Abstract
Besides the regulation of hematopoiesis, granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the expression of a functional program in endothelial cells (ECs) related to angiogenesis and to their survival in the bone marrow microenvironment. ECs express specific GM-CSF high-affinity binding sites, which mediate the proliferative and migratory response. We now report that ECs express the α and β subunits of GM-CSF receptor (GM-CSFR), and that GM-CSF is able to activate the Janus kinase (JAK)2, a member of the cytosolic tyrosine kinase family, which is known to mediate signals of several non–tyrosine kinase receptors. JAK2 tyrosine phoshorylation, as well as activation of its catalytic activity, is induced by subnanomolar concentrations of GM-CSF and occurs within 3 minutes of stimulation and persists at least for 10 minutes. The effect is specific as inferred by the lack of effect of heat-inactivated GM-CSF or neutralized by specific antibodies and by the finding that interleukin-5, which utilizes a specific α chain and the same β chain of GM-CSFR, does not phosphorylate JAK2. Furthermore, we show that the amount of JAK2 physically associated with GM-CSFR β chain is increased after GM-CSF stimulation and that GM-CSF triggers both β chain and JAK2 tyrosine phosphorylation. Taken together, these results suggest that biologic activities of GM-CSF in vascular endothelium may, in part, be elicited by GM-CSFR–mediated JAK2 activation.
GRANULOCYTE-MACROPHAGE colony-stimulating factor (GM-CSF) is a cytokine that regulates proliferation and differentiation of myeloid progenitor cells and functional activation of mature cells.1,2 GM-CSF heterodimeric receptor (GM-CSFR) is composed of two transmembrane glycoprotidic subunits, termed α3 and β.4 The 60- to 90-kD α chain specifically binds the ligand with low affinity and dimerizes with the 120- 140-kD β chain, which is also shared by interleukin-3 (IL-3) and IL-5 receptors.5
Although the GM-CSFR lacks intrinsic kinase activity,5 GM-CSF induces a variety of immediate cellular responses, including activation of components of the Ras signaling pathway,6-8 and the tyrosine phosphorylation of several substrates,8-13 including the β-chain subunit of its receptor.13-17 Furthermore, by using tyrosine kinase inhibitors, it has been established that activation of one or more tyrosine kinases is an early and critical signal-transducing event in GM-CSF–stimulated hematopoietic cells.7 8
The putative connection between GM-CSFR and phosphorylation of tyrosine residues has been identified in a novel family of cytosolic tyrosine kinase, named Janus kinases (JAKs).18-20 Many results obtained studying the activation of these kinases by a number of cytokines, including interferons, erythropoietin, IL-2, IL-3, IL-4, IL-5, and IL-9, support the current opinion that JAKs are physically associated with several members of the cytokine receptor superfamily and are catalytically activated after ligand binding.18-20
In particular, several data demonstrate that JAK2, a member of these nonreceptor tyrosine kinases, is physically associated with the GM-CSFR β chain,17,21,22 and activated upon challenge of myeloid cells with GM-CSF.17,21,22 More recently, it has been reported that in human neutrophils functionally activated by GM-CSF, the association of JAK2 with β chain is ligand-dependent.23 Elegant experiments performed with BaF3 cells expressing α chain and β chain mutated in cytoplasmic domains, have permitted the demonstration of a complete correlation between GM-CSF–mediated JAK2 activation and mitogenic effect.21 Furthermore, GM-CSF–dependent activation of JAK2 in neutrophils seems to regulate the formation of a DNA-binding complex containing both STAT1 and STAT3, which provides evidence on the role of this kinase in the regulation of gene expression in nonproliferating myeloid cells.23
Several studies have suggested that GM-CSF responsiveness may not be limited to hematopoietic lineages.24-26 Furthermore, the reconstitution of GM-CSFR in murine fibroblasts is functional and transduces growth-promoting signals.27 The expression of GM-CSFR has been also described in cancer cells,28,29 trophoblasts,3 oligodendrocytes,30 and endothelial cells (ECs).26,31 Moreover, it has been reported that GM-CSFR–mediated EC activation includes functions related to angiogenesis and inflammation (ie, proliferation, migration, and expression of adhesion molecules).26,31-40 Although GM-CSF is less potent than other more classic angiogenic molecules (ie, fibroblast growth factor) in promoting EC proliferation, it activates a fully migratory phenotype.41 In addition, it has also been shown that GM-CSF interaction with specific binding sites on the EC surface induces the expression of c-fos mRNA26 and a rapid activation of Na+/H+ exchanger.42
In this study, we examined the molecular feature of GM-CSFR in ECs and its ability to associate and activate JAK2.
MATERIALS AND METHODS
Cells.Human ECs from umbilical cord vein, prepared and characterized as previously described,26,31,41 were grown in M199 medium (GIBCO, Grand Island, NY) supplemented with 20% fetal calf serum (Irvine, Santa Ana, CA), EC growth factor (100 μg/mL) (Sigma Chemical, St Louis, MO), and porcine heparin (100 μg/mL) (Sigma). They were used at early passages (II to III) and characterized by using the following primary monoclonal antibodies: anti–Factor VIII (Boehringer, Mannheim, Germany), anti-CD31 (a gift of Dr M. Lampugnani, Istituto “Mario Negri,” Milano, Italy), anti–major histocompatibility complex of class I (clone 0.165) and class II (clone AA 3.84) (a gift of Prof F. Malavasi, Torino, Italy), and CD11b (Becton Dickinson, Mountain View, CA). EC cultures were positive for Factor VIII (93% to 100%), CD31 (98% to 100%), and class I major histocompatibility complex (90% to 93%), and negative for class II major histocompatibility complex and CD11b. In a selected number of experiments, EC cultures were incubated with monoclonal antibody OKM1 (2 μg/mL) (Ortho Diagnostic Systems, Raritan, NJ) phosphate-buffered saline containing 2% human serum for 45 minutes at 4°C. After incubation, the cells were washed twice with phosphate-buffered saline and incubated for 1 hour at 37°C with rabbit complement (Sigma; 30% in M199 containing 20% fetal calf serum) and then washed. This treatment was able to produce more than 98% lysis of monocytes and granulocytes and did not affect EC functions.43 U937 and M-07e cell lines were respectively maintained in RPMI 1640 and in Iscove's medium (GIBCO) supplemented with 10% fetal calf serum.
Cross-linking of [125I]GM-CSF to ECs (A) and U937 cells (B) and displacement by GM-CSF. Quiescent ECs at passage II and U937 cells were incubated in binding medium with 0.5 nmol/L [125I]GM-CSF without or with 100-fold excess of unlabeled ligand in the presence of 1 mmol/L disuccinyl suberate. Proteins were separated by SDS-PAGE and visualized by autoradiography. Three experiments with ECs and 2 with U937 cells have been performed with similar results.
Cross-linking of [125I]GM-CSF to ECs (A) and U937 cells (B) and displacement by GM-CSF. Quiescent ECs at passage II and U937 cells were incubated in binding medium with 0.5 nmol/L [125I]GM-CSF without or with 100-fold excess of unlabeled ligand in the presence of 1 mmol/L disuccinyl suberate. Proteins were separated by SDS-PAGE and visualized by autoradiography. Three experiments with ECs and 2 with U937 cells have been performed with similar results.
Cross-linking experiments.Human recombinant GM-CSF (Immunex, Seattle, WA) was dissolved in 200 μL of 20 mmol/L sodium phosphate buffer pH 7.4 and transferred to iodogen-coated tubes (50 μg/mL) (Pierce Europe, BA Oud Beijerland, The Netherlands), where cytokine was iodinated (5 minutes, 4°C) with 1 mCi 125I (Amersham, Buchs, UK). Twenty microliters of phosphate buffer 20 mmol/L, pH 7.2, containing 1% bovine serum albumin (BSA; lipopolysaccharide-free; Sigma), 0.4 mol/L NaCl, and 0.1% Chaps (Pierce) was added and the reaction products were separated on Sephadex-G10 (Pharmacia Biotech, Uppsala, Sweden). The specific activity of the tracer was 1 μCi/179 fmol. [125I]GM-CSF retained its biologic activity as shown by M-07e cell proliferation.22 Confluent ECs or suspended U937 cells (2 × 107 cells) were incubated in binding medium (Dulbecco's modified Eagle's medium [DMEM] containing 20 mmol/L N-[2-hydroxyethyl]piperazine-N'-2-ethane sulfonic acid, pH 7.4, 0.1% BSA, 100 μg/mL soybean trypsin inhibitor, and bacitracin) with 0.5 nmol/L of [125I]GM-CSF, with or without 100-fold excess of unlabeled ligand for 30 minutes at 4°C, and then 1 mmol/L dissuccinimidyl suberate (Pierce) was added. The cross-linking reaction was allowed to proceed at 4°C for 30 minutes. After two washes with phosphate-buffered saline containing 0.01% NaN3, cells were lysed (30 minutes, 4°C) in 20 mmol/L Tris-HCl, pH 7.4, containing NaCl 140 mmol/L, 1% Triton X-100, pepstatin 10 μg/mL, leupeptin 100 μg/mL, aprotinin 10 μg/mL, phenylmethylsulfonyl fluoride (PMSF) 1 mmol/L, and EDTA 5 mmol/L. After centrifugation (15,000g, 20 minutes, 4°C), soluble proteins were denaturated by boiling for 4 minutes in Laemmli Sample Buffer 5x44 and separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and bands visualized by autoradiography.
Analysis of GM-CSFRα transcript.Total RNA was prepared from ECs at passages II to III or U937 cells by use of RNAzol (Cima/Biotecx Laboratories, San Diego, CA) according to the instructions of manufacturer. One microgram of total RNA was denatured by heating and reverse-transcribed by 20 U Moloney murine leukemia virus reverse transcriptase (RT; Perkin Elmer, Norwalk, CT) into first-strand cDNA using 25 pmol of primers (dt)15. The reaction was performed for 1 hour at 37°C in a 20-μL final volume containing 5 mmol/L dithiothreitol, 40 U RNAsin, 5 mmol/L deoxy-nucleotide triphosphate mixture (Perkin Elmer), and 1× buffer (20 mmol/L Tris, pH 8.3, 4 mmol/L MgCl2). Polymerase chain reaction (PCR) was performed in a Perkin Elmer DNA thermal cycler using 5 μL of the transcription mixture and 2.5 U of Taq polymerase (Perkin Elmer). Deoxy-nucleotide triphosphate mixture (0.2 mmol/L), 1× reaction buffer (10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.01% gelatin), and 35 pmol of each primer were added in a 50-μL reaction volume. The following specific oligomers of GM-CSFRα sequence (Tib Molbiol Berlin GmbH, Berlin, Germany) were used45: GM-CSFRa1 — sense, 5′ CGACGGGAACCTCGGCTCTG 3′ (position 1,100 to 1,120), and antisense, 5′ CAGTTCCCCCGTGCGCGGAGG 3′ (position 1,400 to 1,420); GM-CSFRα2 — sense, 5′ GGTGGAAGACGAGATCATCTG 3′ (position 1,260 to 1,280), antisense, 5′ GAGATGCAATCTTGCTCTG 3′ (position 1,740 to 1,760).
RT-PCR analysis of expression of α chain of GM-CSFR in ECs (lanes 3 to 5) and U937 cell line (lanes 7 to 9). One microgram of total RNA was reverse-transcripted and amplified with GM-CSFRα1 –(lanes 4 and 8), GM-CSFRα2– (lanes 5 and 9), and β-actin– (lanes 3 and 7) specific oligonucleotides. Amplified products were run in an ethidium bromide-agarose gel and visualized by UV. In lane 1, molecular weight have been runned. Lanes 2 and 6 represent two control experiments performed with RT-PCR mixture without cell RNA. This experiment is representative of five performed with similar results.
RT-PCR analysis of expression of α chain of GM-CSFR in ECs (lanes 3 to 5) and U937 cell line (lanes 7 to 9). One microgram of total RNA was reverse-transcripted and amplified with GM-CSFRα1 –(lanes 4 and 8), GM-CSFRα2– (lanes 5 and 9), and β-actin– (lanes 3 and 7) specific oligonucleotides. Amplified products were run in an ethidium bromide-agarose gel and visualized by UV. In lane 1, molecular weight have been runned. Lanes 2 and 6 represent two control experiments performed with RT-PCR mixture without cell RNA. This experiment is representative of five performed with similar results.
Northern blot analysis of α chain of GM-CSFR and of β actin in ECs and in U937 cell lines. Seven micrograms of PolyA+ RNA was run in a formaldehyde-agarose gel and after blotting to Duralon membrane, hybridized to α-chain– and β-actin–specific probes. Transcripts have been visualized by autoradiography. This experiment is representative of three performed with similar results.
Northern blot analysis of α chain of GM-CSFR and of β actin in ECs and in U937 cell lines. Seven micrograms of PolyA+ RNA was run in a formaldehyde-agarose gel and after blotting to Duralon membrane, hybridized to α-chain– and β-actin–specific probes. Transcripts have been visualized by autoradiography. This experiment is representative of three performed with similar results.
The PCR protocol was as follows: 1.5 minutes at 94°C, 2 minutes at 60°C, 3 minutes at 72°C for 35 cycles; 1 minutes at 94°C, 1 minute at 55°C, 10 minutes at 72°C for the last cycle. PCR of β-actin was performed by using specific oligonucleotides (Stratagene, La Jolla, CA) with the following protocol: 1 minute at 94°C, 1 minute at 55°C, 1 minute at 72°C for 30 cycles; 1 minute at 94°C, 1 minute at 55°C, 10 minutes at 72°C for the last cycle. Twenty microliters of the amplified solution was run in an 1.8% agarose gel electrophoresis in Tris-borate-EDTA buffer and stained with 0.5 μg/mL ethidium bromide. The products of PCR were then hybridized with the GM-CSFR α-chain probe, as described later. Alternatively, poly(A)+ RNA was purified by total RNA with oligo d(T) cellulose column (Pharmacia Biotech) according to the manufacturer's instructions. Seven micrograms of poly(A)+ RNA was electrophoresed on a 1% agarose gel containing 6.3% formaldehyde in 3-[N-morpholino]propanesulfonic acid buffer and blotted on a Nylon Duralon-UV membrane (Stratagene) by the traditional capillary system in 10× sodium salt citrate (SSC). Filters were probed with 0.365-kb Kpn-Xho I and 0.317-kb Sac I-Xho I fragments of human GM-CSFRα cDNA, which was inserted in pKH125 plasmid,3 or with 1.15-kb Pst I-Pst I fragment of β-actin cDNA. cDNAs were radiolabeled with a random-primed synthesis kit (Megaprime DNA labeling system; Amersham).
Tyrosine phosphorylation and immunoprecipitation experiments.Confluent ECs (1 × 107 cells per 150-cm2 dish) or U937 cells (1 × 107) cells were made quiescent by 20 hours' starvation in serum-free M199 containing 3% BSA (lipopolysaccharide-free; Sigma) (M199-BSA), preincubated for 10 minutes at 37°C with 1 mmol/L Na3VO4, and then stimulated with GM-CSF as detailed in Results. In some experiments, IL-5 (Sigma), heat-inactivated GM-CSF, or GM-CSF neutralized by overnight incubation at 4°C with rabbit antihuman GM-CSF antibody26 were used as stimuli. Cells were lysed in a 50 mmol/L Tris-HCl buffer, pH 7.4, containing 150 mmol/L NaCl, 1% TritonX-100, and protease and phosphatase inhibitors (pepstatin 50 μg/mL, leupeptin 50 μg/mL, aprotinin 10 μg/mL, PMSF 1 mmol/L, soybean trypsin inhibitor 500 μg/mL, ZnCl2 100 μmol/L, Na3VO4 1 mmol/L). After centrifugation (20 minutes, 10,000g), supernatants were precleared by incubation for 1 hour with Protein A-Sepharose (Pharmacia) and nonimmune serum (1:100). Samples were incubated with rabbit polyclonal antibody anti-JAK2 or anti-JAK1 (Upstate Biotechnology, Lake Placid, NY) (5 μg/mL) or with anti-β chain (1:100)22,23 for 1 hour and immune complexes were recovered on Protein A-Sepharose. Immunoprecipitates were washed four times with lysis buffer, twice with the same buffer without Triton X-100 and once with Tris-buffered saline. In some experiments, immunoprecipitates anti-β chain recovered on Protein A-Sepharose were boiled for 2 minutes in a solubilization buffer containing 0.4% SDS, 50 mmol/L triethanolamine chloride (pH 7.4), 100 mmol/L NaCl, 2 mmol/L EDTA, and 2 mmol/L 2β-mercaptoethanol.46 After boiling, iodacetamide was added to 10 mmol/L. Finally, 0.25 vol of 10% (vol/vol) Triton X-100 was added. These extracts were again immunoprecipitated with anti-JAK2 antibody. Proteins were solubilized in boiling Laemmli buffer,43 separated by SDS-PAGE, and transferred to Immobilon-P sheets (Millipore, Bedford, MA) and probed with a monoclonal antiphosphotyrosine (PTyr) antibody (clone G410; Upstate Biotechnology), or with anti-JAK2, or anti-β chain. The enhanced chemiluminescence technique (Amersham) was used for detection.
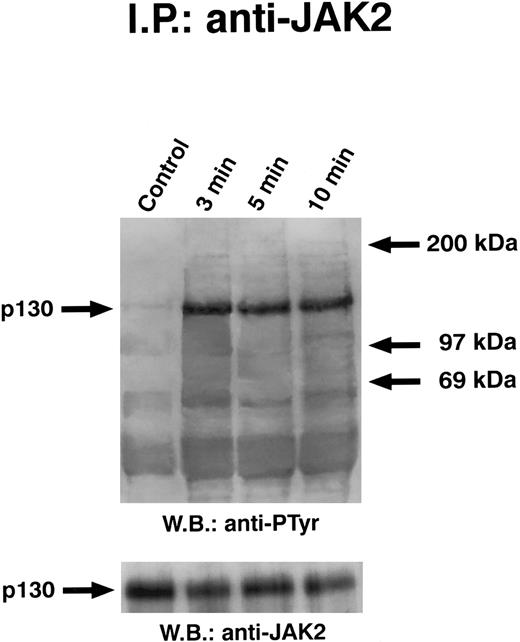
Time course of GM-CSF–induced tyrosine phosphorylation of JAK2. Quiescent ECs were incubated with 10 ng/mL of GM-CSF in M199-BSA at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were reprobed with anti-JAK2 antibody (lower panel). This experiment is representative of five performed with similar results.
Time course of GM-CSF–induced tyrosine phosphorylation of JAK2. Quiescent ECs were incubated with 10 ng/mL of GM-CSF in M199-BSA at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were reprobed with anti-JAK2 antibody (lower panel). This experiment is representative of five performed with similar results.
Time course of GM-CSF–induced tyrosine phosphorylation of JAK1. Quiescent ECs were incubated with 10 ng/mL of GM-CSF in M199-BSA at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were reprobed with anti-JAK1 antibody (lower panel). This experiment is representative of two performed with similar results.
Time course of GM-CSF–induced tyrosine phosphorylation of JAK1. Quiescent ECs were incubated with 10 ng/mL of GM-CSF in M199-BSA at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were reprobed with anti-JAK1 antibody (lower panel). This experiment is representative of two performed with similar results.
To examine the kinase activity of JAK2, washed anti-JAK2 immunoprecipitates were incubated for 15 minutes at 30°C in 100 μL of 50 mmol/L N-[2-hydroxyethyl]piperazine-N'-2-ethane sulfonic acid, pH 7.4, containing 50 mmol/L NaCl, 5 mmol/L MgCl2, 5 mmol/L MnCl2, 0.1 mmol/L Na3VO4, 1 μmol/L adenosine triphosphate (ATP), 25 μCi [γ-32P]ATP, and 5 μg of peptide VLPQDKEYYKVKEPGE, corresponding to amino acids 475 to 491 of mouse JAK2.47 Background incorporation was considered the radioactivity incorporated into the substrate in absence of JAK2, and the values were subtracted. Reaction was stopped by adding 10 μL 0.5-mol/L acetic acid. Aliquots (50 μL) were pipetted onto P81 phosphocellulose papers (Whatman International, Maidston, UK) and washed five times with 0.05 mol/L acetic acid at room temperature. Cherenkov radiation of dried samples was counted.
RESULTS
GM-CSFR in ECs.We have previously demonstrated that ECs express high-affinity binding sites for GM-CSF.25,31 Furthermore, transcript of β chain has been detected in ECs,31,48,49 but that of α chain seems to be present in a human EC line, but not in ECs from umbilical veins.31 48
To further analyze the presence of GM-CSFR expressed on ECs, we performed cross-linking experiments on whole cells. Figure 1 demonstrates that ECs and U937 cells express a major specific labeled band competed by unlabeled GM-CSF at approximately 95 kD. Given a molecular weight of 14 kD for unglycosylated GM-CSF, the implied molecular weight for GM-CSFR in ECs is approximately 80 kD. The size of this band is compatible to α chain of GM-CSFR present in other cell types.50-53 The addition of 100-fold excess of cold cytokine displaced the binding of [125I]GM-CSF, showing its specificity. A second band of 125 kD is present in both cell types and is specifically displaced by an excess of GM-CSF (Fig 1). The presence of a 95-kD protein that specifically was cross-linked to GM-CSF prompted us to reexamine the expression of α-chain mRNA. First, the expression of this gene was studied by RT-PCR by using two pairs of primers (GM-CSFRα1 and GM-CSFRα2). As shown in Fig 2, RT-PCR performed on RNA extracted from ECs and U937 cells gave two products of 300 bp and 500 bp that correspond to the amplified sequence expected with primers GM-CSFRα1 and GM-CSFRα2 and were recognized by the specific probe (not shown). To exclude that the amplified bands in ECs were due to the presence of contaminant leukocytes, cell cultures were treated with OKM1 monoclonal antibody and complement to lyse monocytes and neutrophils eventually present. Also in this experimental condition, RT-PCR showed the presence of GM-CSFR mRNA (not shown). To further confirm these results, PolyA+ mRNA of ECs was probed with a 0.365-kb cDNA fragment corresponding to the position 150 to 505 of cDNA sequence. Figure 3 shows that both ECs and U937 cells expressed a 1.8-kb transcript. Similar results have been obtained with a cDNA fragment corresponding to position 1,071 to 1,349 (not shown).
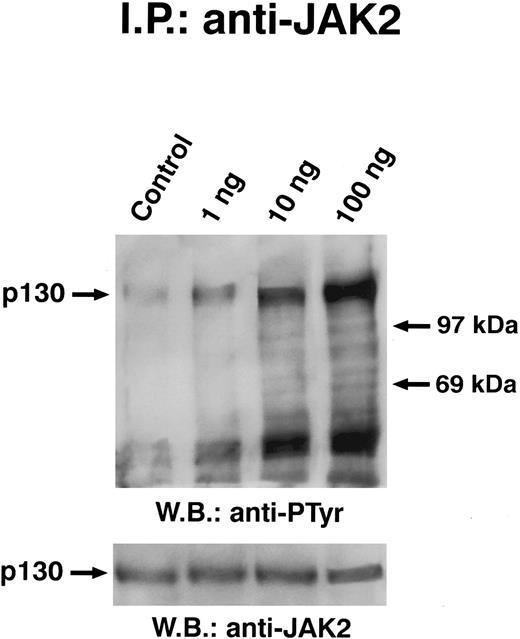
Dose dependence of GM-CSF–induced tyrosine phosphorylation of JAK2. Quiescent ECs were incubated with different amounts of GM-CSF (ng/mL) in M199-BSA for 5 minutes at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were re-probed with anti-JAK2 antibody (lower panel). This experiment is representation of five performed with similar antibody. In this experiment, the 200-kD molecular marker remained at the top of the gel.
Dose dependence of GM-CSF–induced tyrosine phosphorylation of JAK2. Quiescent ECs were incubated with different amounts of GM-CSF (ng/mL) in M199-BSA for 5 minutes at 37°C, lysed, and proteins immunoprecipitated with anti-JAK2 antibody. Immunoprecipitate was analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blots were re-probed with anti-JAK2 antibody (lower panel). This experiment is representation of five performed with similar antibody. In this experiment, the 200-kD molecular marker remained at the top of the gel.
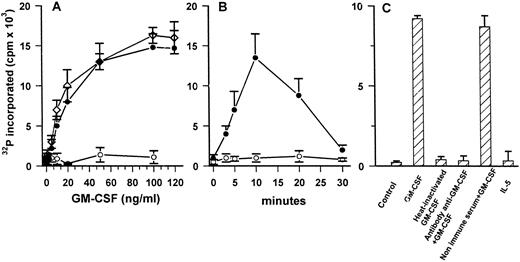
In vitro phosphorylation of VLPQDKEYYKVKEPGE substrate. (A) Dose-dependent effect of GM-CSF–induced activation of JAK2 in ECs (•) and U937 cells (⋄). Cells were stimulated for 5 minutes with increasing amount of cytokine. Anti-JAK2 immune-complexes (•, ⋄) or immune complexes obtained with nonimmune rabbit serum (○), in triplicate, were incubated in kinase buffer containing 5 μg of peptide and processed as described in Materials and Methods. (B) Time course of GM-CSF (50 ng/mL)–dependent activation of JAK2 in ECs. (C) Effect of stimulation of JAK2 catalytic activity in ECs treated for 5 minutes with GM-CSF (50 ng/mL), heat-inactivated GM-CSF (50 ng/mL), GM-CSF (50 ng/mL) neutralized by specific antibody or by nonimmune serum, and IL-5 (50 ng/mL). Mean ± SD of three samples in one experiment representative of three performed.
In vitro phosphorylation of VLPQDKEYYKVKEPGE substrate. (A) Dose-dependent effect of GM-CSF–induced activation of JAK2 in ECs (•) and U937 cells (⋄). Cells were stimulated for 5 minutes with increasing amount of cytokine. Anti-JAK2 immune-complexes (•, ⋄) or immune complexes obtained with nonimmune rabbit serum (○), in triplicate, were incubated in kinase buffer containing 5 μg of peptide and processed as described in Materials and Methods. (B) Time course of GM-CSF (50 ng/mL)–dependent activation of JAK2 in ECs. (C) Effect of stimulation of JAK2 catalytic activity in ECs treated for 5 minutes with GM-CSF (50 ng/mL), heat-inactivated GM-CSF (50 ng/mL), GM-CSF (50 ng/mL) neutralized by specific antibody or by nonimmune serum, and IL-5 (50 ng/mL). Mean ± SD of three samples in one experiment representative of three performed.
GM-CSF stimulates tyrosine phosphorylation of JAK2.To establish that GM-CSFR was able to signal inside ECs, we studied the activation of JAK2, a tyrosine kinase involved in GM-CSF–dependent activation of myeloid cells.17,22 23
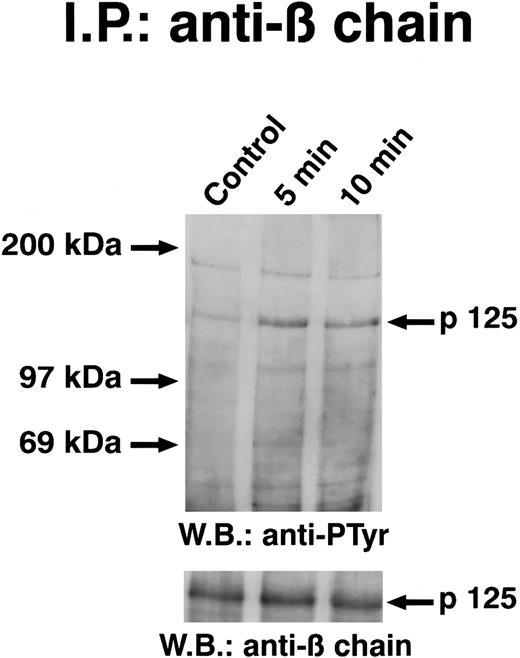
Solubilized proteins from GM-CSF–treated ECs were immunoprecipitated with anti-JAK2 antibody, separated by SDS-PAGE, blotted, and analyzed by antiphosphotyrosine antibody. As depicted in Fig 4, GM-CSF was able to induce the tyrosine phosphorylation of a 130-kD protein specifically recognized by anti-JAK2 antibody. When lysates from GM-CSF–stimulated cells were immunoprecipitated with anti-JAK1 antibody, the antiphosphotyrosine antibody did not detect phosphorylated proteins (Fig 5). Time-course experiments demonstrated that JAK2 tyrosine phoshorylation elicited by GM-CSF reached the maximum after 3 minutes and persisted up to 10 minutes (Fig 4). This effect was clearly evident at subnanomolar concentrations, following a dose-dependent curve (Fig 6). No precipitation of JAK2 was detected with nonimmune rabbit antiserum (not shown).
GM-CSF stimulates catalytic activity of JAK2.To demonstrate that GM-CSF–induced JAK2 tyrosine phosphorylation was due to the activation of its kinase activity, anti-JAK2 immunoprecipitates were incubated with an exogenous substrate identical to the region surrounding Tyr-482 and Tyr-483 of JAK2.47 As shown in Fig 7A, the increase of JAK2 catalytic activity in ECs was dependent on the GM-CSF concentrations. The amount of cytokine inducing the increase of catalytic activity is similar to that active on U937 cells, used as positive control. The effect of GM-CSF reached the maximum after 10 minutes and then declined to basal values (Fig 7B). Moreover, the kinase activity showed a time- and a dose-dependence similar to those observed for tyrosine phosphorylation of the kinase induced by the cytokine (compare Figs 4 and 6 with Fig 7A and B). To validate the specificity of GM-CSF, IL-5, heat-inactivated GM-CSF, or immune-adsorbed GM-CSF were used to evaluate JAK2 catalytic activity. As shown in Fig 7C, they were unable to induce JAK2 activation. Proteins immunoprecipitated from ECs lysated with nonimmune serum did not phosphorylate the exogenous substrate (Fig 7A).
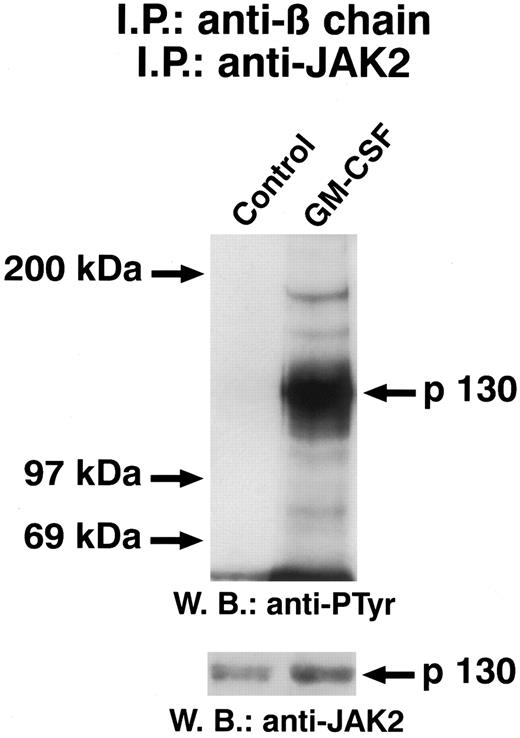
JAK2 is associated with β chain of GM-CSF.To determine whether JAK2 was associated with GM-CSFR, cell lysates from unstimulated and GM-CSF–stimulated ECs were immunoprecipitated with the anti–β-chain antiserum. The presence of JAK2 in the immune complexes was investigated by performing a second immunoprecipitation on solubilized proteins with an anti-JAK2 antibody and immunoblotting with anti-JAK2 antibody. As shown in Fig 8, even in the absence of GM-CSF, JAK2 was present in the anti–β-chain immunoprecipitate, indicating that this kinase is constitutively associated with GM-CSFR. However, the amount of JAK2 associated with β chain was increased somewhat in the presence of GM-CSF. Densitometric analysis demonstrated a 35% ± 7% (n = 5) increase in JAK2 association to β chain.
GM-CSF–induced tyrosine phosphorylation of JAK2 associated with GM-CSFR β chain. Quiescent ECs were incubated for 5 minutes at 37°C in M199-BSA with GM-CSF (50 μg/mL) and cell lysate were immunoprecipitated with β-chain antiserum. After protein solubilization from Protein A-Sepharose, samples were further subjected to a second immunoprecipitation with anti-JAK antibody. Samples were analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blot was reprobed with anti-JAK2 antibody (lower panel). The figure is representative of five similar experiments.
GM-CSF–induced tyrosine phosphorylation of JAK2 associated with GM-CSFR β chain. Quiescent ECs were incubated for 5 minutes at 37°C in M199-BSA with GM-CSF (50 μg/mL) and cell lysate were immunoprecipitated with β-chain antiserum. After protein solubilization from Protein A-Sepharose, samples were further subjected to a second immunoprecipitation with anti-JAK antibody. Samples were analyzed by SDS-PAGE followed by immunoblotting with antiphosphotyrosine antibody. Subsequently, blot was reprobed with anti-JAK2 antibody (lower panel). The figure is representative of five similar experiments.
Given that autophosphorylation is often the earliest event of an activated kinase, the same blots were probed with an antiphosphotyrosine antibody. Figure 9 shows that GM-CSF treatment of ECs induced a rapid phosphorylation in tyrosine residues of JAK2 associated with β chain.
Time course of GM-CSF–induced tyrosine phosphorylation of GM-CSFR β chain. Quiescent ECs were incubated with GM-CSF (20 ng/mL) in M199-BSA at 4°C and cell lysates were immunoprecipitated with β-chain antiserum. Immunoprecipitate was analyzed by SDS-PAGE, followed by immunoblotting with antiphosphotyrosine antibody and then with anti–β-chain antibody. The figure is representative of two identical experiments.
Time course of GM-CSF–induced tyrosine phosphorylation of GM-CSFR β chain. Quiescent ECs were incubated with GM-CSF (20 ng/mL) in M199-BSA at 4°C and cell lysates were immunoprecipitated with β-chain antiserum. Immunoprecipitate was analyzed by SDS-PAGE, followed by immunoblotting with antiphosphotyrosine antibody and then with anti–β-chain antibody. The figure is representative of two identical experiments.
GM-CSF–dependent tyrosine phosphorylation of β chain of GM-CSF.In hematopoietic cells, the GM-CSFR β chain is tyrosine phosphorylated in response to GM-CSF.13-17 To evaluate whether the GM-CSFR β chain became tyrosine phosphorylated in ECs, cells stimulated with GM-CSF at 4°C were lysed and immunoprecipitated with anti–β-chain antiserum. Immunoblots stained with the antiphosphotyrosine antibody showed that GM-CSF caused a tyrosine phosphorylation of β chain that was evident at least for 10 minutes. The immune complexes performed with nonimmune serum did not contain β chain (not shown).
DISCUSSION
The results presented in this study demonstrate that a functional, active GM-CSFR is expressed on ECs and when engaged by GM-CSF is able to activate JAK2, a tyrosine kinase involved in the signal transduction of the cytokine receptor superfamily. This statement is founded on the following five major observations: (1) ECs express on their surface an 80-kD protein that is specifically cross-linked to GM-CSF; (2) the transcript of GM-CSFR α-chain gene, as well as that of β chain,31,48,49 are present in ECs; (3) the activity of JAK2, but not that of JAK1, is significantly enhanced after a few minutes by GM-CSF stimulation at subnanomolar concentrations able to activate proliferation and migration of ECs26,31,41; (4) JAK2 associated with β chain becomes phosphorylated upon ECs challenge with GM-CSF; and (5) GM-CSF induces the tyrosine phosphorylation of the β chain of its receptor, an event occurring in myeloid cells stimulated with the cytokine.13-17 Furthermore, IL-5, which shares the same transducing subunit with GM-CSF, does not activate JAK2 in ECs, which are lacking of the specific IL-5 α chain.31
Biologic activities of endothelium are activated by GM-CSF.23-41 Previous data reported the presence of a high-affinity binding site on human ECs,26,31 as well as the expression of β-chain gene.31,48,49 However, the expression of α chain has not been detected by Northern analysis and by RT-PCR using primers corresponding to the positions 170 to 191 and 916 to 936.31,48 Interestingly, a transformed EC line originated from cell of umbilical veins had high level of α-chain transcript.31 Our results obtained by cross-linking GM-CSF to ECs indicate the presence of two radiolabeled proteins of 95 and 125 kD. The major 95-kD–labeled protein implies a molecular weight of 80 kD for the receptor and corresponds to the low-affinity receptor observed in other cell types.3,5,50-53 It is not clear whether the 125-kD protein is a component of GM-CSFR in ECs or an artifact of the cross-linking in which there has been covalent trimerization of GM-CSF by disuccimidyl suberate, forming a complex with the receptor. However, it has been reported that GM-CSF28,30,53 or IL-354 can bind more than one protein in similar experiments performed with other cells.
The presence of low-affinity GM-CSFR in ECs is confirmed by the analysis of the specific transcript. By using two different experimental approaches, our data clearly indicate that human ECs express α chain mRNA of GM-CSFR: (1) in RT-PCR, two different primers that amplify a gene region encoding the extracellular portion of the subunit gave products corresponding to the positive control and recognized by the specific probe; and (2) PolyA+ mRNA contains a transcript that hybridizes with two cDNA fragments encoding a cytoplasmic and an extracellular portion of the protein. A simple explanation of the discrepancy between these data and that earliest reported31 48 could be the different technical conditions used (eg, conditions of cell culture and of PCR, or primer selection). Alternatively, we cannot exclude the intriguing possibility of a different isoform of α chain in human ECs.
A second finding arising from the results reported in this study concerns the activation of JAK2 associated with β chain of GM-CSFR, after GM-CSF stimulation of ECs. Although the cytoplasmic tail of α chain of GM-CSFR is involved in GM-CSF–dependent tyrosine phosphorylation,13 studies performed with deletion mutants of α chain have demonstrated the pivotal role of this subunit in signal transduction.16,17,55 A domain near to the COOH terminus amino acid is necessary for the antiapoptotic effect of GM-CSF17,55 and a membrane-proximal domain is required both for phosphorylation and activation of JAK2 and for mitogenesis.21 55 These findings compared with our results suggest that activation of JAK2 is likely to be a critical early event in signal transduction through the GM-CSFR in ECs.
Besides JAK2, other transducing molecules can be associated with β chain, including cytokine-inducible SH2-containing protein,56 Grb2,57 p53/56lyn,11 and p92fes.23,58 The association event may occur constitutively or require ligand binding, but the functional activation of these molecules is strictly dependent on the formation of the receptor-ligand complex.11,17,21-23,56,58 In resting ECs, JAK2 is physically associated with the GM-CSFR, but GM-CSF seems to trigger the recruitment of additional JAK2 molecules by the β chain and the activation of JAK2 catalytic activity, as previously reported for stimulated prolactin receptor.59 Moreover, the association of JAK2 with β chain in neutrophils is strictly dependent on GM-CSF stimulation.23 In studies performed on erythropoietin receptor, it has been demonstrated that JAK2 association is ligand-independent.60 In contrast, growth hormone binding to its receptor is required for the formation of a complex between growth hormone receptor and JAK2.61 In SF21 insect cells expressing both JAK2 and the β chain of GM-CSFR, Quelle et al21 have demonstrated the constitutive association of the two molecules. However, the high concentrations of molecules expressed in insect cells are quite different from that present in mammalian cells and can preclude the detection of small changes in protein association. It has been shown that in a cell-free system, the JAK2-β chain association requires the amino-terminal portion of the kinase that binds to the 36–amino acid domain in the membrane proximal region of GM-CSFRβ chain.62 However, it is not possible to speculate on a molecular model concerning the formation of the complex between JAK2 and GM-CSFR in living cells. Interestingly, our data suggest that, at least in ECs, specific ligand stimulation recruits more kinase molecules by an unknown mechanism.
Besides a role in hematopoietic cells stimulated with GM-CSF, our results support the hypothesis that JAK2 participates in the flux of mitogenic signals in ECs. In bone marrow, ECs provide GM-CSF and other soluble polypeptides that regulate proliferation and commitment of hematopoietic precursors.1 Experiments performed in chick and mouse embryo suggest a common origin of ECs and hematopoietic cells in blood islands.63 Indeed, many angiogenic molecules have positive or negative effect on hematopoiesis64 and many cytokines, which act primarily on hematopoietic cells, affect EC functions.48,65-67 Therefore, the ability of GM-CSF to induce migration and proliferation of ECs,26,31,34,36,40,41 including that of bone marrow,34 38 could be essential for the survival and renewal of the bone marrow microenvironment.
ACKNOWLEDGMENT
We thank Dr A. Graziani for helpful discussions, Dr F. Malavasi and Dr Lampugnani who provided monoclonal antibodies, and Dr S. Gillis for recombinant GM-CSF.
Supported by the CNR (Targets Project: Applicazioni Cliniche della Ricerca Oncologica); by Istituto Superiore della Sanita' (ISS, National AIDS Project); by the Associazione Italiana per la Ricerca sul Cancro (AIRC); and by the European Community (Biomed-2 project). R.S. and L.P. are recipients of a fellowship from Istituto Superiore della Sanita' and from AIRC.
Address reprint requests to Federico Bussolino, MD, Dipartimento di Genetica, Biologia e Chimica Medica, Via Santena 5bis, 10126 Torino, Italy.

![Fig. 1. Cross-linking of [125I]GM-CSF to ECs (A) and U937 cells (B) and displacement by GM-CSF. Quiescent ECs at passage II and U937 cells were incubated in binding medium with 0.5 nmol/L [125I]GM-CSF without or with 100-fold excess of unlabeled ligand in the presence of 1 mmol/L disuccinyl suberate. Proteins were separated by SDS-PAGE and visualized by autoradiography. Three experiments with ECs and 2 with U937 cells have been performed with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.863/8/m_bl_0039f1.jpeg?Expires=1767889550&Signature=J7Y6gzuH1~eLl~J7X38wowqiiHayEShOGI91Ef6e2pQCsrxH68kL1etbEzWNseaIBII1UHBDI6kc2waezkdHtRs4D8vNKJIchqqemI3a77aHy2rKcvi6bmj689N0FZbpkisUAivZ-s2NB4M7sd9nf5zi3mGxVhglySSzxQRJ5VovLs4FfrHO6G9HbkGI8HkktJBO5O6yIt-TEgHo~JuGixplTH~UWWmGJCYE3jekvEND56SIA7upMFaW90vgGQR3pwD3u7L9jnkkUg87q~R0hHqVL0dYkPKxzSeP2QU80CZNTdPSa3drktHbMBCKOCDo1leA0M97hVkwLfr19S0Lsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal