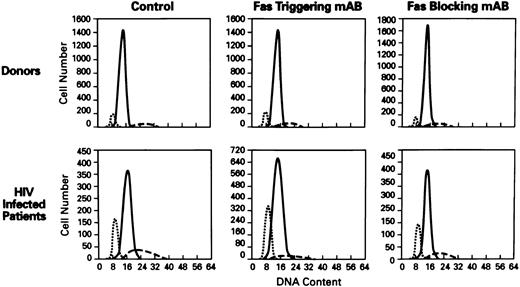
Cell-cycle analysis of lymphocytes treated with anti-Fas MoAb (CH11). Flow cytometric analysis of nuclei of lymphocytes stained with propidium iodide (N = 60). Hypoploid peak reflects the percentages of apoptotic cells. X axis, DNA content; Y axis, cell number. The histograms of PBMCs from normal donor (top) and HIV+ patient (bottom) showed the following: control (untreated lymphocytes), treated with Fas triggering antibody (CH11), treated with Fas blocking MoAb (ZB4). (⋅⋅⋅) The apoptotic peak; ( — ) the G1 phase; (- - -) the G2 phase of cell cycle.
Cell-cycle analysis of lymphocytes treated with anti-Fas MoAb (CH11). Flow cytometric analysis of nuclei of lymphocytes stained with propidium iodide (N = 60). Hypoploid peak reflects the percentages of apoptotic cells. X axis, DNA content; Y axis, cell number. The histograms of PBMCs from normal donor (top) and HIV+ patient (bottom) showed the following: control (untreated lymphocytes), treated with Fas triggering antibody (CH11), treated with Fas blocking MoAb (ZB4). (⋅⋅⋅) The apoptotic peak; ( — ) the G1 phase; (- - -) the G2 phase of cell cycle.
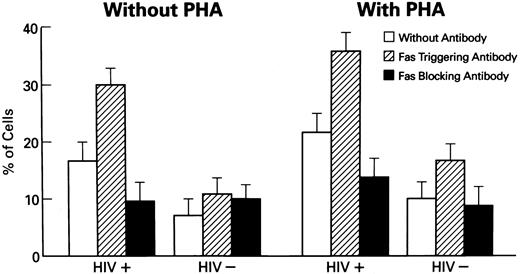
Induction of apoptosis in lymphocytes from HIV-1–infected patients. Columns represent the percentages of apoptotic cells in untreated cells and cells cocultured with CH11, Fas-R triggering MoAb (N = 50). Statistical analysis shows the following relationships: in cultures of HIV-1+ lymphocytes: untreated v PHA-stimulated lymphocytes showed significantly increased numbers of apoptotic cells (P < .01); as was seen in study of untreated v CH11 MoAb-treated (P < .01); untreated v ZB4 MoAb-treated (P < .01); PHA-treated v PHA-treated cells + CH11 MoAb (P < .01). Normal HIV− controls did not show statistically significant relationships (Wilcoxon two-sample test or one-sample test where appropriate).
Induction of apoptosis in lymphocytes from HIV-1–infected patients. Columns represent the percentages of apoptotic cells in untreated cells and cells cocultured with CH11, Fas-R triggering MoAb (N = 50). Statistical analysis shows the following relationships: in cultures of HIV-1+ lymphocytes: untreated v PHA-stimulated lymphocytes showed significantly increased numbers of apoptotic cells (P < .01); as was seen in study of untreated v CH11 MoAb-treated (P < .01); untreated v ZB4 MoAb-treated (P < .01); PHA-treated v PHA-treated cells + CH11 MoAb (P < .01). Normal HIV− controls did not show statistically significant relationships (Wilcoxon two-sample test or one-sample test where appropriate).
Stimulation of PBMC by PHA further increased the number of apoptotic cells (P < .01). In contrast, treatment with a blocking anti-Fas MoAb, ZB4, decreased the number of apoptotic cells in cultures of PBMCs from HIV-1–infected persons, in comparison with CH11 MoAb-treated and untreated cultures. No significant changes under these conditions were noted in cultures of normal PBMCs. Enhanced apoptosis in cultures from HIV-1–infected PBMCs was also documented using the method of LMW DNA electrophoresis with ethidium bromide staining (data not shown).
Although increased expression of Fas-R was found on both CD4+ and CD8+ lymphocytes derived from patients with HIV-1 infection, CD4+ cells preferentially underwent apoptosis after CH11 treatment, as shown by combined phenotyping and TdT assay. Using two-color flow cytometry, apoptosis could be visualized and affected cells could be phenotypically characterized. Although apoptosis was also observed within the CD8+ population, significantly higher numbers of CD4+ (PE-stained) lymphocytes showed a positive TdT reaction (FITC staining), indicative of apoptosis (Fig 4A). Because Fas-mediated apoptosis preferentially affected CD4+ cells in cultures of patients' PBMCs, the proportion of CD4+ cells would be anticipated to decrease relative to CD8+ cells (after the addition of CH11 MoAbs) triggering Fas-R. Flow cytometric analysis of lymphocytes cultured in the presence of CH11 MoAb showed a significantly decreased CD4:CD8 ratio as compared with cells derived from untreated cultures (P < .05; Fig 4B).
Selectivity of Fas-mediated apoptosis in cultures of lymphocytes from HIV-1–infected patients. (A) Fas-related apoptosis of CD4+ cells in culture of lymphocytes from patients with HIV-1 infection. Samples of lymphocytes from HIV-1–infected patients were untreated (ø) or cocultivated with CH11 MoAb (F ) for 3 days. The number of apoptotic cells were determined using two-color flow cytometry. Apoptotic cells were detected using TdT reaction; phenotype of cells was determined using CD4 or CD8 MoAb. Cells were gated to include only either CD4+ or CD8+ cells. The standard deviation of the mean is seen in brackets. (B) CD4/CD8 ratio of lymphocytes cocultivated with CH11 MoAb. Samples of PBMCs from the HIV-1–infected patients who were either untreated or cocultivated with CH11 MoAb. Columns represent the CD4+/CD8+ ratio of untreated (ø) and CH11-treated cells (F ). The standard deviation of the mean is shown in brackets.
Selectivity of Fas-mediated apoptosis in cultures of lymphocytes from HIV-1–infected patients. (A) Fas-related apoptosis of CD4+ cells in culture of lymphocytes from patients with HIV-1 infection. Samples of lymphocytes from HIV-1–infected patients were untreated (ø) or cocultivated with CH11 MoAb (F ) for 3 days. The number of apoptotic cells were determined using two-color flow cytometry. Apoptotic cells were detected using TdT reaction; phenotype of cells was determined using CD4 or CD8 MoAb. Cells were gated to include only either CD4+ or CD8+ cells. The standard deviation of the mean is seen in brackets. (B) CD4/CD8 ratio of lymphocytes cocultivated with CH11 MoAb. Samples of PBMCs from the HIV-1–infected patients who were either untreated or cocultivated with CH11 MoAb. Columns represent the CD4+/CD8+ ratio of untreated (ø) and CH11-treated cells (F ). The standard deviation of the mean is shown in brackets.
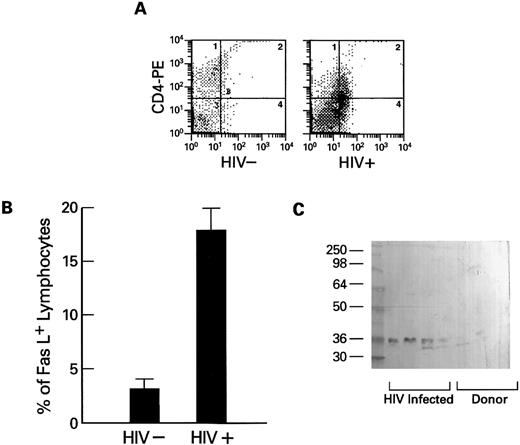
Expression of Fas-ligand in PBMCs obtained from HIV-1–infected persons.The extent of Fas-related apoptosis depends on at least two factors: the presence of Fas-R and the availability of Fas-L.26 When the expression of Fas-L (which also occurs in a membrane-bound form27 ) on lymphocytes was measured using flow cytometry, Fas-L was detected on substantial numbers of lymphocytes from HIV-1–infected patients, but not from normal volunteers (Fig 5A and B). Because high levels of spontaneous apoptosis were seen in cultures of lymphocytes derived from HIV-1–infected patients and stimulation of the cultured cells with mitogens or IL-2 increased the rate of programmed cell death, we tested whether Fas-L expression could be detected in cultures of PBMCs from HIV-1+ patients. Using Western blot and a specific MoAb directed against human Fas-L, we found Fas-L in filtered supernatants of lymphocytes cultured in media for 2 days and plasma obtained from HIV-1 infected persons, but not from cultures of normal control cells (Fig 5C) or normal plasma.
Expression of cell-free soluble and membrane-associated Fas-L by lymphocytes from HIV-1–infected persons. (A and B) Expression of Fas-L on normal (HIV−) and HIV-1–infected lymphocytes. Lymphocytes from HIV-1–infected patients (N = 10) and normal controls (N = 10) were dually stained with PE-conjugated CD4 or CD8 MoAb and FITC-conjugated anti–Fas-L. Columns at the left represent the numbers of CD4 or CD8 cells that reacted with Fas-L (B). An example scattergram (A) showing binding of CD4 PE on the Y axis and anti–Fas-L (FITC) on the X axis for both an HIV-1–infected patient and a normal control are represented. (C) Western blot of cell-free suspension from cultures from normal donors and lymphocytes from HIV-1–infected patients. PBMCs from normal controls (N = 20) and HIV-1–infected patients (N = 20) were cultured as previously described for 72 hours. Cell-free supernatants were prepared as described in Materials and Methods, and samples were analyzed by Western blotting using MoAb to Fas-L.
Expression of cell-free soluble and membrane-associated Fas-L by lymphocytes from HIV-1–infected persons. (A and B) Expression of Fas-L on normal (HIV−) and HIV-1–infected lymphocytes. Lymphocytes from HIV-1–infected patients (N = 10) and normal controls (N = 10) were dually stained with PE-conjugated CD4 or CD8 MoAb and FITC-conjugated anti–Fas-L. Columns at the left represent the numbers of CD4 or CD8 cells that reacted with Fas-L (B). An example scattergram (A) showing binding of CD4 PE on the Y axis and anti–Fas-L (FITC) on the X axis for both an HIV-1–infected patient and a normal control are represented. (C) Western blot of cell-free suspension from cultures from normal donors and lymphocytes from HIV-1–infected patients. PBMCs from normal controls (N = 20) and HIV-1–infected patients (N = 20) were cultured as previously described for 72 hours. Cell-free supernatants were prepared as described in Materials and Methods, and samples were analyzed by Western blotting using MoAb to Fas-L.
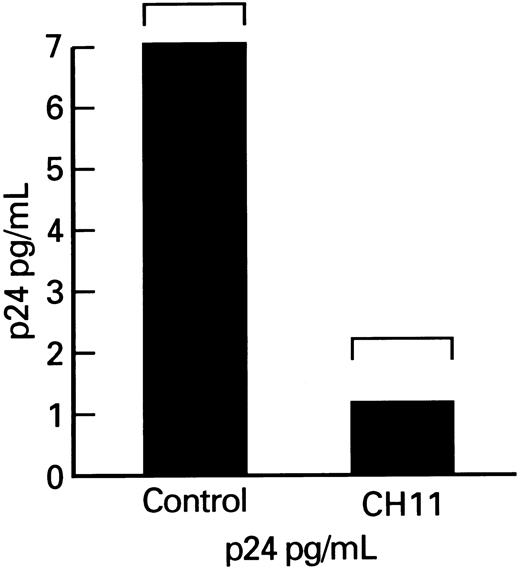
Effects of Fas-receptor triggering on p24 production by lymphocytes obtained form HIV-1–infected persons.Although enhancement of lymphocyte apoptosis by anti-Fas MoAb could result in nonselective depletion of all infected and uninfected CD4+ cells in cultures, there may be a selective process relatively restricted either to HIV-1–infected cells or to activated lymphocytes. To determine the effect of Fas-mediated apoptosis on HIV-1 replication, we measured the production of viral p24 core protein in lymphocytes. Total p24 levels were significantly decreased in cultures of patient's PBMCs that were supplemented with the Fas-R agonist antibody, CH11. Because the observed decrease in p24 production might primarily reflect lower numbers of viable cells in cultures treated with MoAb triggering Fas-R, we measured p24 production, which was expressed per 106 viable cells. The p24 output (normal to constant number of ) for the number of viable cells was significantly decreased in CH11-supplemented cultures (P < .01; Fig 6).
p24 production by lymphocytes from HIV-infected patients after cocultivation with CH11. Columns indicate p24 production adjusted per 105 viable PBMCs.
p24 production by lymphocytes from HIV-infected patients after cocultivation with CH11. Columns indicate p24 production adjusted per 105 viable PBMCs.
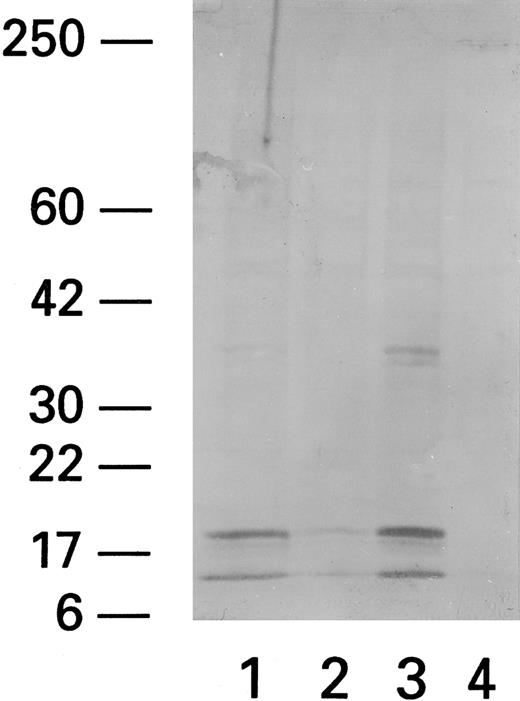
Expression of ICE in PBMCs from HIV-infected patients stimulated with Fas triggering antibody, CH11.Recent data indicate that Fas-related apoptosis is mediated by ICE and ICE-like proteases. We therefore studied the effect of Fas triggering antibody and PHA on ICE levels. Western blot analysis (using anti-pan ICE polyclonal antibody) showed that patient PBMCs exposed to Fas R agonist CH11 had more prominent 20-kD and 10-kD bands, previously identified as ICE proteins,25 whereas unexposed PBMCs did not (Fig 7). Normal PBMCs did not show any ICE protein even after coculture with CH11.
ICE expression in lymphocytes after cocultivation with CH11. Western blot of CH11-treated (Fas triggering MoAb) (1), ZB4-treated (Fas blocking MoAb) (2), CH11/PHA-treated (3), and untreated (4) subjects shows two bands at 10 and 20 kD MW. The higher MW band at 36 to 38 kD probably represents a nonspecific band that has been reported by the manufacturer (Kamyia).
ICE expression in lymphocytes after cocultivation with CH11. Western blot of CH11-treated (Fas triggering MoAb) (1), ZB4-treated (Fas blocking MoAb) (2), CH11/PHA-treated (3), and untreated (4) subjects shows two bands at 10 and 20 kD MW. The higher MW band at 36 to 38 kD probably represents a nonspecific band that has been reported by the manufacturer (Kamyia).
DISCUSSION
The Fas-R/Fas-L system is involved in the regulation of the immune response and lymphocyte senescence.10,13,14 Fas-mediated killing is, in addition to the granzyme/perforin pathway, another mechanism by which cytotoxic T cells kill target cells.26-29 During viral infections, activation of the Fas pathway may result in the elimination of infected cells as well as the destruction of uninfected bystander cells. Previous investigators have shown increased Fas-R expression in lymphocytes of patients with HIV-1 infection, along with an increase in apoptosis after Fas-R triggering.17-21
We have extended this prior data in a large cohort of patients and examined in greater depth the component of the Fas-R/Fas-L axis. In this large group of HIV-1–infected patients, we confirmed17-19,21 that higher percentages of both CD4+ and CD8+ lymphocytes expressed Fas-R and that the lymphocytes from HIV-1–infected patients were more sensitive to Fas triggering than cells from normal uninfected donors. Our new data showed that CD4+ lymphocytes were more likely to undergo apoptosis after Fas triggering than CD8+ cells. Although symptomatic patients with low CD4+ counts expressed more Fas-R than did asymptomatic patients with higher CD4+ counts, lymphocytes from HIV-1–infected patients produced more Fas-L than did cells from normal controls. Patient plasma also contained more Fas-L than normal controls. Triggering the Fas-R of lymphocytes from HIV-1–infected patients with CH11 was associated with an increase in ICE and a decrease in p24 antigen production that could not be explained by cell death.
Lymphocytes expressing the Fas-R were also found in normal individuals and in the majority of CD4+ as well as CD8+ cells from HIV-1+ individuals. Activation of normal lymphocytes is known to be associated with the induction of Fas-R expression, and high percentages of Fas+ lymphocytes in HIV-1 infection may be a result of sustained or repeated activation of the immune system. Only a limited number of normal lymphocytes expressing Fas-R show susceptibility to Fas-mediated apoptosis. Expression of the Fas ligand is also needed to induce apoptotic cell death in lymphocytes via the Fas pathway. We observed that the Fas ligand was produced in much greater quantities in lymphocytes from HIV-1–infected patients than in normal controls.
The decreased ratio of CD4/CD8 cells treated with anti-Fas MoAb is consistent with our observation that CH11 MoAb preferentially triggers apoptosis in CD4+ cells. No differences in susceptibility of CD4+ and CD8+ cells to this Fas-R agonist were observed in cultures from uninfected controls. However, in lymphocytes from HIV-infected individuals, CD4+ cells were more likely to undergo apoptosis than CD8+ cells. Estaquier et al21 recently showed increased Fas-R expression on CD4+ and CD8+ cells from HIV-infected patients and showed that apoptosis was dependent on the presence of Fas ligand. CD4+ and CD8+ cells were equally susceptible to apoptosis induced by exposure to Fas ligand or by Fas triggering antibody. In our study, CD4+ cells were much more likely to undergo apoptosis after cocultivation with Fas agonist antibody than CD8+ cells. Differences in patient selection may explain these different findings. Whereas Estaquier et al21 studied HIV-1–infected asymptomatic patients, all patients studied in our cocultivation experiments had a history of opportunistic infection and most had CD4 counts less than 200 cells/mL.
Our preliminary studies of the role of ICE in Fas-related apoptosis indicate that this enzyme is modulated in HIV infection. ICE appears to play a major role in Fas-mediated apoptosis; overexpression of ICE induces apoptosis in transfected cell lines.25 The finding of increased ICE expression in lymphocytes stimulated with CH11 directly extends the findings of Estaquier et al,21 who showed that inhibitors of ICE interfere with Fas-mediated apoptosis in CD4+ cells.
Fas-mediated apoptosis of virus infected cells has been reported to be a means of controlling viral production in infections other than HIV-1.16 Our study shows that Fas-R triggering was associated with decreases in HIV-1 propagation. Although lower p24 levels could partially be explained by an increased death rate in CH11-treated cultures, when the p24 level was adjusted for numbers of viable cells, viral production was still decreased. This effect may be due to more selective killing and higher susceptibility of virus-infected (ie, CD4+) cells or to downregulation of HIV-1 production after Fas-R triggering. Prior in vitro data showed that PBMCs infected with HIV-1 increased their p24 production in the presence of TNF-α, whereas those pretreated with an anti-Fas blocking MoAb failed to produce virus.30
In addition to Fas-R, Fas-L must be present to induce apoptosis via the Fas-R pathway. Jurkat cells and activated normal lymphocytes in culture produce Fas-L.30 In HIV-1 infection, increased spontaneous and activation-induced apoptosis28 could result from increased Fas-L production. We observed that inhibition of the Fas pathway with a specific blocking MoAb (ZB4) decreased spontaneous apoptosis in cultures of lymphocytes from HIV-1–infected but not normal individuals, suggesting that Fas-L was present in cultures of patients' PBMCs. We also found enhanced expression of Fas-L by fresh and cultured PBMCs derived from patients with HIV-1 infection. Expression of Fas-L may be intrinsic to the pathophysiology of HIV infection or may result from activation of T cells and monocytes26 by concurrent opportunistic infection. In either case, Fas-L produced by lymphocytes from HIV-1–infected patients may induce apoptosis in activated neighboring cells in an paracrine fashion.
Recently, Effros et al31 showed shortening of telomere length in CD28−CD8+ cells in HIV-infected persons. She speculated that this finding, which is also present in the elderly, is a consequence of repeated expansion of this chronically stimulated cell line. She further speculated that clonal exhaustion results and leads to loss of the immune response to HIV as the disease progresses. Preliminary findings from other investigators did not show similar shortened telomere length in CD4+ cells in HIV-infected persons.32 This finding is unexpected, because apoptosis and cell death should cause significant depletion of CD4+ cells, with compensatory expansion of surviving CD4 cell lines resulting in telomere shortening. Some investigators have contended that failure to show shortened telomere length in CD4+ cells argues against their in vivo rapid destruction. Much work still needs to be performed in this area to clarify these issues.
Our findings prompt several important questions. First, what is the mechanism of enhanced Fas selectivity of normal versus patient lymphocytes? Although additional factors such as TNF-α or interferon-γ are expressed in abundance in AIDS and could contribute to this effect,30,33,34 HIV-1 infection per se may enhance apoptosis by its activation of CD4+ lymphocytes. It is also possible that gradual depletion of CD4+ cells results in decreased production of an apoptosis inhibitor, resulting in a perpetuating process in the course of disease. Second, it is unclear which factors in AIDS determine the relative sensitivity of CD4 cells to Fas-mediated apoptosis. Although tropism of HIV-1 for CD4+ cells may, in part, account for this relative sensitivity, the rate of apoptosis is likely higher than the number of infected cells. A recent study, showing that cross-linking of the CD4 molecule may, under certain circumstances, result in upregulation of Fas-R and/or induction of apoptosis,35 may help explain why CD4+ cells are depleted and CD8+ cells are not. Such cross-linking would likely occur by binding of gp120 to CD4 molecules on uninfected cells with subsequent ligation with anti-gp120 MoAb present in serum.36 Finally, the role of ICE in Fas-mediated apoptosis in the HIV-infected patient still needs to be clarified. It is hoped that a full understanding of the factors that mediate viral triggering of apoptotic cell death will lead to integrated treatment that will help interrupt this process. Addressing these issues may offer insights into novel strategies to sustain T-cell numbers and increase immune function in AIDS.
Address reprint requests to J.P. Maciejewski, MD, University of Nevada, School of Medicine, Department of Microbiology-320, Howard Medical Building, Reno, NV 89557-0046.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal