Abstract
Hematopoiesis in the mouse conceptus begins in the visceral yolk (VYS), with primitive erythroblasts first evident in blood islands at the headfold stage (E8.0). VYS erythropoiesis is decreased or abrogated by targeted disruption of the hematopoietic transcription factors tal-1, rbtn2, GATA-1, and GATA-2. To better understand the potential roles of these genes, and to trace the initial temporal and spatial development of mammalian embryonic hematopoiesis, we examined their expression patterns, and that of βH1-globin, in normal mouse conceptuses by means of in situ hybridization. Attention was focused on the 36-hour period from mid-primitive streak to early somite stages (E7.25 to E8.5), when the conceptus undergoes rapid morphologic changes with formation of the yolk sac and blood islands. Each of these genes was expressed in extraembryonic mesoderm, from which blood islands are derived. This VYS expression occurred in a defined temporal sequence: tal-1 and rbtn2 transcripts were detected earlier than the others, followed by GATA-2 and GATA-1, and then by βH1-globin. Transcripts for all of these genes were present in VYS mesoderm cell masses at the neural plate stage (E7.5), indicating commitment of these cells to the erythroid lineage before the appearance of morphologically recognizable erythroblasts. By early somite stages (E8.5), GATA-2 mRNA expression is downregulated in VYS blood islands as terminal primitive erythroid differentiation proceeds. We conclude that primitive mammalian erythropoiesis arises during gastrulation through the ordered temporal expression of tal-1, rbtn2, GATA2, and GATA-1 in a subset of extraembryonic mesoderm cells. During the stages analyzed, tal-1 and rbtn2 expression was also present in posterior embryonic mesoderm, while GATA-1 and GATA-2 expression was evident in extraembryonic tissues of ectodermal origin.
BLOOD CELL DEVELOPMENT occurs in two waves during mammalian embryogenesis. The first and less well-characterized wave, primitive hematopoiesis, takes place in the visceral yolk sac (VYS). The murine VYS arises during gastrulation (E6.5 to E8.5), as mesoderm cells traverse the posterior primitive streak and migrate to extraembryonic sites. Extraembryonic mesoderm cells proliferate to form blood islands by the early headfold stage (E8.0).1 The yolk sac at this stage also contains α-globin transcripts,2 while nucleated hemoglobin-containing cells have been detected by the early somite stage (E8.5).3 Primitive hematopoiesis is followed by a second wave, definitive hematopoiesis, beginning in the liver at mid-gestation. At the end of gestation, definitive hematopoiesis shifts to the bone marrow, where it remains throughout adult life.
Hematopoietic lineage differentiation is regulated in part by the differential expression of transcription factors. Targeted disruption of several genes, including the hematopoietic transcription factors tal-1, rbtn2, GATA-1, and GATA-2, diminishes or completely abrogates primitive hematopoiesis. The disruption of the tal-14 or the rbtn25 gene results in mouse conceptuses entirely lacking primitive erythroid cells. Disruption of the GATA-2 gene results in mutant conceptuses containing far fewer primitive erythroid cells than wild-type conceptuses.6 Like the rbtn2 and tal-1 mutants, GATA-2 null mutants die by mid-gestation. A requirement for GATA-1 in normal primitive erythroid development has been established from in vitro differentiation studies with GATA-1 null embryonic stem (ES) cells.7,8 These cells fail to mature beyond the proerythroblast stage of primitive erythroid differentiation; however, there is no effect on macrophage colony formation.9 Similar results have been reported with rbtn2 null ES cells.5 tal-1 null ES cells have a more profound hematopoietic defect and produce no erythroid and only rare macrophage colonies in vitro.4
The expression of tal-1, rbtn2, GATA-1, and GATA-2 in hematopoietic progenitors makes them useful markers to trace the initial development of hematopoiesis in the mammalian embryo. Comparison of their normal expression patterns can also provide insights regarding their potential roles in the regulation of embryonic hematopoiesis. We examined tal-1, rbtn2, GATA-1, GATA-2, and βH1-globin mRNA accumulation in the mouse conceptus during gastrulation by in situ hybridization. Our analysis focused on a 36-hour period (E7.25 to E8.5), from mid-primitive streak to early somite stages, when the conceptus undergoes rapid morphologic changes with formation of the yolk sac and blood islands. We found that tal-1, rbtn2, GATA-1, and GATA-2 mRNAs are expressed in a defined temporal sequence before the onset of βH1-globin accumulation. Furthermore, the expression of these genes was spatially restricted; however, all were localized to subsets of extraembryonic mesoderm cells before the development of morphologically identifiable erythroid cells.
MATERIALS AND METHODS
Isolation of embryonic tissue and preparation of sections.CD-1(ICR) mice (Charles River Laboratories, Wilmington, MA) were maintained in a 12-hour light, 12-hour dark cycle. Vaginal plugs were checked the morning after natural overnight matings (E0.3). At specified times, mice were killed by cervical dislocation and the uteri were removed from the peritoneum. The decidua were transected in cold PB-1.10 The embryos resting in half-decidua were rinsed in phosphate-buffered saline (PBS) and fixed overnight in freshly prepared, cold 4% paraformaldehyde/PBS. They were dehydrated through ethanol into xylene and embedded in paraffin using a Tissue-Tek V.I.P. automatic processor (Miles, Mishawaka, IN). Three serial 5-μm sections from each examined developmental stage were placed onto triethylaminopropyl silane–coated slides. Only embryos whose stage11 was consistent with developmental time were analyzed.
In situ hybridization.The procedure used was essentially that of Wilkinson and Green,12 as previously described.13 Briefly, sections were dewaxed, rehydrated, and treated with proteinase K to enhance probe accessibility and with acetic anhydride to reduce nonspecific background. Single-stranded 33P-labeled antisense RNA probes were prepared by standard techniques14 with specific activities of 1.5 to 5 × 108 dpm/μg, and hydrolyzed by alkaline treatment to approximately 200 bp.15 Sense probes were synthesized to the same specific activity as antisense probes and served as controls for nonspecific background. Sections were hybridized at −25°C, washed at −7°C, and treated with RNase A to further diminish nonspecific adherence of probe. Multiple embryos and exposures of different lengths were examined for each sense and antisense probe. Autoradiography with NTB-2 emulsion (Eastman Kodak, Rochester, NY) was performed for 2 to 25 days. Slides were developed in D19 (Eastman Kodak), and the tissues were counterstained with toluidine blue. Photography was performed using a Nikon Optiphot microscope (Nikon, Garden City, NY) equipped with darkfield illumination (Darklight; Micro Video Instruments, Avon, MA).
Probes.A 1.78-kb mouse GATA-1 cDNA clone was provided by S. Orkin,16 from which a 1.25-kb EcoRI fragment was subcloned into pBluescript KS+. A 0.7-kb mouse GATA-2 cDNA clone, pBS7∼CR2 clone 1.1, was also provided by S. Orkin.6 A full-length, 1.35-kb mouse rbtn2 cDNA clone, pMR2A12N, was obtained from the HGMP Resource Centre Probe Bank, Hinxton, UK.17,18 A 0.6-kb mouse tal-1 cDNA clone was provided by S. Brandt. A 0.37-kb mouse βH1-globin cDNA clone, provided by A. McMahon,19 was subcloned into pBluescript SK+.
RESULTS
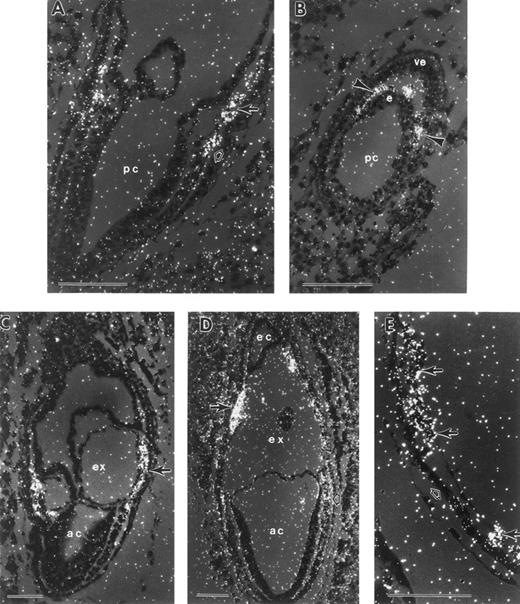
During gastrulation, which begins at E6.5 in the mouse, epiblast cells traverse the primitive streak, becoming mesoderm. Epiblast cells exiting the posterior portion of the streak migrate proximally to become extraembryonic mesoderm and contribute to the formation of (1) the allantois; (2) the amnion, with embryonic ectoderm; (3) the chorion, with extraembryonic ectoderm; and (4) the VYS, with visceral endoderm. At the mid-primitive streak stage, E7.25, small cavities can be discerned in the extraembryonic mesoderm (Fig 1A). These cavities expand (Fig 1B) and coalesce to form the exocoelomic cavity, which separates the amniotic cavity from the ectoplacental cavity (Fig 1C). By the neural plate stage (Fig 1C), E7.5, the VYS mesoderm in close proximity to extraembryonic ectoderm has proliferated to form mesodermal cell masses. These consist of a mesothelial lining and morphologically indistinguishable mesoderm cells1 in apposition to visceral endoderm cells (Fig 1D). Within the next 24 hours, the mesodermal cell masses differentiate into the morphologically distinguishable erythroid and endothelial cells, which constitute the yolk sac blood islands (Fig 1F ). Concomitantly, the yolk sac expands to surround most of the embryo proper by early somite stages, E8.5 (Fig 1E).
Development of the mouse conceptus from the mid-primitive streak to early somite stages. All sections are longitudinal with the exception of (E) and (F ), which are frontal. (A) Mid-primitive streak stage conceptus (E7.25) with the proamniotic cavity (pc) lined by ectoderm cells. Mesoderm cells are beginning to form a cavity (arrow) that will expand to form the exocoelom (ex). (B) Late-primitive streak stage conceptus with an expanded exocoelomic cavity (arrow) in extraembryonic mesoderm. (C) Neural plate stage conceptus (E7.5). Extraembryonic mesoderm cells forming the lateral wall of the exocoelom are proliferating to form a mesodermal cell mass (arrow). (D) Higher magnification of a mesodermal cell mass, consisting of flattened mesothelial cells (arrowhead) lining the exocoelom and proliferating undifferentiated mesoderm cells (closed arrow). The mesodermal cell mass is apposed to the single cell layer of visceral endoderm (open arrow) with its brush border facing the yolk sac cavity. (E) Early somite stage conceptus with multiple yolk sac blood islands (2 blood islands are indicated with arrowheads). Trophoblast giant cells (arrow) are in contact with the maternal decidual tissue (d). (F ) Blood islands at the early somite stage consisting of differentiating erythroid cells (open arrow) and endothelial cells (closed arrow). ac, amniotic cavity; d, deciduum; ec, ectoplacental cavity; epc, ectoplacental cone; ex, exocoelomic cavity; pc, proamniotic cavity; ep, embryo proper. Each scale bar represents 100 μm and the magnification of (F ) is the same as (D).
Development of the mouse conceptus from the mid-primitive streak to early somite stages. All sections are longitudinal with the exception of (E) and (F ), which are frontal. (A) Mid-primitive streak stage conceptus (E7.25) with the proamniotic cavity (pc) lined by ectoderm cells. Mesoderm cells are beginning to form a cavity (arrow) that will expand to form the exocoelom (ex). (B) Late-primitive streak stage conceptus with an expanded exocoelomic cavity (arrow) in extraembryonic mesoderm. (C) Neural plate stage conceptus (E7.5). Extraembryonic mesoderm cells forming the lateral wall of the exocoelom are proliferating to form a mesodermal cell mass (arrow). (D) Higher magnification of a mesodermal cell mass, consisting of flattened mesothelial cells (arrowhead) lining the exocoelom and proliferating undifferentiated mesoderm cells (closed arrow). The mesodermal cell mass is apposed to the single cell layer of visceral endoderm (open arrow) with its brush border facing the yolk sac cavity. (E) Early somite stage conceptus with multiple yolk sac blood islands (2 blood islands are indicated with arrowheads). Trophoblast giant cells (arrow) are in contact with the maternal decidual tissue (d). (F ) Blood islands at the early somite stage consisting of differentiating erythroid cells (open arrow) and endothelial cells (closed arrow). ac, amniotic cavity; d, deciduum; ec, ectoplacental cavity; epc, ectoplacental cone; ex, exocoelomic cavity; pc, proamniotic cavity; ep, embryo proper. Each scale bar represents 100 μm and the magnification of (F ) is the same as (D).
Terminal differentiation of erythroid cells is marked by the synthesis of hemoglobin. βH1-globin serves as the major β-globin gene product in early yolk sac erythroblasts.20 To determine the initial time and place of globin transcription during murine embryogenesis, we performed in situ hybridization studies with βH1-globin. The earliest consistently detectable expression of globin transcripts was at the neural plate stage, E7.5 (Fig 2A and B). βH1-globin levels accumulated above background specifically in the mesodermal cell masses of the VYS. At the early somite stage (E8.5), most of the cells in the blood islands were strongly positive (Fig 2C). In the early somite stage embryo shown in Fig 2C, a few circulating blood cells are evident both in the embryo proper and in the allantois. A βH1-globin sense probe gave no localized signal above background (Fig 2D, E, and F ).
In situ hybridization with βH1-globin antisense (A through C) and sense probes (D through F ) of late-primitive streak (A and C), neural plate (B and D) and early somite stage (C and F ) conceptuses. (A) Late-primitive streak stage conceptus with no signal above background. (B) Neural plate stage conceptuses (E7.5), with βH1-globin transcripts localized to the mesodermal cell mass (closed arrow). (C) Early somite stage conceptuses with high levels of βH1-globin mRNA present in yolk sac blood islands (closed arrows), and blood cells within the allantois (arrowhead) and the dorsal aorta of the embryo proper (white arrow). (D through F ) Sections of equivalent staged embryos probed with sense βH1-globin gave no signal above background. ac, amniotic cavity; ex, exocoelomic cavity; m, mesoderm; pc, proamniotic cavity. Each scale bar represents 100 μm.
In situ hybridization with βH1-globin antisense (A through C) and sense probes (D through F ) of late-primitive streak (A and C), neural plate (B and D) and early somite stage (C and F ) conceptuses. (A) Late-primitive streak stage conceptus with no signal above background. (B) Neural plate stage conceptuses (E7.5), with βH1-globin transcripts localized to the mesodermal cell mass (closed arrow). (C) Early somite stage conceptuses with high levels of βH1-globin mRNA present in yolk sac blood islands (closed arrows), and blood cells within the allantois (arrowhead) and the dorsal aorta of the embryo proper (white arrow). (D through F ) Sections of equivalent staged embryos probed with sense βH1-globin gave no signal above background. ac, amniotic cavity; ex, exocoelomic cavity; m, mesoderm; pc, proamniotic cavity. Each scale bar represents 100 μm.
To further trace the development of hematopoietic lineages during gastrulation, the expression patterns of GATA-1 and GATA-2 were examined by in situ hybridization. No GATA-1 or GATA-2 transcripts were detected in the VYS mesoderm at the mid-primitive streak stage (Figs 3A and 4A). Expression in the VYS was first seen in a subset of extraembryonic mesoderm cells at the late-primitive streak stage (Figs 3B and 4B), just before the initiation of βH1-globin transcript accumulation (Fig 2A). By the neural plate stage, most cells in the VYS mesoderm cell masses were positive for GATA-1 and GATA-2 (Fig 4C and data not shown). At the early somite stage, there was continued expression of GATA-1 (Figs 3C and D, and 5A), but not of GATA-2 (Figs 4D and 5B), in blood island erythroblasts.
In situ hybridization with a GATA-1 antisense probe. (A) Mid-primitive streak stage conceptus with no localized signal above background. (B) Late-primitive streak stage conceptus with GATA-1 mRNA expression present in the mesoderm cell mass (closed arrow). There is expression evident in extraembryonic ectoderm cells of the chorion (open arrow). (C) Early somite stage conceptus with localized signal in yolk sac blood islands (arrow). (D) Higher magnification of an early somite stage conceptus, with GATA-1 mRNA present in yolk sac blood islands (arrow). Low levels of GATA-1 transcripts are also evident in extraembryonic ectoderm cells of the chorion (open arrow) compared to yolk sac endoderm (closed arrow). GATA-1 sense probes gave similar high generalized background with no localized signal (data not shown). ep, embryo proper; m, mesoderm; pc, proamniotic cavity. Each scale bar represents 100 μm.
In situ hybridization with a GATA-1 antisense probe. (A) Mid-primitive streak stage conceptus with no localized signal above background. (B) Late-primitive streak stage conceptus with GATA-1 mRNA expression present in the mesoderm cell mass (closed arrow). There is expression evident in extraembryonic ectoderm cells of the chorion (open arrow). (C) Early somite stage conceptus with localized signal in yolk sac blood islands (arrow). (D) Higher magnification of an early somite stage conceptus, with GATA-1 mRNA present in yolk sac blood islands (arrow). Low levels of GATA-1 transcripts are also evident in extraembryonic ectoderm cells of the chorion (open arrow) compared to yolk sac endoderm (closed arrow). GATA-1 sense probes gave similar high generalized background with no localized signal (data not shown). ep, embryo proper; m, mesoderm; pc, proamniotic cavity. Each scale bar represents 100 μm.
In situ hybridization with a GATA-2 antisense probe. (A) Mid-primitive streak stage conceptus with no detectable signal over background in the embryo proper. (B) Late-primitive streak stage conceptus with GATA-2 mRNA expression evident both in the ectoplacental cone (epc) and in trophoblast cells (arrowhead). There is also expression evident in the mesoderm cell masses (arrow). (C) Neural plate stage conceptus with GATA-2 mRNA expression in trophoblast cells (arrowhead) and in mesodermal cell masses (arrow). (D) Early somite stage conceptus with continued signal in trophoblast giant cells (arrowhead). GATA-2 mRNA is detected in the extraembryonic ectoderm of the chorion (open arrow), but has been downregulated in yolk sac blood islands (closed arrow). (E) Higher magnification of the lower region of (D), showing GATA-2 mRNA expression in the chorion (open arrow) and trophoblast giant cells (arrowhead). Sense controls had high generalized background, but no localized signal (data not shown). ac, amniotic cavity; ex, exocoelomic cavity; pc, proamniotic cavity; m, mesoderm; ep, embryo proper. Each scale bar represents 100 μm.
In situ hybridization with a GATA-2 antisense probe. (A) Mid-primitive streak stage conceptus with no detectable signal over background in the embryo proper. (B) Late-primitive streak stage conceptus with GATA-2 mRNA expression evident both in the ectoplacental cone (epc) and in trophoblast cells (arrowhead). There is also expression evident in the mesoderm cell masses (arrow). (C) Neural plate stage conceptus with GATA-2 mRNA expression in trophoblast cells (arrowhead) and in mesodermal cell masses (arrow). (D) Early somite stage conceptus with continued signal in trophoblast giant cells (arrowhead). GATA-2 mRNA is detected in the extraembryonic ectoderm of the chorion (open arrow), but has been downregulated in yolk sac blood islands (closed arrow). (E) Higher magnification of the lower region of (D), showing GATA-2 mRNA expression in the chorion (open arrow) and trophoblast giant cells (arrowhead). Sense controls had high generalized background, but no localized signal (data not shown). ac, amniotic cavity; ex, exocoelomic cavity; pc, proamniotic cavity; m, mesoderm; ep, embryo proper. Each scale bar represents 100 μm.
Differential expression patterns of GATA-1 and GATA-2 in yolk sac blood islands and giant trophoblast cells at the early somite stage. (A) In situ hybridization with a GATA-1 antisense probe, showing signal in yolk sac blood islands (arrowheads), and lack of signal in trophoblast giant cells (arrows). (B) In situ hybridization with a GATA-2 antisense probe, showing lack of signal in yolk sac blood islands (arrowheads), and presence of signal in trophoblast cells (arrows). Scale bars represent 100 μm.
Differential expression patterns of GATA-1 and GATA-2 in yolk sac blood islands and giant trophoblast cells at the early somite stage. (A) In situ hybridization with a GATA-1 antisense probe, showing signal in yolk sac blood islands (arrowheads), and lack of signal in trophoblast giant cells (arrows). (B) In situ hybridization with a GATA-2 antisense probe, showing lack of signal in yolk sac blood islands (arrowheads), and presence of signal in trophoblast cells (arrows). Scale bars represent 100 μm.
GATA-1– and GATA-2–positive cells were also identified in other extraembryonic tissues. Transcripts for both were found in the extraembryonic ectoderm at the late streak stage (Figs 3B and 4B). Low, but reproducible, levels of GATA-1 (Fig 3D) and more detectable levels of GATA-2 (Fig 4D and E) were evident at early somite stages in the chorion, which derives in part from the extraembryonic ectoderm. Furthermore, GATA-2 was expressed in the ectoplacental cone and trophoblast giant cells from late streak to early somite stages (Fig 4B through E, arrowheads). It is noteworthy that at the early somite stage, GATA-1 was expressed in blood island erythroid cells, but not in trophoblast giant cells (Fig 5A), while GATA-2 was expressed in trophoblast giant cells, but not in blood islands (Fig 5B). These contrasting expression patterns demonstrate that GATA-1 and GATA-2 are differentially regulated during the onset of primitive hematopoiesis.
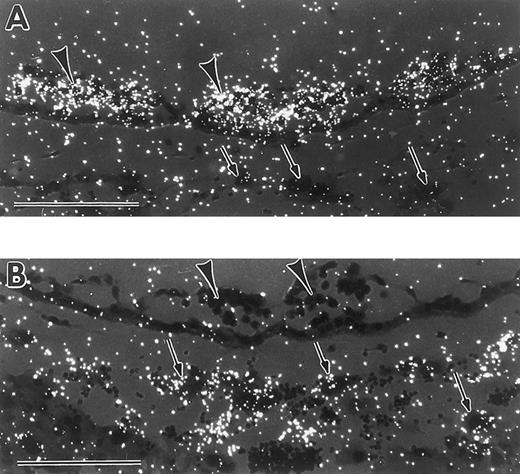
In the earliest embryos probed for tal-1 expression, at the mid-primitive streak stage, a small number of positive cells were reproducibly observed in the extraembryonic mesoderm (Fig 6A). By the late-primitive streak stage, tal-1 transcripts were present in extraembryonic mesoderm cells of the forming yolk sac, but not extraembryonic mesoderm cells contributing to formation of the chorion or the amnion (Fig 6B). tal-1 transcripts were also detected in the most posterior embryonic mesoderm cells (Fig 6B). At the mid-neural plate stage, tal-1 expression persisted in VYS mesoderm and posterior embryonic mesoderm (Fig 6C). By the early somite stage, there was a variably low signal in both blood cells and endothelial cells of the VYS blood islands, as well as in embryonic mesoderm (Fig 6D). Thus, tal-1 transcripts were detected earlier than GATA-1 transcripts, and in a wider range of mesoderm cells. No tal-1 expression was found in extraembryonic ectoderm, visceral endoderm, or trophoblast giant cells.
In situ hybridization with a tal-1 antisense probe. (A) Mid-primitive streak stage conceptus with tal-1 mRNA expression localized to 2 extraembryonic mesoderm cells. Serial sections probed with antisense tal-1 gave similar results. (B) Late-primitive streak stage conceptus with tal-1 signal restricted to VYS mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (C) Neural plate stage conceptus with tal-1 mRNA present in both VYS mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (D) Early somite stage conceptus with low levels of tal-1 mRNA expression in yolk sac blood islands (closed arrow) and in regions of embryonic mesenchyme (open arrows). ac, amniotic cavity; ex, exocoelomic cavity; pc, proamniotic cavity; ep, embryo proper. Each scale bar represents 100 μm.
In situ hybridization with a tal-1 antisense probe. (A) Mid-primitive streak stage conceptus with tal-1 mRNA expression localized to 2 extraembryonic mesoderm cells. Serial sections probed with antisense tal-1 gave similar results. (B) Late-primitive streak stage conceptus with tal-1 signal restricted to VYS mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (C) Neural plate stage conceptus with tal-1 mRNA present in both VYS mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (D) Early somite stage conceptus with low levels of tal-1 mRNA expression in yolk sac blood islands (closed arrow) and in regions of embryonic mesenchyme (open arrows). ac, amniotic cavity; ex, exocoelomic cavity; pc, proamniotic cavity; ep, embryo proper. Each scale bar represents 100 μm.
The earliest conceptuses examined for rbtn2 expression were at mid- to late-primitive streak stage. rbtn2 transcripts were present both in the extraembryonic yolk sac mesoderm and in posterior embryonic mesoderm (Fig 7A). A transverse section at this stage showed rbtn2 expression in midline mesoderm and the mesodermal wings, but no expression in embryonic ectoderm or visceral endoderm (Fig 7B). The rbtn2 expression pattern at the late-primitive streak stage was similar to that of tal-1, with transcripts throughout the VYS mesoderm and in the posterior embryonic mesoderm (Fig 7C). At the mid-neural plate stage, rbtn2 transcripts persisted in both yolk sac and posterior embryonic mesoderm, while mesoderm cells contributing to the formation of the chorion and amnion were negative (Fig 7D). At early somite stages, yolk sac blood cells expressed moderate levels of rbtn2 (Fig 7E). rbtn2 transcripts were also detected in the head mesenchyme of the embryo proper (data not shown).
In situ hybridization with an rbtn2 antisense probe. (A) Mid-late primitive streak stage conceptus with rbtn2 mRNA expression in both extraembryonic mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (B) Transverse oblique section of a mid-primitive streak stage conceptus with rbtn2 mRNA confined to mesoderm cells located between the inner epiblast cells (e) and the outer visceral endoderm cells (ve). rbtn2 transcripts are present both in the axial mesoderm and in the mesodermal wings (arrowheads). (C) Late-primitive streak stage conceptus with rbtn2-positive extraembryonic mesoderm cells (arrow). (D) Neural plate stage conceptus with rbtn2 transcripts present in both mesodermal cell masses (closed arrow) and posterior embryonic mesoderm (open arrow). (E) Early somite stage yolk sac with rbtn2 mRNA accumulation in cells within blood islands (closed arrows), but not in the yolk sac endoderm (open arrow). There was no signal above background in the sense controls. ac, amniotic cavity; e, epiblast; ec, ectoplacental cavity; ex, exocoelomic cavity; pc, proamniotic cavity; ve, visceral endoderm. Each scale bar represents 100 μm.
In situ hybridization with an rbtn2 antisense probe. (A) Mid-late primitive streak stage conceptus with rbtn2 mRNA expression in both extraembryonic mesoderm (closed arrow) and posterior embryonic mesoderm (open arrow). (B) Transverse oblique section of a mid-primitive streak stage conceptus with rbtn2 mRNA confined to mesoderm cells located between the inner epiblast cells (e) and the outer visceral endoderm cells (ve). rbtn2 transcripts are present both in the axial mesoderm and in the mesodermal wings (arrowheads). (C) Late-primitive streak stage conceptus with rbtn2-positive extraembryonic mesoderm cells (arrow). (D) Neural plate stage conceptus with rbtn2 transcripts present in both mesodermal cell masses (closed arrow) and posterior embryonic mesoderm (open arrow). (E) Early somite stage yolk sac with rbtn2 mRNA accumulation in cells within blood islands (closed arrows), but not in the yolk sac endoderm (open arrow). There was no signal above background in the sense controls. ac, amniotic cavity; e, epiblast; ec, ectoplacental cavity; ex, exocoelomic cavity; pc, proamniotic cavity; ve, visceral endoderm. Each scale bar represents 100 μm.
DISCUSSION
We examined mouse conceptuses from the late-primitive streak to early somite stages for expression of βH1-globin, the first β-globin cluster gene to be expressed in primitive erythroid cells.20,21 Globin transcripts were first consistently detected in mesodermal cell masses at the neural plate stage (E7.5), earlier in development than previously recognized.2,3 Globin transcripts were localized to mesodermal cell masses,1 which consist of a small number of extraembryonic mesoderm cells adjacent to visceral endoderm. Since globin transcription is a molecular marker of terminal erythroid differentiation, these results suggest that commitment of extraembryonic mesoderm cells to erythropoiesis has occurred before the appearance of morphologically recognizable erythroblasts. By early somite stages, βH1-globin transcripts became upregulated dramatically. We hypothesize that this early expression and massive upregulation of globin transcripts is required to fill the developing vascular network of the rapidly growing embryo with hemoglobin-containing cells.
A number of transcription factors known to be expressed in definitive bone marrow progenitors have also been shown by targeted disruption experiments to have functional roles in primitive hematopoiesis. These genes to date consist of tal-1, rbtn2, GATA-1, and GATA-2.22 We examined the normal expression patterns of these genes during murine gastrulation by in situ hybridization to further trace and better understand the development of embryonic hematopoiesis. GATA-1 transcripts were first detected in VYS mesoderm at the late-primitive streak stage, before the onset of βH1-globin mRNA accumulation. GATA-1 mRNA expression was found in the VYS mesoderm cell masses at the neural plate stage and in the VYS blood islands at the early somite stage. The temporal and spatial expression pattern of GATA-1 mRNA is consistent with the protein's putative function as a transcriptional activator of globin.8 GATA-1 expression was also detected at low, but reproducible, levels in extraembryonic ectoderm at the late-primitive streak, neural plate, and early somite stages. These tissues contribute to the formation of the placenta, suggesting that GATA-1 may also perform additional nonhematopoietic roles during these stages of development.
As with GATA-1, we found GATA-2 transcripts in the VYS mesoderm at the late-primitive streak stage, before the onset of βH1-globin transcription. Thus, both GATA-2 and GATA-1 may be involved in the activation of globin transcription in primitive erythroid cells. The expression pattern in VYS mesoderm is consistent with the observation that GATA-2 transcription is required in a cell autonomous fashion for primitive hematopoiesis.6 Unlike GATA-1, GATA-2 mRNA was not detected in VYS mesoderm at early somite stages, reminiscent of the downregulation of GATA-2, but not GATA-1, during terminal differentiation of definitive erythroid cells.23 Our results are consistent with the hypothesis that GATA-1 downregulates GATA-2.8 GATA-2 transcripts were also present in the extraembryonic ectoderm at the neural plate and early somite stages. The presence of both GATA-1 and GATA-2 transcripts in the extraembryonic ectoderm, a nonhematopoietic tissue, suggests that the concomitant expression of these genes is not sufficient to specify erythroid development. Furthermore, GATA-2 mRNA was also expressed in the ectoplacental cone and in trophoblast giant cells, consistent with a role for GATA-2 in the regulation of placental lactogen.24
It is noteworthy that GATA-1 and GATA-2 homologs have been found in nonmammalian vertebrates. In Xenopus,25-27 GATA-2 transcripts are initially found at high levels in the ventral and lateral ectoderm, and later, at low levels in presumptive ventral blood island (VBI) cells. GATA-1 mRNA is also detected in presumptive VBI cells immediately preceding the appearance of embryonic globin transcripts. GATA-2, but not GATA-1, transcripts are then downregulated in the VBI as embryonic globin transcription increases. Likewise, in zebrafish, GATA-2 is expressed in the ventral ectoderm early in embryogenesis, and is subsequently found with GATA-1 in mesodermal hematopoietic progenitors in the intermediate cell mass, located in the posterior dorsal region of the embryo.28 Zebrafish GATA-2 expression is also downregulated during primitive erythroid differentiation. In the chick, GATA-2, GATA-1, and embryonic globin are all expressed in the zona opaca when blood islands initially form.29 These findings, taken together, suggest that the roles of both GATA-1 and GATA-2 in the initiation of primitive erythropoiesis are evolutionarily conserved among vertebrates. Furthermore, the initial ectodermal expression of GATA-2 raises the possibility that the ventral ectoderm of fish and amphibian embryos and the embryonic portion of the mammalian placenta are evolutionarily related.
Unlike GATA-1 and GATA-2, the expression of tal-1 and rbtn2 was confined to mesoderm at all the stages analyzed in this study. We identified both tal-1 and rbtn2 transcripts in extraembryonic mesoderm cells as early as the mid-primitive streak stage before detectable GATA-1 or GATA-2 transcripts. This early expression is consistent with tal-1 and rbtn2 being upstream of GATA-1 and GATA-2. This hypothesis is supported for tal-1 both by the lack of GATA-1 and embryonic globin transcription in tal-1 null conceptuses and by the more severe hematopoietic defects found in tal-1 null ES cells4 compared with GATA-1 null and GATA-2 null ES cells.9,30 While it was initially reported that rbtn2 null ES cells produce normal numbers of macrophage colonies, more recent experiments apparently suggest deficient macrophage differentiation consistent with an early role in primitive hematopoietic differentiation.22
We found that the temporal and spatial expression patterns of tal-1 and rbtn2 transcripts during mouse gastrulation are similar. It has been noted that the tal-1 and rbtn2 proteins are complexed with each other in nuclear extracts of erythroleukemia cells31 and that they are both found in the nuclei of fetal liver erythroblasts.5 These data are consistent with the hypothesis that the tal-1 and rbtn2 proteins also interact in a subset of embryonic and extraembryonic mesoderm cells during early stages of gastrulation.
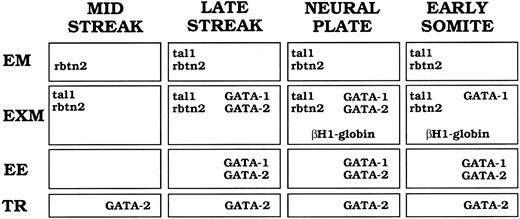
Summary of tal-1, rbtn2, GATA-1, GATA-2, and βH1-globin mRNA expression patterns during murine gastrulation. Captions at the top of the figure indicate developmental stages. Abbreviations at the side of the figure indicate the different tissues in which signal was detected. EM, embryonic mesoderm; EXM, extraembryonic mesoderm; EE, extraembryonic ectoderm; TR, trophoblast giant cells or their precursors. Genes expressed at a particular stage and in a particular tissue are designated in the appropriate box.
Summary of tal-1, rbtn2, GATA-1, GATA-2, and βH1-globin mRNA expression patterns during murine gastrulation. Captions at the top of the figure indicate developmental stages. Abbreviations at the side of the figure indicate the different tissues in which signal was detected. EM, embryonic mesoderm; EXM, extraembryonic mesoderm; EE, extraembryonic ectoderm; TR, trophoblast giant cells or their precursors. Genes expressed at a particular stage and in a particular tissue are designated in the appropriate box.
We have localized tal-1, rbtn2, GATA-1, and GATA-2 transcripts to mesodermal cell masses in the developing yolk sac at the neural plates stage. GATA-1 and rbtn2 proteins have been found to be complexed in erythroleukemia cell lysates, while GATA-2 and rbtn2 can also associate.32 tal-1 and GATA-1 are coexpressed in human bone marrow-derived erythroid, megakaryocytic, and mastocytic lineages.33 These data taken together raise the possibility that tal-1, rbtn2, GATA-1, and GATA-2 physically interact in VYS mesoderm cells during their commitment to the primitive erythroid lineage. Other regulatory interactions may also be occurring at this time of development. Regulatory elements of globin genes, as well as most other erythroid-specific genes, contain GATA motifs.34The GATA-1 gene itself contains an upstream double GATA-binding site, thought to be involved with GATA-1 autoregulation.35 One of the two tal-1 promoters contains a GATA site that is required for full promoter activity.36 These findings suggest that GATA-1 and GATA-2 may regulate a large number of genes, including tal-1, in mesodermal cell masses of the neural plate stage murine embryo.
tal-1 transcripts (our study) and protein37 are expressed in both endothelial cells and blood cells of E8.5 blood islands, consistent with the possibility that these two cell types arise from a common precursor (hemangioblast). Both tal-1 and rbtn2 are expressed not only in VYS mesoderm, but also in posterior embryonic mesoderm at late streak stages and within the embryo proper at early somite stages (Fig 6D and data not shown). The expression of these genes in posterior mesoderm, similar to that of flk-1 during gastrulation,38 may reflect roles in intraembryonic endothelial network formation. Alternatively, the intraembryonic expression of tal-1 and rbtn2 may be related to development of intraembryonic blood cells. Experiments in chick and Xenopus have demonstrated that definitive blood cells arise from intraembryonic sites, particularly the aorta-mesonephros region (reviewed by Dzierzak and Medvinsky39 ). Hematopoietic progenitors have been identified at a similar site in the mouse.40-42 While the origin of these intraembryonic hematopoietic progenitors in the mouse has not yet been elucidated, it is possible that they arise from tal-1- and rbtn2-expressing mesoderm cells that have migrated through the posterior primitive streak during gastrulation.
The expression patterns of murine tal-1, rbtn2, GATA-1, GATA-2, and βH1-globin transcripts during gastrulation are summarized in Fig 8. We hypothesize that a subset of epiblast cells become committed to the hematopoietic pathway as they pass through the posterior primitive streak and express rbtn2 and tal-1. By the late-primitive streak stage, expression of GATA-1 and GATA-2, as well as tal-1 and rbtn2, is evident in VYS mesoderm cells. At the neural plate stage (E7.5), transcripts of all four transcription factors are present in mesodermal cell masses as globin transcription begins, indicating commitment to the erythroid lineage. By E8.5, GATA-2 mRNA expression is downregulated in yolk sac blood islands as terminal primitive erythroid differentiation proceeds. Thus, primitive mammalian erythropoiesis arises during gastrulation through the ordered temporal expression of tal-1, rbtn2, GATA-2, and GATA-1 in a subset of extraembryonic mesoderm cells.
ACKNOWLEDGMENT
We are grateful to P. Kingsley, L. Angerer, and R. Angerer for advice on in situ hybridization; to S. Orkin, S.J. Brandt, and A. McMahon for providing cDNA clones; to F. Pendola for subcloning βH1-globin cDNA; and to D. Penney and K. Maltby of the University of Rochester Cancer Center Experimental Pathology and Ultrastructure Facility for use of paraffin and darkroom facilities and for excellent assistance with figure preparation. We thank S. Orkin, L. Zon, and K. McGrath for critical review of the manuscript and for many helpful comments.
Supported by National Institutes of Health Grant No. R29HL45573 (J.P.) and American Cancer Society Grant No. DHP-155 (J.P.).
Address reprint requests to James Palis, MD, University of Rochester Medical Center, Department of Pediatrics and Cancer Center, 601 Elmwood Ave, Rochester, NY 14642.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal