Abstract
Relatively little is known about the relationship of lymphoid-associated gene expression to the proliferation and differentiation potential of early human bone marrow lymphoid progenitors. Surface expression of interleukin-7 (IL-7) receptor-α (IL-7Rα), a component of the high-affinity receptor for the lymphoid precursor growth factor IL-7, defined a CD34+ progenitor subset lacking the CD19+ pro-B phenotype but demonstrating markedly enhanced lymphoid clonogenic capacity and the ability to differentiate into pro-B cells in short-term culture. These progenitors expressed mRNA for the lymphoid-associated genes Igβ, RAG-1, and PAX-5, and were uniformly TdT-positive (TdT+). In contrast, IL-7Rα−/CD19−/CD34+ progenitors had a 50-fold reduced lymphoid clonogenic capacity and did not differentiate into pro-B cells in short-term culture. Expression of TdT and the lymphoid-associated genes Igβ and RAG-1, but not PAX-5, was detected in this fraction, although at lower levels than in the IL-7Rα+ progenitors. In contrast to IL-7Rα, loss of the stem cell factor receptor c-kit was associated with enhanced lymphoid clonogenic potential and increased B-lineage differentiation potential. These results indicate that IL-7Rα expression defines entry into a developmental stage characterized by upregulation of multiple lymphoid-associated genes and enhanced fitness for B-lymphoid differentiation. The onset of IL-7Rα and PAX-5 expression immediately before acquisition of CD19 is consistent with evidence suggesting upregulation of CD19 through pathways involving PAX-5 and IL-7.
BONE MARROW is the source of multiple lymphohematopoietic lineages, including B lymphocytes. The earliest recognizable committed B-cell precursor is the pro-B cell, which expresses the progenitor marker CD34, the B-lineage–associated marker CD19, the lymphoid-associated protein CD10, and the lymphoid precursor–associated DNA polymerase terminal deoxynucleotidyltransferase (TdT).1,2 Progression to the pre–B-cell stage is accompanied by the loss of CD34 and TdT and the expression of Ig μ-chain predominantly within the cytoplasm. The expression of TdT prior to CD19 or CD103 and the identification of a CD10+/CD19−/CD34+ population4 suggests that cells prior to the pro-B stage may be in the process of early lymphoid differentiation. Although in vitro culture systems adapted for the propagation of human CD19+ pro-B or pre-B cells have been developed,5-8 less is known about the lymphoid differentiation potential of CD34+ cells before definitive lymphoid lineage commitment signaled by acquisition of CD19. We have previously shown that early lymphoid progenitors from adult human bone marrow expressing CD34 but lacking CD10 can be preferentially cultured in a serum-free stromal-dependent short-term lymphoid progenitor colony assay.9 We therefore hypothesize that bone marrow stroma in the absence of added growth factors may provide a supportive microenvironment for the earliest stages of human lymphoid differentiation prior to definitive lineage commitment.
Phenotypic characterization of these early lymphoid developmental stages would permit separation of cells for correlation of lymphoid-associated gene expression with proliferation and differentiation potential in culture. Since development of human lymphoid progenitors in several stromal-dependent culture systems is dependent on interleukin-7 (IL-7) secreted by the stroma,10,11 we hypothesized that these progenitors may express a high-affinity IL-7 receptor (common γc-chain plus IL-7–specific IL-7Rα chain). The hypothesis that IL-7R expression may be an obligate step in early lymphoid development is supported by mouse knockout experiments in which disruption of the IL-7Rα gene blocks early B- and T-cell development.12 Expression of IL-7Rα and TdT by bone marrow progenitors in the absence of other lymphoid-specific markers in the EBF transcription factor knockout mouse, which is B-cell deficient,13 also suggests that IL-7Rα appears early in B-cell development. We therefore used a monoclonal antibody to IL-7Rα to separate and characterize CD34+ progenitor subpopulations with lymphoid differentiation potential. Our results show that in the absence of exogenous factors, bone marrow stroma preferentially supports the proliferation of lymphoid progenitors that have not yet expressed lineage-specific B- or T-lymphocyte markers. Acquisition of IL-7Rα is accompanied by a markedly increased potential for clonogenic proliferation and B-lineage differentiation. The IL-7Rα+ progenitor population shares many phenotypic features with a CD10+/CD19−/CD34+ human multilineage progenitor with natural killer (NK), dendritic, and T- and B-cell differentiation potential recently described by Galy et al,14 suggesting that cytokines signaling through the IL-7R α-chain may be involved in development of early progenitors of several related hematopoietic lineages.
MATERIALS AND METHODS
Cell preparation.Bone marrow cells were obtained from normal adult donors (N = 21) with informed consent and approval by the institutional Research Subjects Review Board. Light-density cells were separated by Ficoll/Hypaque density-gradient centrifugation. CD34+ cells were enriched using the CEPRATE LC affinity column (CellPro Inc, Bothell, WA). Fibroblast-like bone marrow stromal cells, previously characterized,15 16 were grown to confluence from the CD34-depleted fraction (CD34 affinity column–nonadherent) of human bone marrow (using > 10 different bone marrow donors) in McCoy's medium plus 10% fetal calf serum without steroids, and were passaged once before seeding into 8-well Lab-Tek chamber slides (Nunc Inc, Naperville, IL). The medium was replaced with serum-free McCoy's medium immediately before seeding CD34+ progenitor cells into the wells.
Antibodies and reagents.Anti–IL-7Rα antibody M21 was a generous gift from Dr Richard Armitage.17 Goat anti-rabbit fluorescein isothiocyanate (FITC) was obtained from Tago Immunologicals (Burlingame, CA), rabbit anti-TdT was from Supertechs (Bethesda, MD), and control rabbit and goat IgGs were from Jackson ImmunoResearch Laboratories Inc (West Grove, PA). CD10 FITC, CD38 phycoerythrin (PE), CD5 PE, CD19 FITC, CD34 (HPCA-2) FITC, CD34 (HPCA-2) PE, CD11b PE, and CD45RO PE antibodies were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). FITC-conjugated goat anti-mouse IgG1 was purchased from Fisher Scientific (Pittsburgh, PA). CD3 EDC (PE–Texas red conjugate), CD19 ECD, CD33 PE, CD10 PE, CD19 PE, CD20 FITC, CD45RA PE, CD13 PE, CD2 FITC, CD3 FITC, CD15 FITC, CD7 FITC, CD4 FITC, and FITC- PE, and ECD-conjugated mouse isotype controls were purchased from Coulter Immunology (Marietta, GA). Anti–c-kit PE was purchased from Immunotech (Westbrook, ME). PE-Cy5–conjugated CD3, CD4, and CD8 and control IgG were purchased from Caltag Laboratories (South San Francisco, CA). Recombinant human cytokine IL-7 was purchased from R&D Systems (Minneapolis, MN).
Four-color flow cytometry.Affinity-column–enriched CD34+ cells, light-density marrow, or Ficoll-Hypaque–separated blood mononuclear cells were analyzed for expression of IL-7Rα by four-color immunophenotyping. Cells were first stained (at 4°C) with a combination of M21 or control IgG1 (1 μg/mL), CD19 ECD, CD3 PE-Cy5, CD4 PE-Cy5, CD8 PE-Cy5, and PE-conjugated CD10, CD5, CD33, or CD45RO (all non-IgG1 isotypes). After three washes, cells were stained with FITC-conjugated goat anti-mouse IgG1, washed thrice, and analyzed on a Coulter XL flow cytometer. For analysis of expression of CD13, CD45RA, CD34, CD38, c-kit, or CD24 (IgG1 antibodies), cells were stained with a combination of M21 or control IgG1 (1 μg/mL), CD19 ECD, CD3 PE-Cy5, CD4 PE-Cy5, and CD8 PE-Cy5, washed, and then stained with FITC-conjugated goat anti-mouse IgG1. After three washes, cells were incubated with irrelevant murine IgG1 (3 μg/mL) for 30 minutes to block unoccupied goat anti-mouse IgG1. Without washing, PE-conjugated CD13, CD45RA, CD34, CD38, c-kit, CD24, or control IgG1 was added for 30 minutes. This protocol prevented nonspecific binding of PE-conjugated IgG1 antibody to residual binding sites of anti-mouse IgG1 attached to anti–IL-7Rα antibody on the cell surface. In some experiments with cells expressing higher levels of CD19 (eg, peripheral blood), cells were stained with control or M21, followed by FITC-conjugated goat anti-mouse IgG1, then IgG1 blocking, and finally CD19 ECD, CD3 PE-Cy5, CD4 PE-Cy5, and CD8 PE-Cy5. This eliminated the slight amount of cross-reactive binding of goat anti-mouse IgG1 to IgG2 isotype antibody CD19 ECD. Cells in the lymphoid/blast light-scatter gate were analyzed for four colors of fluorescence: FITC, PE, ECD, and PE-Cy5. Electronic subtraction to account for overlap of emission spectra among the four fluorochromes was optimized using mixtures of NALM-6 cells stained with either CD19 FITC, CD19 PE, CD19 ECD, or CD19 PE-Cy5.
Cell sorting.Adherent cells from the CEPRATE CD34 column (250,000 to 750,000 cells) were stained with the indicated combinations of directly conjugated antibodies for sterile sorting on an EPICS C flow cytometer. For sorting based on IL-7Rα expression, enriched CD34+ cells were stained with M21 antibody plus CD19 ECD and CD3 ECD, followed by FITC-conjugated goat anti-mouse IgG1. The majority of CD3+ cells could be excluded from the CD19+ sorted fractions on the basis of higher red fluorescence intensity. For sorting based on IL-7Rα and CD10, CD10 PE was used in place of CD19 ECD and CD3 ECD (antibodies were selected based on availability of appropriately conjugated non-IgG1 isotypes). Cells were sorted at 100 to 300 cells/s with three-droplet sorting and no coincidence correction.
Lymphoid progenitor colony assay.Sorted cells (200 to 10,000 sorted directly into each well depending on CD34+ fraction analyzed, to yield an expected colony number of ∼5 to 50 per well) were added to triplicate wells containing confluent stroma. At the end of 2 weeks, the supernatant medium was aspirated and the slides containing BM-FB and adherent lymphoid progenitor colonies were stained with TdT antibody as previously described.9 Culture areas were examined under a fluorescence microscope for colonies of TdT+ cells, defined as groupings of at least 20 cells with characteristic speckled nuclear fluorescence. The mean total number of TdT+ colonies per experiment was 75.1 (n = 23). Experiments in which colonies were identified but fewer than 10 colonies were grown from any sorted subpopulation (n = 3) were excluded from analysis.
Two-color immunofluorescence of lymphoid precursor colonies.After coculture of separated marrow cells with BM-FB in 8-well slide chambers as described, the medium was aspirated and replaced with primary antibody (CD10, CD19, CD34, CD2, CD7, isotype-specific controls) diluted in culture medium. The slides were incubated at room temperature for 60 minutes, washed three times in culture medium, and then incubated in biotin-conjugated horse anti-mouse Ig (Vector Laboratories, Burlingame, CA) diluted 1:20 in culture medium for 60 minutes at room temperature. After this step, slides were processed as described for TdT immunofluorescence staining, except that rhodamine-conjugated avidin was added with the anti-TdT antibody to develop the biotin-stained cells. At least 100 TdT+ cells were counted for each data point.
Myeloid/erythroid colony assay.Sorted cells were resuspended in 0.9% methylcellulose and cultured at 37°C in the presence of IL-3 (20 ng/mL; R&D Systems), IL-6 (20 ng/mL; R&D Systems), erythropoietin (4 U/mL; Amgen, Thousand Oaks, CA), and stem cell factor (10 ng/mL; Amgen). Granulocyte/monocyte, erythroid, and mixed colonies were scored at 2 weeks.
Polymerase chain reaction.Cells were sorted directly into tubes containing guanidinium lysis reagent (TriReagent LS separation kit; Molecular Research Center Inc, Cincinnati, OH) at 6,000 to 50,000 cells/mL lysis reagent, with the addition of 1 μg of Escherichia coli tRNA as carrier prior to RNA extraction. Total RNA was extracted according to the manufacturer's instructions. Reverse transcription (RT) was performed using the Promega Reverse Transcription System (Promega Corp, Madison, WI) with random hexamer primers according to the manufacturer's instructions. To compare different sorted populations, aliquots of cDNA preparation equivalent to a specific number of sorted cells (3 to 1,000 cells) were added to each polymerase chain reaction (PCR) tube. PCR volume was 50 μL (final concentrations: 150 μmol/L each dNTP, 10 mmol/L Tris hydrochloride, pH 8.4, 50 mmol/L KCl, 2.5 mmol/L, MgCl2 [1.5 mmol/L for GAPDH], 1.5 μmol/L PCR primers, and 50 U/mL AmpliTaq polymerase [Perkin-Elmer/Cetus, Norwalk, CT]) for amplification (preincubation at 95°C for 4 minutes, and then 30 cycles of denaturation at 95°C 1 minute, annealing at 60°C unless otherwise indicated for 1 minute, elongation at 72°C for 2 minutes, and then incubation at 72°C for 10 minutes) in a Hybaid thermal cycler (National Labnet, Woodbridge, NJ). For nested PCR (used for all genes except common γc-receptor and GAPDH), 1 μL of the first reaction product was amplified in a second amplification reaction similar to the first but with a nested primer set. Primers were as follows: GAPDH, sense GAAATCCCATCACCATCTTCCAGG, and antisense CGCGGCCATCACGCCACAGTTTCC, 390-bp expected PCR product; RAG-1, sense CCTTACCATGAGTCCTGTGGAAGAACTG, antisense CAGGACATCTTCCATCTCATAGCATTTG, nested sense ATGAATGGCAACTTTGCCAGGAAG, and nested antisense GGAGCTCAGCAAAACGCTGTGAAT, 213 bp; IL-7Rα, sense GGGGCCCTCGTGGAGGTAAA, antisense CTCTGCAGGAGTGTCAGCTTTGTG, nested sense GTGCCTGAATTTCAGGAAACTACAAG, and nested antisense CTGGATAAATTCACATGCGTCCAT, annealing temperature for both reactions 65°C, 311 bp; Igβ, sense TTGGCACAGCTGAAGCAGAGGAAC, antisense AGGTGGCTGTCTGGTCAATGTCCA, nested sense CGCTGCTGATCATCCTCTTCATCA, nested antisense AGGTGGCTGTCTGGTCAATGTCCA, 124 bp; TdT, sense TTACCAGCCCAGGATCAACAGAGG, antisense CCAGAATCATCTTCCGCTCATGTG, nested sense ATGACCTTGTGGAGTCAACATTTG, nested antisense TAGGGGCACAGAACTAAATCCACA, annealing temperature of nested reaction 65°C, 186 bp; PAX-5, sense CCAGTCCCAGCTTCCAGTCACAG, antisense GGAGACTCCTGAATACCTTCGTCTC, nested sense GCATAGTGTCCACTGGCTCCGT, nested antisense GTCTCTCTTGCGCTTGTTGGTGTC, 130 bp; and common γc-receptor, sense GGATCTTGTTACTGAATACCACGG and antisense CCAAATCAGCCACAGTGGGGTGAGG, 336 bp. PCR primers for Igβ and PAX-5 spanned a large intron, preventing amplification of genomic DNA in the first PCR, as confirmed by testing on purified genomic DNA. Primers for TdT spanned a 140-bp intron, allowing distinction between cDNA and residual genomic DNA. RAG-1 primers did not span an intron. Specificity of RT-PCR for RNA transcripts of each lymphoid/B-lineage gene studied was confirmed by negative results of nested PCR at the highest template concentration when the RT step was omitted. An aliquot of PCR product was electrophoresed in minigels of 3% FMC agarose/1% NuSieve (FMC, Rockland, ME) in comparison to a 123-bp molecular-weight ladder (GIBCO) and stained with ethidium bromide for viewing under UV illumination. A sample containing all reagents except for template cDNA was always amplified with the test samples to demonstrate absence of false-positive PCR product. All PCR products were the expected size based on comparison to 123-bp ladder assayed with each analysis.
RESULTS
Phenotypic characterization of a CD34+/CD19− cell expressing IL-7R.In accordance with previous indications that most cells capable of giving rise to TdT+ colonies (CFU-TdT) in short-term stromal-dependent culture are at an earlier differentiation stage than the pro-B cell,9 in initial sorting experiments most CFU-TdT were contained in the CD34+/CD19− population (68% to 86% of CD34+ CFU-TdT, n = 2). Based on these results, a distinguishing phenotype correlating with lymphoid differentiation potential was sought in the CD34+/CD19− progenitor fraction. Since nearly all CFU-TdT are dependent on IL-7 for clonogenic growth,10 expression of the IL-7–specific IL-7Rα was tested as a possible early marker of lymphoid potential.
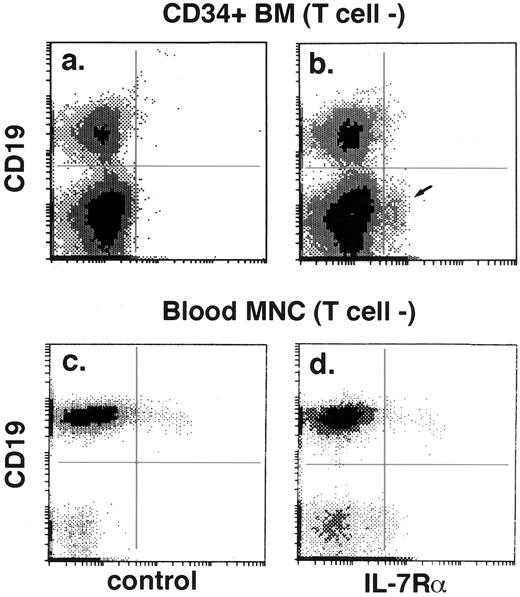
Using the M21 anti–IL-7Rα monoclonal antibody, a small but clearly recognizable population of IL-7Rα+ cells (3.3% ± 1.4%, n = 5) was identified in affinity-column–enriched CD34+ bone marrow cells gated to exclude T cells (Fig 1b, arrow). Consistent with their in vitro responsiveness to IL-7,18 most T cells expressed IL-7Rα (data not shown). Most IL-7Rα+ non-T cells did not express CD19 (Fig 1b). Conversely, most CD19+ cells, whether in CD34+-enriched bone marrow (Fig 1b) or peripheral blood (Fig 1d), lacked IL-7Rα expression. This suggested that IL-7Rα may be a marker of an immature lymphoid population, and therefore the phenotype of the IL-7Rα+/CD19−/T-cell marker− subset (referred to later herein as the IL-7Rα+ progenitor) in an enriched CD34+ population was further examined by four-color flow cytometry.
Expression of IL-7Rα in bone marrow progenitors but not blood B cells. Histogram of IL-7Rα v CD19 (b and d) or control IgG1 v CD19 (a and c) in affinity-column–enriched CD34+ marrow cells (a and b) and peripheral blood mononuclear cells (MNC) (c and d). Both axes on all flow cytometry histograms indicate relative fluorescence intensity in a logarithmic scale (4 decades for Figs 1 and 2, and 3 decades for Figs 4 and 5). In all histograms, dashed lines indicate the fluorescence cutoff for positivity based on cells stained with control IgG. T cells were gated out on the basis of CD3, CD4, or CD8 expression. A small subpopulation (2.8%) of CD19-cells in the CD34+ bone marrow (b, arrow) are specifically stained by anti–IL-7Rα antibody. The proportion of cells in the four quadrants of (a) is 14.5%, 0.4%, 84.6%, and 0.5%, and of (b), 14.4%, 0.5%, 81.9%, and 3.3%. CD19+ (mature B) cells in peripheral blood are IL-7Rα− (d), although some non-B cells in the blood show weak IL-7Rα expression. The proportion of cells in the four quadrants of (c) is 49.9%, 2.2%, 47.6%, and 0.3%, and of (d), 50.7%, 1.7%, 44.5%, and 3.1%.
Expression of IL-7Rα in bone marrow progenitors but not blood B cells. Histogram of IL-7Rα v CD19 (b and d) or control IgG1 v CD19 (a and c) in affinity-column–enriched CD34+ marrow cells (a and b) and peripheral blood mononuclear cells (MNC) (c and d). Both axes on all flow cytometry histograms indicate relative fluorescence intensity in a logarithmic scale (4 decades for Figs 1 and 2, and 3 decades for Figs 4 and 5). In all histograms, dashed lines indicate the fluorescence cutoff for positivity based on cells stained with control IgG. T cells were gated out on the basis of CD3, CD4, or CD8 expression. A small subpopulation (2.8%) of CD19-cells in the CD34+ bone marrow (b, arrow) are specifically stained by anti–IL-7Rα antibody. The proportion of cells in the four quadrants of (a) is 14.5%, 0.4%, 84.6%, and 0.5%, and of (b), 14.4%, 0.5%, 81.9%, and 3.3%. CD19+ (mature B) cells in peripheral blood are IL-7Rα− (d), although some non-B cells in the blood show weak IL-7Rα expression. The proportion of cells in the four quadrants of (c) is 49.9%, 2.2%, 47.6%, and 0.3%, and of (d), 50.7%, 1.7%, 44.5%, and 3.1%.
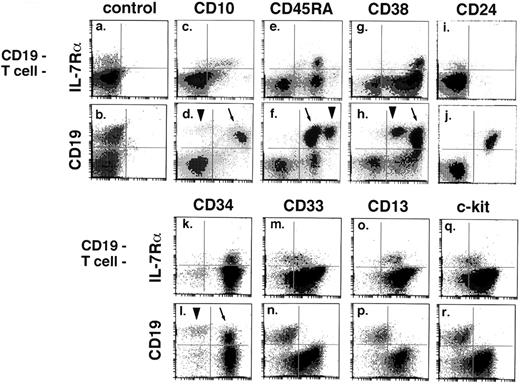
Most IL-7Rα+ progenitors from CD34-enriched marrow expressed the lymphoid-associated marker CD10 (Fig 2c), but at a lower intensity than CD19+/CD34+ pro-B cells (Fig 2d, arrow). CD45RA was expressed at moderate intensity by nearly all IL-7Rα+ progenitors (Fig 2e) and pro-B cells (Fig 2f, arrow), whereas expression was higher in mature B cells (Fig 2f, arrowhead). IL-7Rα+ progenitors expressed CD38 (Fig 2g) at similar levels as pro-B cells (Fig 2h, arrow), with diminished but still detectable expression in the mature B-cell population (Fig 2h, arrowhead). CD38 expression by clonogenic early lymphoid progenitors in short-term culture is also supported by an experiment in which CFU-TdT were present in sorted CD34+/CD38+ but not CD34+/CD38− cells (data not shown). CD24 was not expressed by IL-7Rα+ progenitors (Fig 2i), but was strongly positive in all CD19+ cells (Fig 2j).
Surface phenotype of IL-7Rα+ progenitors and CD19+ B-lineage cells. Affinity-column–enriched CD34+ marrow cells were stained with a 4-color combination. For each surface marker, paired histograms are presented: IL-7Rα v marker in the CD19−/T− gated fraction, and CD19 v marker. The CD19+ fraction in CD34+ enriched marrow is mostly CD34+ pro-B cells, with a smaller population of contaminating CD34−/CD19+ mature B cells (pre–B-cell contamination is infrequent in affinity-column–enriched CD34+ cells). Where distinguishable, the pro–B-cell population is indicated by an arrow and the B-cell population by an arrowhead. Antigen expression in the pro–B- and B-cell CD19+ fractions was verified by staining with CD34 in a separate experiment. Panels include results from three different experiments (IgG controls were performed in each experiment). Each staining result was confirmed by three to eight replicate experiments on different bone marrow specimens. IL-7Rα+ progenitors are CD10+/CD45RA+/CD38++/CD24−/CD34+/CD33dim+/CD13+/c-kitdim+.
Surface phenotype of IL-7Rα+ progenitors and CD19+ B-lineage cells. Affinity-column–enriched CD34+ marrow cells were stained with a 4-color combination. For each surface marker, paired histograms are presented: IL-7Rα v marker in the CD19−/T− gated fraction, and CD19 v marker. The CD19+ fraction in CD34+ enriched marrow is mostly CD34+ pro-B cells, with a smaller population of contaminating CD34−/CD19+ mature B cells (pre–B-cell contamination is infrequent in affinity-column–enriched CD34+ cells). Where distinguishable, the pro–B-cell population is indicated by an arrow and the B-cell population by an arrowhead. Antigen expression in the pro–B- and B-cell CD19+ fractions was verified by staining with CD34 in a separate experiment. Panels include results from three different experiments (IgG controls were performed in each experiment). Each staining result was confirmed by three to eight replicate experiments on different bone marrow specimens. IL-7Rα+ progenitors are CD10+/CD45RA+/CD38++/CD24−/CD34+/CD33dim+/CD13+/c-kitdim+.
As expected, IL-7Rα+ progenitors were CD34+ (Fig 2k), and thus did not represent contaminating CD34− cells in the enriched CD34+ fraction. IL-7Rα+ cells expressed myeloid-associated proteins CD33 (Fig 2m) and CD13 (Fig 2o) at a slightly lower intensity than IL-7Rα−/CD19− cells (right lower quadrant of Fig 2m and o). The receptor for stem cell factor, c-kit, was dimly expressed by IL-7Rα+ progenitors (Fig 2q). In contrast, CD19+ cells from the CD34-enriched fraction did not show detectable expression of CD33, CD13, or c-kit (Fig 2n, p, and r). IL-7Rα+ progenitors did not express CD5 (although a small percentage of CD19+ cells were CD5+, as expected; data not shown). IL-7Rα+ progenitors lacked the thymocyte/dendritic marker CD1a, as well as NK-associated markers CD16 or CD56 (n = 2; data not shown). IL-7Rα+/CD3−/CD19− cells were 98% TdT+ (±2%, n = 3), as determined by immunofluorescence of sorted cells from CD34-enriched marrow. TdT expression could also be demonstrated in a small proportion of IL-7Rα−/CD3−/CD19− cells (mean, 3.2% ± 0.5%, n = 3).
Because of the reported demonstration of a common lymphoid/dendritic/NK progenitor in the CD10+/CD19−/CD34+ fraction, the phenotype of the entire CD10+/CD19− population (including both IL-7Rα+ or IL-7Rα−) was examined using four-color flow cytometry in a CD34-enriched sample in which 34% of CD10+/CD19−/CD3− cells were IL-7Rα+. Over 94% of CD10+/CD19−/CD3− cells showed the same phenotype as the IL-7Rα+ progenitor, CD34+/ -C D 4 5 R A ;pl / C D 3 8 ;pl / H L A - D R ;pl / C D 7 ;ms / C D 2 4 ;ms / C D 1 a ;ms / C D 5 ;ms -(data not shown). CD43, an early B-cell marker in the mouse,19 was expressed by more than 95% of the CD10+/CD19−/CD3− fraction and by CD19+/CD10+ pro-B cells, but not by CD19+/CD10− B cells (data not shown).
As estimated by forward-angle light-scatter measurement, the cell size of both the early progenitor/stem cell CD34+/CD33− population (mean ± SD channel of linear light-scatter intensity, 32.1 ± 1.5, n = 6) and the total CD34+ population lacking lymphoid-related markers (IL-7Rα−/CD19−, 33.9 ± 1.8) was not significantly different from that of the IL-7Rα+ fraction (31.1 ± 1.7). However, IL-7Rα−/CD19+/CD10+ pro-B cells and IL-7Rα−/CD19+/CD10− mature B cells were significantly smaller (24.2 ± 1.1 and 24.1 ± 1.2, respectively) than the IL-7Rα+ population.
Clonogenic and maturation potential of CD34+ B-lymphoid progenitor subpopulations defined by IL-7R expression.To define the clonogenic and B-lineage differentiation potential of this IL-7Rα+ progenitor population, CD34+-enriched cells were sorted on the basis of IL-7Rα and CD19 expression and cultured in the stromal-dependent TdT colony assay. Sorting gates were set conservatively so that weakly staining cells were not included in the IL-7Rα− population. The IL-7Rα+/CD19− subpopulation was 50-fold enriched in CFU-TdT compared with all other fractions (Table 1). IL-7Rα+ fractions tended to contain more total CFU-TdT than IL-7Rα− fractions, but one third of total CFU-TdT were contributed by IL-7Rα− fractions (Table 1). Few CFU-TdT were identified in CD19+ fractions.
Enhanced Lymphoid Clonogenic Potential of IL-7Rα+ Progenitors
| Fraction . | Colonies/10,000 Cells (Cloning Efficiency) . | % Colonies in Sorted Fraction* . |
|---|---|---|
| IL-7Rα+/CD19− | 448 ± 213† | 61.2 ± 8.4 |
| IL-7Rα−/CD19− | 9 ± 3 | 37.7 ± 7.8 |
| IL-7Rα+/CD19+ | 8 ± 8 | 0.9 ± 0.9 |
| IL-7Rα−/CD19+ | 0.5 ± 0.5 | 0.2 ± 0.2 |
| Fraction . | Colonies/10,000 Cells (Cloning Efficiency) . | % Colonies in Sorted Fraction* . |
|---|---|---|
| IL-7Rα+/CD19− | 448 ± 213† | 61.2 ± 8.4 |
| IL-7Rα−/CD19− | 9 ± 3 | 37.7 ± 7.8 |
| IL-7Rα+/CD19+ | 8 ± 8 | 0.9 ± 0.9 |
| IL-7Rα−/CD19+ | 0.5 ± 0.5 | 0.2 ± 0.2 |
The percentage of total lymphoid progenitor colonies in each sorted fraction was calculated from the number of colonies/10,000 sorted cells multiplied by the relative size of each sorted fraction within the total CD34+ population.
All results are the mean ± SEM as an estimate of variation of means of 3 replicate wells assayed 3 times. Cloning efficiency of IL-7Rα+/CD19− fraction was significantly higher than all other fractions (P < .05, paired t-test). Percent total colonies in the sorted IL-7Rα+/CD19− fraction is higher than in the IL-7Rα−/CD19− fraction at P = .07 (t-test).
The enrichment of CFU-TdT in the IL-7Rα+ fraction was supported by additional experiments in which CD34+ cells were sorted on the basis of IL-7Rα and CD10 expression. CFU-TdT were markedly enriched in the IL-7Rα+ fraction (2.5% ± 0.7% SEM of sorted cells formed TdT+ colonies, n = 3) versus the IL-7Rα− fraction (0.16% ± 0.05% SEM, n = 3). In one experiment in which IL-7Rα−/CD10+ and IL-7Rα−/CD10− cells were separately cultured, only 0.15% of IL-7Rα−/CD10− and 0.2% of IL-7Rα−/CD10+ cells were CFU-TdT, as opposed to 3.9% of the IL-7Rα+ fraction (of which most CFU-TdT were IL-7Rα+/CD10+). In this experiment, 73% of total CFU-TdT were contained in the IL-7Rα+ fractions, and only 7% were found in the IL-7Rα−/CD10+ fraction.
Although we have previously shown that proliferation of lymphoid progenitors cultured with stroma under serum-free conditions was dependent on IL-7 secreted by the stroma, addition of exogenous IL-7 to these cultures had variable effects,10 possibly related to indirect signaling through accessory cells in the cultures. IL-7 was added to the culture medium to assess the response of IL-7Rα+ and IL-7Rα− cells sorted from CD34+ marrow to exogenous IL-7. Exogenous IL-7 resulted in a modest but not statistically significant increase in lymphoid progenitor colonies derived from the IL-7Rα+ fraction (194% ± 64% of control, n = 4), but had no detectable effect on clonogenic potential of the IL-7Rα− fraction (100% ± 20% of control, n = 4).
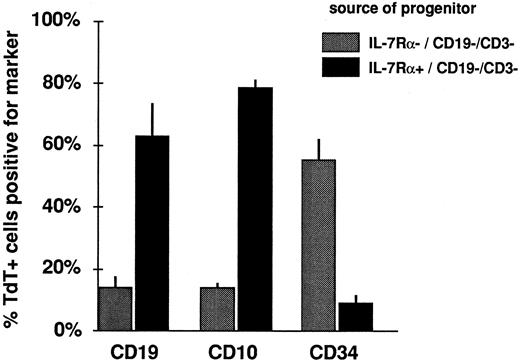
To assess the relative capability for in vitro B-lineage differentiation of cells derived from IL-7Rα+ or IL-7Rα− progenitors, the phenotype of cultured TdT+ cells derived from IL-7Rα+/CD19−/CD3− or IL-7Rα−/CD19−/CD3− fractions was examined by two-color immunofluorescence. TdT+ cells cultured from the IL-7Rα− fraction showed little acquisition of CD10 or CD19, but retained expression of CD34 in a majority of cells (Fig 3). TdT+ cells derived from IL-7Rα+ progenitors, in contrast, lost CD34, acquired CD19, and expressed CD10. Addition of exogenous IL-7 did not induce CD10 or CD19 expression in TdT+ colonies derived from sorted IL-7Rα− progenitors (data not shown).
IL-7Rα expression correlates with ability to differentiate into pro-B cells in short-term culture. Adherent stromal layers were seeded with sorted subpopulations of CD34+-enriched marrow stained with anti–IL-7Rα FITC v CD19 PE v CD3 ECD. The percentage of TdT+ cells after a 2-week culture expressing each differentiation marker is indicated (mean ± SEM, n = 3). The IL-7Rα+ population gave rise to TdT+ cells with significantly lower CD34 but higher CD10 and CD19 expression than the IL-7Rα− population (P < .05, paired t-test).
IL-7Rα expression correlates with ability to differentiate into pro-B cells in short-term culture. Adherent stromal layers were seeded with sorted subpopulations of CD34+-enriched marrow stained with anti–IL-7Rα FITC v CD19 PE v CD3 ECD. The percentage of TdT+ cells after a 2-week culture expressing each differentiation marker is indicated (mean ± SEM, n = 3). The IL-7Rα+ population gave rise to TdT+ cells with significantly lower CD34 but higher CD10 and CD19 expression than the IL-7Rα− population (P < .05, paired t-test).
The myeloid/erythroid differentiation potential of IL-7Rα+ progenitors was evaluated by culture in semisolid medium in the presence of a cytokine mix optimized for myeloid, monocytic, and erythroid differentiation. The IL-7Rα−/CD19−/CD3− fraction contained 3% and 2% CFU-GM (cloning efficiency) in two experiments, whereas the IL-7Rα+/CD19−/CD3− fraction contained only 0.25% and 0.01% CFU-GM. The IL-7Rα−/CD19+ fraction (mostly pro-B cells), as expected, contained few (0% and 0.03%) CFU-GM. Rare erythroid and mixed colonies were found in the IL-7Rα−/CD19−/CD3− fraction in one experiment, but not in the IL-7Rα+/CD19−/CD3− fraction.
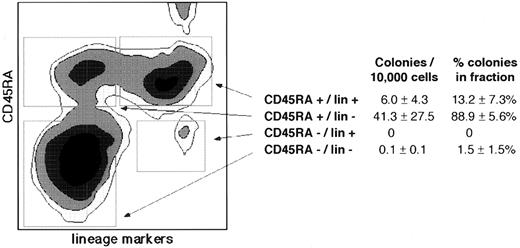
TdT+ cells and CFU-TdT are CD45RA+.CD45RA is the earliest surface protein expressed by murine B-cell precursors,19 appearing prior to CD19 expression.20 To determine whether all CFU-TdT express the related surface protein CD45RA found on IL-7Rα+ progenitors, enriched CD34+ cells were sorted after staining with a mixture of FITC-conjugated antibodies to lineage (lin)-associated markers (CD10, CD19, and CD20, B cell; CD2, CD3, and CD4, T cell; and CD15, myeloid) and PE-conjugated CD45RA. This antibody combination revealed four major populations, of which the CD45RA+/lin− fraction contained the majority of CFU-TdT, with smaller numbers of CFU-TdT in the CD45RA+/lin+ population and almost none in the CD45RA− populations (Fig 4). Immunofluorescence staining of sorted cells indicated that although 19% (±3%, n = 3) of CD45RA+/lin− cells expressed TdT (25% ± 4% of TdT+ cells in all fractions), only 0.05% (±.05%, n = 4) of CD45RA−/lin− cells were TdT+ (0.01% ± 0.01% of total TdT+ cells). Therefore, virtually all TdT+ cells and cells capable of giving rise to TdT+ progeny in culture were CD45RA+.
Clonogenic lymphoid progenitors express CD45RA. Affinity-column–separated CD34+ normal bone marrow cells were stained with PE-conjugated CD45RA and a mixture of FITC-conjugated lineage-associated differentiation markers (CD10, CD19, CD20, CD2, CD3, CD4, and CD15− lin). The subpopulations sorted for lymphoid progenitor colony assay are indicated by boxes. The small CD45RA++/lin+ subpopulation (upper right) was sorted and found not to contain CFU-TdT. The cloning efficiency and proportion of total CFU-TdT within each sorted population, calculated as for Table 1, is indicated at right. Differences in colonies/10,000 cells and % colonies in the fraction between CD45RA+/lin−v CD45RA−/lin− or CD45RA+/lin+ were significant (P < .05 by paired t-test). Errors are ± SE as an estimate of variation of means of three replicate wells in three to four separate experiments. Most lymphoid progenitor colonies originate from the CD45RA+/lin− fraction, and virtually none from the CD45RA− fractions. Similarly, only 0.05% (±0.1% SD, n = 4) of CD45RA−/lin− cells were TdT+ while 19.3% (±5.5% SD, n = 3) of CD45RA+/lin− and 55.7% (±22.1% SD, n = 3) of CD45RA+/lin+ cells were TdT+. In a separate experiment, 94% of the CD45RA−/lin− fraction was shown by staining with CD34 antibody to be CD34+.
Clonogenic lymphoid progenitors express CD45RA. Affinity-column–separated CD34+ normal bone marrow cells were stained with PE-conjugated CD45RA and a mixture of FITC-conjugated lineage-associated differentiation markers (CD10, CD19, CD20, CD2, CD3, CD4, and CD15− lin). The subpopulations sorted for lymphoid progenitor colony assay are indicated by boxes. The small CD45RA++/lin+ subpopulation (upper right) was sorted and found not to contain CFU-TdT. The cloning efficiency and proportion of total CFU-TdT within each sorted population, calculated as for Table 1, is indicated at right. Differences in colonies/10,000 cells and % colonies in the fraction between CD45RA+/lin−v CD45RA−/lin− or CD45RA+/lin+ were significant (P < .05 by paired t-test). Errors are ± SE as an estimate of variation of means of three replicate wells in three to four separate experiments. Most lymphoid progenitor colonies originate from the CD45RA+/lin− fraction, and virtually none from the CD45RA− fractions. Similarly, only 0.05% (±0.1% SD, n = 4) of CD45RA−/lin− cells were TdT+ while 19.3% (±5.5% SD, n = 3) of CD45RA+/lin− and 55.7% (±22.1% SD, n = 3) of CD45RA+/lin+ cells were TdT+. In a separate experiment, 94% of the CD45RA−/lin− fraction was shown by staining with CD34 antibody to be CD34+.
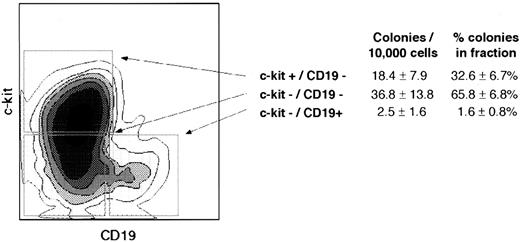
Expression of c-kit in CD19− progenitors is inversely related to lymphoid clonogenic potential and B-lineage maturation in short-term culture.Since stem cell factor is an important B-lymphopoietic cytokine in the mouse and a significant proportion of both IL-7Rα− and IL-7Rα+ progenitors expressed the stem cell factor receptor c-kit, the relationship between c-kit expression and lymphoid proliferation and differentiation potential was studied. Staining of enriched CD34+ cells with CD19 FITC and c-kit PE showed that few CD19+ cells coexpressed c-kit, but a substantial proportion of CD19− cells were c-kit+ (Fig 5). In short-term lymphoid culture, the c-kit−/CD19− fraction was significantly more enriched in CFU-TdT than the c-kit+/CD19− fraction, with few CFU-TdT in the CD19+ fraction (Fig 5).
c-kit expression in CD19− progenitors is inversely related to lymphoid clonogenic potential in short-term culture. Affinity-column–enriched CD34+ cells were stained with c-kit PE and CD19 FITC. The cloning efficiency and proportion of total CFU-TdT within each sorted population, calculated as for Table 1, is indicated at right. The difference in colonies and % total colonies between each of three populations was significant (P < .05 by paired t-test); errors are ± SE as an estimate of variation of means of three replicate wells in seven separate experiments on different marrow specimens.
c-kit expression in CD19− progenitors is inversely related to lymphoid clonogenic potential in short-term culture. Affinity-column–enriched CD34+ cells were stained with c-kit PE and CD19 FITC. The cloning efficiency and proportion of total CFU-TdT within each sorted population, calculated as for Table 1, is indicated at right. The difference in colonies and % total colonies between each of three populations was significant (P < .05 by paired t-test); errors are ± SE as an estimate of variation of means of three replicate wells in seven separate experiments on different marrow specimens.
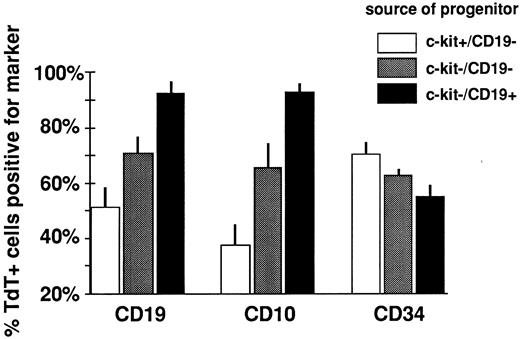
The in vitro differentiation capability of these subsets of cells was examined after short-term culture. A higher proportion of TdT+ cells derived from c-kit−/CD19− progenitors expressed CD19 and CD10 than TdT+ cells cultured from c-kit+/CD19− progenitors (Fig 6). CD34 expression was not significantly different in TdT+ cells arising from these two progenitor fractions. As a control for efficiency of CD10 and CD19 staining in the cultured cells, c-kit−/CD19+ progenitors (already CD19+/CD10+ when cultured) gave rise to TdT+ cells that were nearly all CD19+/CD10+, as expected. T-lineage–associated markers CD2 and CD7 were expressed by less than 5% of cultured TdT+ cells derived from CD19−/c-kit+ progenitors.
c-kit expression in CD19− progenitors is inversely related to B-lymphoid differentiation potential in short-term culture. The percentage of TdT+ cells from different progenitor sources expressing each differentiation marker after culture is indicated (mean ± SEM, n = 4 to 5 for CD19 and CD34 and n = 3 to 5 for CD10). The % CD10+ and CD19+ cells from all three populations was significantly different (P < .05, t-test). The % CD34+ cells from the c-kit−/CD19+ fraction was significantly different versus c-kit+/CD19− (P < .05, paired t-test). The % CD34+ cells from c-kit+/CD19− and c-kit−/CD19− fractions was not significantly different.
c-kit expression in CD19− progenitors is inversely related to B-lymphoid differentiation potential in short-term culture. The percentage of TdT+ cells from different progenitor sources expressing each differentiation marker after culture is indicated (mean ± SEM, n = 4 to 5 for CD19 and CD34 and n = 3 to 5 for CD10). The % CD10+ and CD19+ cells from all three populations was significantly different (P < .05, t-test). The % CD34+ cells from the c-kit−/CD19+ fraction was significantly different versus c-kit+/CD19− (P < .05, paired t-test). The % CD34+ cells from c-kit+/CD19− and c-kit−/CD19− fractions was not significantly different.
Lymphoid- and B-lineage–related genes appear in CD34+/CD19− subpopulations and are highly expressed in IL-7Rα+ progenitors.These results suggest a differentiation sequence in which the CD34+/CD45RA− population lacks clonogenic progenitors capable of expressing TdT in short-term culture. The CD34+/CD45RA+/IL-7Rα− population contains an early lymphoid progenitor with little capacity for B-lineage differentiation in short-term culture (Fig 3). Coexpression of IL-7Rα and CD10 signals an enhanced potential for proliferation of TdT+ progenitors (Table 1) that are capable of differentiating into pro-B cells in short-term culture (Fig 3). Acquisition of CD19 characterizes the previously described pro–B, pre–B, and mature (naive) B-cell subsets. To further characterize this proposed differentiation sequence, gene expression in sorted cell fractions containing proposed progenitor subpopulations was studied. Semiquantitative analysis of gene expression for comparative purposes was accomplished by performing replicate RT-PCR reactions with titered amounts of cDNA template.
Three types of sorting experiments (a total of nine sortings on seven separate adult marrow samples) were performed to obtain a sequential series of populations containing progenitors with increasing fitness for lymphoid differentiation in short-term culture. To test whether lymphoid-associated gene expression precedes acquisition of lymphoid differentiation potential, CD34+ cells lacking CD45RA, CD10, CD19, and other lineage-related markers (CD20, CD11c, and CD5) were sorted for RT-PCR (Fig 7a, n = 4). To assess the significance of mRNA expression detectable by such a sensitive method in relation to the expected purity of sorted populations, the opposite population (CD34+/lin+) from each experiment was also sorted and analyzed by RT-PCR. To test whether lymphoid-associated gene expression is found in the earliest cells with clonogenic lymphoid potential in short-term culture, CD34+ cells lacking CD10, CD19, and other lineage-related markers (CD20, CD11c, and CD5) were sorted for RT-PCR (Fig 7b, n = 2). This fraction would also exclude most IL-7Rα+ progenitors, which are CD10+, and include both CD45RA+ and CD45RA− cells lacking lineage marker expression. In a similar fashion, the quantitative significance of the results was assessed by analysis of the opposite sorted population from each experiment. Gene expression at the IL-7Rα+ lymphoid progenitor stage was assessed by sorting CD19−/IL-7Rα+ cells (Fig 7c, n = 2). CD34+ enriched marrow samples were sorted for CD34+/CD19+ pro-B cells (Fig 8d, n = 2). Figure 7a, b, c, and d therefore represent a hypothesized sequence of increasing lymphoid/B-lineage potential.
Lymphoid- and B-lineage–related genes appear in CD34+/CD19− subpopulations and are highly expressed in IL-7Rα+ progenitors. Sorting experiments were performed to isolate four sequential progenitor subpopulations with increasing lymphoid potential, representing populations containing no CFU-TdT (a), early CFU-TdT lacking differentiation potential (b), IL-7Rα+ CFU-TdT (c), and pro-B cells (d). For clarity, only the early B-lineage markers CD10 and CD19 are listed in the figure among the lineage markers used, which included CD10, CD19, CD20, CD11c, and CD5. The indicated quantity of cDNA (cell equivalents based on the original number of cells sorted) was used as template in the nested PCR reaction. For each gene, results of replicate experiments on a total of two to four separate bone marrow samples were similar to the representative experiments shown, except where noted in the text.
Lymphoid- and B-lineage–related genes appear in CD34+/CD19− subpopulations and are highly expressed in IL-7Rα+ progenitors. Sorting experiments were performed to isolate four sequential progenitor subpopulations with increasing lymphoid potential, representing populations containing no CFU-TdT (a), early CFU-TdT lacking differentiation potential (b), IL-7Rα+ CFU-TdT (c), and pro-B cells (d). For clarity, only the early B-lineage markers CD10 and CD19 are listed in the figure among the lineage markers used, which included CD10, CD19, CD20, CD11c, and CD5. The indicated quantity of cDNA (cell equivalents based on the original number of cells sorted) was used as template in the nested PCR reaction. For each gene, results of replicate experiments on a total of two to four separate bone marrow samples were similar to the representative experiments shown, except where noted in the text.
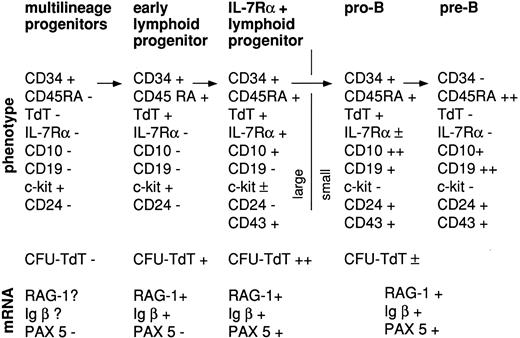
Proposed sequence of gene expression in progenitors with increasing potential for lymphoid and B-lineage differentiation in short-term stromal-dependent in vitro culture.
Proposed sequence of gene expression in progenitors with increasing potential for lymphoid and B-lineage differentiation in short-term stromal-dependent in vitro culture.
GAPDH expression was similar in the sorted populations. Poor recovery of mRNA, as assessed by loss of GAPDH expression, was observed in some initial pro-B samples, but was resolved by maintenance of samples on ice during the sorting. Since mRNA expression of any gene, even those considered “housekeeping” genes, may vary with cell differentiation or activation stage, for comparative analysis in all experiments the actual counted number of sorted cells was the basis for addition of comparable amounts of cDNA template in each PCR reaction. Smaller cell equivalents of cDNA were used for cell populations in Fig 7c, since RNA expression of lymphoid genes was generally higher in this population.
TdT expression was minimal in the earliest fraction (Fig 7a, TdT), consistent with a lack of TdT protein expression by immunofluorescence. Expression at the 1,000-cell–equivalent level only was seen in two of three experiments, in each case when the alternate sorted population in the same experiment showed expression of this gene at the 10-cell level (ie, 100 times greater). Since the level of admixture of CD34+/lin+ cells in the CD34+/lin− population occurring during sorting using this protocol was 0.4%, this degree of mRNA expression is at about the level of sensitivity imposed by the sorting procedure itself. TdT expression in the fraction containing early lymphoid progenitors was higher (Fig 7b), consistent with immunofluorescence detection of TdT+ cells in IL-7Rα−/CD10− cells (data not shown). TdT mRNA expression is highest in the IL-7Rα+ lymphoid progenitor and pro-B cell (Fig 7c and d). RAG-1 expression was detected at the 10- to 1,000-cell level in all experiments in the early lymphoid progenitor, IL-7Rα+, and pro-B populations (Fig 7b to d), but was infrequently observed (in one of four experiments, at the 1,000-cell level only) in the earliest CD45RA− population (Fig 7a). Expression of the B-cell antigen-receptor complex component Igβ was seen in two of two experiments in the early CD45RA− population (Fig 7a), but in each experiment, as with TdT, the positive signal was at the limit of sensitivity dictated by the purity of sorted populations. Igβ expression was identified in the early lymphoid progenitor at the 100- to 1,000-cell level (Fig 7b), and in the IL-7Rα+ progenitor (Fig 7c) and pro-B cell (Fig 7d) at the 10- to 100-cell level in all experiments. IL-7Rα gene expression was consistent with immunofluorescence results, in that the earliest CD45RA− progenitor population, which lacks IL-7Rα surface protein expression, was negative in all four experiments (Fig 7a), whereas the CD10−/CD19− lymphoid progenitor–containing population was weakly positive in two of two experiments (Fig 7b). As expected, sorted IL-7Rα+ cells showed strong expression of IL-7Rα transcripts (Fig 7c), whereas pro-B cells were weakly reactive, showing expression in one of two experiments at the 100-cell level (Fig 7d). PAX-5 was not expressed in any experiment in the CD45RA− or early lymphoid progenitor populations (Fig 7a and b), but appeared in the IL-7Rα+ progenitor (Fig 7c) and in pro-B cells (Fig 7d) at high levels (3 to 100 cells) in all experiments.
DISCUSSION
The data presented here indicate that expression of lymphoid-associated genes and the capacity for clonogenic proliferation of cells expressing these genes occur before the definitive pro-B or prothymocyte stage. Expression of IL-7Rα and CD10 by these progenitors that lack definitive lineage-specific markers indicates progression to a higher degree of readiness or fitness for at least the B-lymphoid lineage, as shown by the enhanced clonogenic potential in short-term culture, increased expression of lymphoid- and B-lineage–associated genes, and ability to differentiate into pro-B cells in culture (Fig 8).
The colony assay used in this study to explore the early stages of lymphoid development preferentially detects clonogenic progenitors before the acquisition of the definitive pro-B marker CD19 (Table 1 and Figs 4 and 5). Detection of lymphoid progenitor colonies directly in the adherent stromal layer by in situ staining for TdT may enhance the identification of smaller numbers of lymphoid progenitors that are present in adult bone marrow as opposed to fetal tissues. TdT expression, which is the end point of the colony assay, was used as a relatively sensitive indicator of early lymphoid potential, since TdT is universally expressed by normal and neoplastic pro-B cells and prothymocytes. This approach is supported by our data showing mRNA expression of TdT occurring at least as early as any lymphoid-associated gene tested (Fig 7).
Based on the substantial evidence linking IL-7 with early lymphoid development, we tested the hypothesis that IL-7R expression is an important indicator of early lymphoid development potential. In contrast to the relatively broad expression of the common γc-chain in CD34+/CD45RA− and CD34+/CD45RA+ subpopulations as detected by RT-PCR (data not shown), expression of IL-7Rα is limited to a small number of CD34+ cells with distinct immunophenotypic (Figs 1 and 2) and functional (Table 1 and Fig 3) characteristics. The restricted expression of IL-7Rα (Fig 1b) was unexpected in light of the known effect of IL-7 on proliferation of early myeloid progenitors, suggesting that this effect may be indirectly mediated. Interestingly, IL-7Rα may be more broadly expressed in fetal bone marrow, in which most pro-B and pre-B cells are IL-7Rα+,21 although only the pro-B fraction is IL-7–responsive.21 Although some of the differences in fetal versus adult IL-7Rα expression may be related to methodologic considerations (for instance, use of the streptavidin staining procedure by Dittel and LeBien21 ), the marked difference in IL-7Rα protein (Fig 1) and mRNA (Fig 7) expression between IL-7Rα+ and CD19+ cells suggests significant downregulation at this differentiation stage in adult marrow.
IL-7Rα+ progenitors showed a 50-fold higher cloning efficiency for lymphoid progenitor colonies in comparison to IL-7Rα− progenitors (Table 1), suggesting that this surface marker signals an enhanced fitness of the progenitor for lymphoid development in short-term stromal culture lacking exogenous cytokines. However, although the cloning efficiency of the CD34+/IL-7Rα− fraction is low, in absolute numbers over a third of CFU-TdT arise from this large fraction. The enhanced cloning efficiency of the IL-7Rα+ population is unlikely to be the result of removal of inhibitory cells from the IL-7Rα+ fraction, since the total number of lymphoid colonies derived from all fractions of sorting experiments involving CD45RA or c-kit (17.1 ± 6.3 colonies/10,000 cells, n = 10) was comparable to that observed in all fractions of IL-7Rα sorting experiments (17.5 ± 4.5 colonies/10,000 cells, n = 3). It is unlikely that the enhanced cloning efficiency in the IL-7Rα+ population was secondary to activation due to cross-linking of IL-7Rα by antibody, since colony growth is modestly inhibited, not stimulated, when anti–IL-7Rα antibody was continually present during culture of total CD34+ cells (data not shown). Addition of exogenous IL-7 in addition to that already secreted by stroma may have complex dose-related effects on lymphoid progenitors,10 possibly due to direct inhibition22 or indirect signaling via IL-7–responsive accessory cells. IL-7 tended to stimulate IL-7Rα+ progenitors, but with significant variation among experiments as previously observed in studies of total CD34+ cells.10 As expected, exogenous IL-7 had no significant effect on proliferation of IL-7Rα− progenitors.
These data suggest that expression of IL-7Rα is a component of the differentiation program specifying the progressive development of lymphoid potential. In addition to a marked increase in clonogenic potential, IL-7Rα expression is accompanied by increased expression of multiple lymphoid-associated genes, including RAG-1, TdT, Igβ, and PAX-5 (Fig 7), which are maintained in the pro-B stage. The small minority of CD34+ cells that express IL-7Rα and the distinct phenotypic and functional features of this subpopulation, all of which point to increasing lymphoid developmental potential, support the hypothesis that among CD34+ hematopoietic progenitors, IL-7Rα expression is a developmentally regulated phenotype as opposed to a marker of lineage-nonspecific cell activation.
Only IL-7Rα+ progenitors are able to acquire the pro–B-cell marker CD19 during short-term culture with human marrow stroma (Fig 3). In contrast, IL-7Rα− progenitors show little evidence of differentiation, largely retaining CD34 and failing to express either CD19 or CD10. In vitro B-lineage differentiation even in the IL-7Rα+ progenitor fraction is incomplete, consistent with incomplete maturation of human pro-B cells6,8,21 or cord blood progenitors7 in culture, indicating a requirement for additional uncharacterized interactions not provided in the in vitro microenvironment.
Although more than 80% of human pro-B cells are TdT+,1 23 it is possible that some early B-cell precursors are TdT−, and these would not be included in the analysis of TdT+ colonies in the in vitro assay. However, the almost uniform TdT positivity of the IL-7Rα+/CD34+ population (98%), which is an earlier differentiation stage than the CD19+ pro-B cell on several grounds, does suggest (along with the gene expression data) that TdT appears early in lymphoid progenitors and the TdT− cells observed in the CD19+ pro–B-cell subset are “late” pro-B cells, reflecting some overlap in the pro-B to pre-B transition. TdT therefore represents a reasonably sensitive marker for examining the transition from uncommitted progenitor to pro-B cell. We also considered the possibility that IL-7Rα−/CD19− cells may differentiate beyond the pro-B stage all the way to pre-B cells and possibly escape detection in the colony assay, since pre-B cells would be TdT−. However, in two experiments, sorted CD34+/CD10−/CD19− cells cultured for 2 weeks and analyzed by flow cytometry after resuspension of the entire culture well remained 98% to 99% CD19−. These results suggest not only the enhanced differentiation capability of IL-7Rα+ progenitors, but the ability of IL-7Rα− progenitors to survive and proliferate under these culture conditions without evident ongoing differentiation.
In contrast to IL-7Rα, expression of c-kit is associated with decreased lymphoid progenitor potential and lower potential for differentiation into pro-B cells in short-term culture. Loss of c-kit may reflect increasing commitment to the lymphoid lineage, culminating in the c-kit−/CD19+ pro-B cell. In the mouse, c-kit has a slightly earlier pattern of expression in the B lineage than IL-7Rα.24,25 A requirement for stem cell factor in human B lymphopoiesis was not evident in our previous in vitro studies,10 although stem cell factor probably plays an accessory role in mouse B lymphopoiesis that is variably demonstrable in culture.26,27 Therefore, c-kit is regulated during B-lineage development, but its functional role as a synergistic factor may not be apparent in the in vitro microenvironment, which may lack one or more of the multiple factors implicated in B-lineage development.28
The strong correlation between B-lymphoid differentiation potential and IL-7Rα expression is supportive of evidence that IL-7 is involved in human B lymphopoiesis. This conclusion is consistent with defective B-lymphoid development in mice lacking IL-7Rα,12 γc-chain,29 or JAK3, a key component of the IL-7R signaling pathway.30 However, the presence of relatively normal numbers of phenotypically mature (albeit functionally impaired) B cells31 in human patients with defective γc-chain (X-linked SCID32 ) argues against an absolute requirement for the γc-component of the IL-7R in human B lymphopoiesis. A growth factor (TSLP) with a spectrum of activity similar to IL-7 has been described in the mouse,33 but it is not known if it binds to a receptor containing IL-7Rα as has been proposed.34,35 The variable presence of uncharacterized factors with functional similarity to IL-7 may underlie conflicting results in culture models testing the IL-7 dependence of human B-cell progenitor development.7,10,11 In our in vitro culture system, the degree of inhibition of TdT+ colonies by anti–IL-7 antibody10 (87%) is at least as high as the percentage of total CFU-TdT that are IL-7Rα+ (62%), suggesting that alternate growth-stimulatory ligands that could possibly substitute for IL-7 in interactions involving B-cell progenitor receptors containing IL-7Rα are not playing a major role in our in vitro cultures.
The model of early human lymphoid development proposed here is consistent with that recently presented by Galy et al.14 A CD19− progenitor with lymphoid differentiation potential recently identified in the mouse20 is also similar in phenotype (CD45R+/CD19−/CD43+/CD24−) to the IL-7Rα+ progenitor cell. The common T/B/NK/dendritic progenitor described by Galy et al shares with the IL-7Rα+ progenitor described here the lack of lineage-specific markers (ie, CD19) and expression of CD45RA and CD38, distinguishing it from both lymphoid-committed precursors (which are CD19+4) and primitive multilineage progenitors (which are CD45RA−36 or CD38−37). Expression of CD45RA by all progenitors capable of forming TdT+ colonies in stromal-dependent culture (Fig 4) is consistent with early expression of the related protein CD45R (B220) by early murine B-cell precursors.19 CD10 expression, a defining feature of the common progenitor described by Galy et al and previously suspected to precede the pro-B stage,38-40 is clearly associated in the current study with IL-7Rα expression among CD34+/CD19− progenitors. The only difference among the markers tested is expression of c-kit detected in IL-7Rα+ progenitors in this report. However, c-kit expression by IL-7Rα+ progenitors is quantitatively weak (Fig 2q), and its detectability may be subject to methodologic variables.
We found lymphoid-associated gene expression and lymphoid clonogenic potential in both IL-7Rα−/CD10−/CD19− and IL-7Rα+/CD10+/CD19− progenitor fractions, populations similar in phenotype to the CD10−/CD19− and CD10+/CD19− fractions shown to be capable of multilineage differentiation,14 thus raising the question as to whether these populations contain true multilineage progenitors or are mixtures of lineage-committed progenitors with similar surface phenotype. Another important question is whether expression of IL-7Rα is a common feature of differentiation into B, T, NK, and dendritic lineages. This possibility is supported by the lack of NK differentiation in the IL-7Rα knockout mouse.12 However, since the onset of IL-7Rα and CD10 expression is not completely synchronous, resulting in the presence of IL-7Rα+/CD10− and IL-7Rα−/CD10+ cells (each of which shares a similar immunophenotype with the IL-7Rα+/CD10+ cell), these possibilities will need to be tested directly by assessing differentiation of sorted subpopulations under culture conditions specific for definitive lineage development.
The IL-7Rα+ subset does express two markers considered to reflect myeloid differentiation, CD13 and CD33. Expression of CD1338 and (variably) CD3338,39 by CD34+/CD10+ and CD34+/CD19+ cells has been reported. Our results clearly indicate a progressive diminution of CD13, CD33, and c-kit expression from CD34+/IL-7Rα− → CD34+/IL-7Rα+ → CD34+/CD19+ fractions (Fig 2m to r), consistent with evolving commitment to the lymphoid lineage at the expense of myeloid/monocytic differentiation potential. Expression of CD13 and CD33 by some B-cell precursor lymphoblastic leukemias41 may therefore reflect normal phenotypic patterns rather than aberrant (mixed-lineage) differentiation.
CD19 expression at the pro-B stage is immediately preceded by PAX-5 (Fig 7c) and IL-7Rα (Fig 7c) expression in the IL-7Rα+ progenitor, consistent with a proposed role for each of these proteins in upregulation of CD19. PAX-5, a paired-box transcription factor,42 is expressed in B-lineage and neural cells,43 which are affected in the PAX-5 knockout mouse.44 PAX-5 activates promoters of early B-lineage genes, including CD1945 and the surrogate Ig light-chain VpreB,46 whose expression is abolished in the knockout mouse.44 CD19− myeloma cells can be induced to express CD19 by transfection with a PAX-5 expression vector.47 It is possible therefore that PAX-5 expression in the early pro-B cell may directly induce CD19 synthesis, determining entry into the pro-B differentiation stage. IL-7Rα expression is evidently not required for expression of TdT or the earliest development of lymphoid potential. However, IL-7 in the context of other B-lineage signals may promote B-cell progenitor maturation by upregulation of CD19 expression,48 and may regulate recombinase gene expression.49
These results demonstrate that several genes considered to be lymphoid lineage-associated or -specific are expressed in two early progenitor compartments prior to the pro-B or thymocyte differentiation stages. IL-7R expression defines entry into a developmental stage characterized by upregulation of multiple lymphoid lineage-associated genes and enhanced fitness for B-lymphoid differentiation. The relationship of this IL-7Rα+ progenitor to the phenotypically similar common lymphoid/NK/dendritic progenitor and the possible role of lymphoid-associated genes in the early development of these related lineages can now be directly tested. Early lymphoid progenitors expressing TdT before acquisition of IL-7Rα can be maintained in culture in a phenotypically undifferentiated state for 2 weeks. This provides an opportunity to directly manipulate at a genetic level normal early human bone marrow progenitors with differentiation potential for lymphoid and possibly multiple additional lineages. It is possible that leukemic stem cells in patients with B-cell precursor lymphoblastic leukemia may reside within minor subpopulations resembling the normal early progenitor populations described here.
ACKNOWLEDGMENT
We acknowledge the generous help of Dr Richard Armitage (Immunex Corp, Seattle, WA) for providing M21 anti–IL-7Rα antibody and for helpful comments. We also acknowledge Drs Beth Martin and Sigrun Reykdal for obtaining bone marrow samples, and Drs Beerelli Seshi and Charles Sparks for helpful comments.
Supported by American Cancer Society Grant No. DHP-40, the Department of Pathology and Laboratory Medicine at the University of Rochester, and the Strong Children's Research Center.
Address reprint requests to Daniel H. Ryan, MD, University of Rochester, School of Medicine and Dentistry, Box 608, 601 Elmwood Ave, Rochester, NY 14642.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal