Abstract
Despite numerous studies investigating the action of c-mpl ligand, no reports have defined the in vivo changes in megakaryocytopoiesis in response to a single injection of this cytokine. Here we compare the kinetics of the megakaryocytopoietic response in C57Bl/6J mice administered 25 μg/kg or 250 μg/kg of pegylated (PEG) murine megakaryocyte growth and development factor (MGDF ) as a single intravenous injection. Megakaryocytes of mice treated with MGDF had normal ultrastructure, showing a typical distribution of the demarcation membrane system, α-granules, and other cytoplasmic organelles. Megakaryocyte ploidy, size, and frequency were markedly increased with both MGDF doses. Megakaryocyte ploidy was maximally increased from a modal value of 16N to 64N on day 3, with both doses of MGDF. Similarly, a comparable increase in megakaryocyte size occurred in the two MGDF groups. Increased megakaryocyte size was coupled to the increase in megakaryocyte ploidy, and no evidence for independent regulation of megakaryocyte size within individual ploidy classes was apparent. In contrast to megakaryocyte ploidy and size, the increase in megakaryocyte frequency was markedly different with the two doses of MGDF. The proportion of 2N and 4N cells was increased from a baseline of 0.035% to 0.430% by day 4 in mice treated with the higher dose of MGDF, but only to 0.175% in mice administered 25 μg/kg of MGDF. The marked increase in the pool of these immature megakaryocytes translated to a sustained elevation in the frequency of polyploid megakaryocytes (8N cells and greater). In contrast to the sustained increase in the frequency of polyploid cells, the level of polyploidization was downregulated on days 6 to 10, but normalized by day 14. We conclude that a single injection of MGDF is able to expand the megakaryocytic pool in a dose-dependent manner, which, with subsequent maturation, should lead to an increased rate of platelet production.
THE RECENT purification and cloning of the c-mpl ligand1 or thrombopoietin (TPO),2-4 and the production of recombinant forms such as megakaryocyte growth and development factor (MGDF ),5 have provided the opportunity to unambiguously define the regulatory effects of this cytokine on megakaryocytopoiesis. Reports on this cytokine, have described its ability to cause a profound increase in circulating platelet levels in mice,1,2,6 rats,4 and nonhuman primates,7,8 presumably as a result of expansion of the megakaryocyte progenitor population7,9-12 and enhanced megakaryocyte maturation.4,8,10 12
In vitro studies have shown that c-mpl ligand is capable of inducing megakaryocyte colony formation and augmenting megakaryocyte maturation. c-mpl ligand has been shown to specifically induce colony-forming unit-megakaryocyte (CFU-Meg) formation from unfractionated murine bone marrow (BM) cells,10,13 murine fetal liver cells,14 and from human peripheral blood (PB) or BM CD34+ cells.11,12,15-17 Addition of c-mpl ligand to human megakaryocytes in culture can increase platelet-specific antigens,1,5 increase megakaryocyte polyploidy,12,15 and allow immature megakaryocytes to develop into morphologically normal, mature megakaryocytes capable of forming proplatelet processes.18 However, the actual process of proplatelet formation appears to be inhibited by the presence of c-mpl ligand in the culture medium.19
In vivo, daily administration of c-mpl ligand to mice caused an increase in de novo platelet synthesis, as determined by 35S incorporation into platelets,1 and resulted in the circulating platelet count being increased to three to four times the baseline values.2,20 In nonhuman primates, daily administration of MGDF raised the platelet count threefold within 5 to 7 days7,8 as a result of increases in megakaryocyte progenitors,7 megakaryocyte numbers, volume, and ploidy.8
In various models of myelosuppression, daily administration of MGDF or thrombopoietin is able to enhance recovery from thrombocytopenia6,20 and reduce mortality.6 The ability of thrombopoietin to enhance platelet recovery in myeloablative models has varied. Some studies report an accelerated recovery of platelet numbers in animals administered thrombopoietin after BM or PB progenitor cell transplantation,21,22 whereas others have not observed this effect.23,24 If, however, lethally irradiated recipients were transplanted with marrow collected from animals pretreated with thrombopoietin, the thrombocytopenia in the transplanted animals was less marked and shorter in duration.23 Similarly, irradiated mice receiving PB progenitor cells (PBPC) mobilized by pegylated MGDF22 were thrombocytopenic for fewer days, compared with recipients of unmobilized PBPC.
In all but one study,20 MGDF or thrombopoietin has been administered on a daily injection schedule, ranging in duration from 2 to 10 days,2,4,6,7,20 to 4 weeks.8 The choice of a daily injection schedule has been based on the use of other hematopoietic cytokines25-27 rather than the pharmacokinetics of thrombopoietin. More importantly, no detailed analysis of the in vivo kinetics of megakaryocytopoiesis in response to a single administrative dose has been reported. To address this issue and with the rationale that an understanding of megakaryocyte kinetics would aid the design of a clinically effective administration schedule, we have defined the megakaryocytic response in C57Bl/6J mice administered a single intravenous injection of pegylated recombinant murine MGDF (PEG-rmMGDF ).
MATERIALS AND METHODS
Young adult C57Bl/6J male mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and ranged between 10 and 20 weeks of age at the time of study.
PEG-rmMGDF was kindly supplied by Amgen Inc (Thousand Oaks, CA). The stock solution of PEG-rmMGDF (usually 0.63 mg/mL) was diluted with 1% normal (homologous) mouse serum (NMS) in phosphate-buffered saline (PBS) on the day of administration. A standard 2.5 μg/mL and 25 μg/mL solution of rmMGDF was made for injection at the 25-μg/kg and 250-μg/kg doses, respectively. Each mouse received 0.1 mL per 10 g body weight, whereas control mice received an equivalent volume of 1% NMS. MGDF or carrier were administered intravenously via the lateral tail vein on day 0, after inducing vasodilation by warming the mice for 10 to 20 minutes under an examination lamp.
Flow cytometric analysis of megakaryocytes.Mice were anesthetized with methoxyflurane and killed by exsanguination at frequent intervals from 12 hours to 14 days after MGDF administration. Marrow from one femur and one tibia was flushed into 2 mL of CATCH medium28 containing 8 μmol/L prostaglandin-E1 (PGE1 ) (Sigma, St Louis, MO). Marrow cells were gently resuspended with a plastic, standard-bulb transfer pipette (Fisher, St Louis, MO) and kept on ice. Marrow suspensions were filtered through 105-μm monofilament nylon mesh (Small Parts Inc, Miami, FL) to remove cell clumps and debris. The cells were pelleted at 400g for 5 minutes at room temperature (RT), and then resuspended in 1 mL of CATCH diluted 1:1 with PBS containing 5% normal goat serum (NGS) (GIBCO-BRL, Life Technologies Inc, Grand Island, NY). NGS was used to block nonspecific binding of goat-derived secondary antibody.
Megakaryocytes were labeled for 60 minutes on ice with a saturating concentration of a rat monoclonal antibody to mouse platelets (4A5), and shown to have a high specificity for murine megakaryocytes29 (kindly provided by Dr S. Burstein, University of Oklahoma, Oklahoma City). Cells were pelleted at 400g for 5 minutes at RT and resuspended in 1 mL of CATCH diluted 1:1 with PBS containing 5% NGS. The megakaryocyte-bound 4A5 antibody was indirectly fluoresceinated with fluorescein isothiocyanate (FITC)-goat anti-rat IgG F(ab′)2 (Biosource, Camarillo, CA) for 30 minutes on ice. Marrow cells labelled with only FITC-goat anti-rat IgG F(ab′)2 served as the negative control. In MGDF-treated animals, an increased concentration of 4A5 and FITC-labeled second antibody was added to the samples when megakaryocyte frequency and size were increased to ensure saturation of 4A5 binding sites, as monitored by the level of green fluorescence.
After labeling with primary and secondary antibody, the cells were pelleted by centrifugation at 400g for 5 minutes at RT, and resuspended in 2 mL of hypotonic propidium iodide (50 μg/mL in 0.1% sodium citrate).30 Before flow cytometric analysis, the cells were treated with RNAase (50 μg/mL, bovine pancreas; Calibiochem Corp, San Diego, CA), and filtered through 105-μm nylon mesh.
DNA content of 4A5-positive cells was analyzed by two-color flow cytometry31 on a FACScan (Becton-Dickinson, San Jose, CA). DNA contents of all 4A5-positive cells were measured (DNA contents 2N-128N). 4A5 positivity was defined by setting the horizontal (FL-1, green fluorescence) window at a level to minimize 6N events. We have previously shown31 that most all 6N events are aggregates of other marrow cells and thus serve as an internal negative control for the green window selection. Marrow cells from control animals were always used to set this gate and the same gate used to define 4A5-positive cells in marrow from MGDF-treated animals. This level of green fluorescence was associated specifically with 4A5-binding, as marrow labeled with FITC-second antibody alone showed few if any cells in the defined 4A5-positive window. The proportion of cells in each ploidy class was determined by integrating the number of cells under each DNA peak. Megakaryocyte frequency was calculated from the flow cytometric profiles as the percentage of cells expressing 4A5. Cell size was indexed by forward-angle light-scatter (FSC) and the mean megakaryocyte size of polyploid cells was determined from the two-color profile.
Collection and preparation of BM for transmission electron microscopy.Mice were killed 4 days after administration of 25 μg/kg, 250 μg/kg rmMGDF, or 1% NMS. BM was gently flushed from the tibia of each mouse into 2 mL of fixative composed of 1.5% glutaraldehyde + 1.5% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4. Two-percent dimethyl sulfoxide (DMSO) was added, and samples promptly microwaved for 10 seconds and fixed overnight at 4°C. The marrow was rinsed in 0.1 mol/L sodium cacodylate buffer, pH 7.4, and then postfixed in 2% osmium tetroxide in 0.1 mol/L sodium cacodylate buffer for 1.5 hours. The samples were dehydrated through a series of graded ethanol, infiltrated with toluene and subsequently with resin composed of DDSA, NMA, and DMP-30 (Ted Pella, Redding, CA) and LX-112 (Ladd Research Industries, Burlington, VT), and finally embedded in 100% of the above resin. Thin sections were cut and stained with uranyl acetate and lead citrate and viewed with a Philips 301 transmission electron microscope (Philips Electronic Instruments, Inc, Mahwah, NJ).
Statistical analyses.Statistical comparison of megakaryocyte ploidy distributions is complex and previous studies performed on megakaryocyte ploidy have either neglected to report statistics, compared the proportion of cells in each class using univariate two-sample t32 or Mann-Whitney rank sum33-38 test, or used a summary statistic of all ploidy classes, such as the geometric mean,39 again compared using univariate test procedures.4,40,41 However, these approaches do not consider that the proportion of cells in each of the DNA classes are interrelated. A more appropriate procedure, and one we have implemented in our studies, is the Hotelling's T2 test,42 which is a multivariate test procedure for comparing two population mean vectors and takes into account the underlying covariance structure. This allowed us to analyze megakaryocyte ploidy by comparing the distribution (all DNA classes simultaneously) in the control group and the distribution at each of the time points for each MGDF dosage. Hotelling's T2 test is appropriate if the underlying distribution function for the two populations can be assumed to be multivariate normal. However, due to the relatively small sample sizes of our data, we did not wish to make this assumption, and therefore used a Permutation test43 of Hotelling's T2 to evaluate the differences in the ploidy distributions. The following is an example of how the data were analyzed using the permutation/Hotelling's T2 test. For comparison of the ploidy distributions for the control group (13 mice) and the group of mice killed 12 hours after the administration of 25 μg/kg of rmMGDF (5 mice), we first obtained the value of Hotelling's T2 statistic for the original sample and denoted it by T20. The Hotelling's T2 statistic was then calculated for all possible samples that could be obtained by allocating 13 of the 18 mice into the control group and the remainder to the treatment group. The total number of permutations for this Hotelling's T2 test was 8,568. The P value was obtained by counting the number of times we observed a value of T2 greater than T20 and dividing it by the total number of permutations (8,568). When the total number of permutations was less than 17,000 for a particular comparison, then all the possible permutations were used in obtaining the P value (a permutation test by exact methods). If not, a random sample (Monte Carlo modication) of 17,000 permutations was chosen to obtain the P value. With 17,000 permutations, we were assured that the estimated P value would be within a 1% margin of error of the true P value with 99% confidence.44 A program was written in MINITAB45 (MINITAB, Inc, PWS-KENT Publishing Co, Boston, MA) to calculate Hotelling's T2 and implement the permutation test. Because 13 multiple comparisons were made (comparing ploidy distribution at each time point to the control), a Bonferroni correction46 was used to maintain the overall experimental type I error level of α = .05 (a 95% confidence limit of detecting a real difference when there is one). Therefore, in each comparison the observed P value had to be less than .004 (.05/13) to be judged significant. Type I error is the probability of rejecting the null hypothesis and concluding that there is a significant difference between the two groups when in fact there is none.
The effect of MGDF treatment with time on megakaryocyte frequency (of both 2N/4N and 8N-128N cells) and size in mice treated with 25 μg/kg and 250 μg/kg rmMGDF was analyzed using the Kruskal-Wallis test,47 a nonparametric test procedure. Once an effect of rmMGDF on a given megakaryocyte parameter was detected, a posthoc analysis comparing the control group to all other (13) time points was done using Wilcoxon-Mann-Whitney rank sum test.47 Because of the small sample sizes, the permutation test as implemented in the statistical software package STATXACT48 (CYTEL Software Corp, Cambridge, MA) was used. When the total number of permutations was large, the Monte Carlo estimate of the P value was based on 10,000 permutations. With 10,000 permutations, we can be 99% confident that the estimated P value will be within 1.3% margin of error of the true P value. Once again the Bonferroni correction was used so that the posthoc comparisons were performed at level where the P value had to be less than .004 (.05/13) to be significant.
All results are graphed showing the arithmetic mean ± standard error of the mean (SEM), although most of the statistical procedures used are nonparametric in nature and involve comparison of medians rather than means.
RESULTS
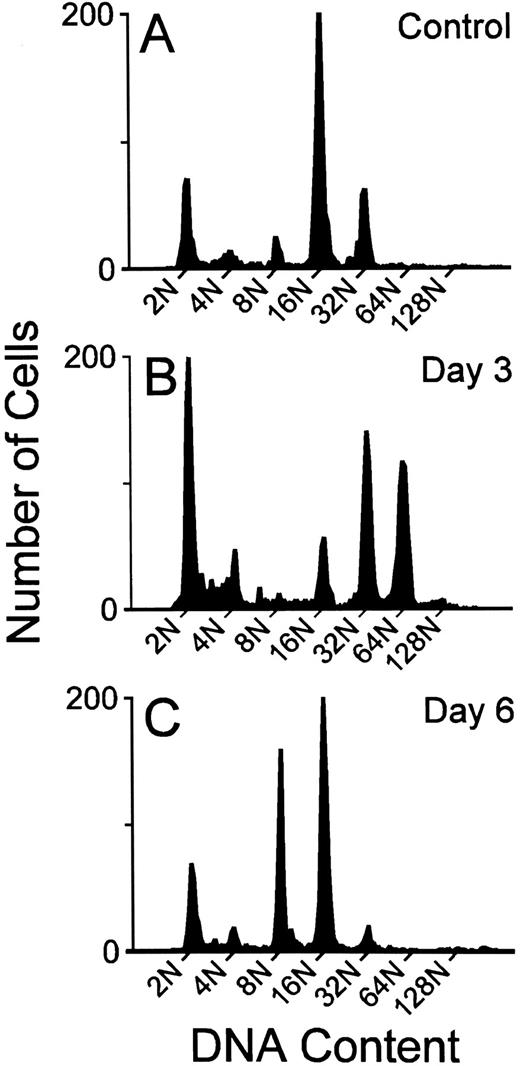
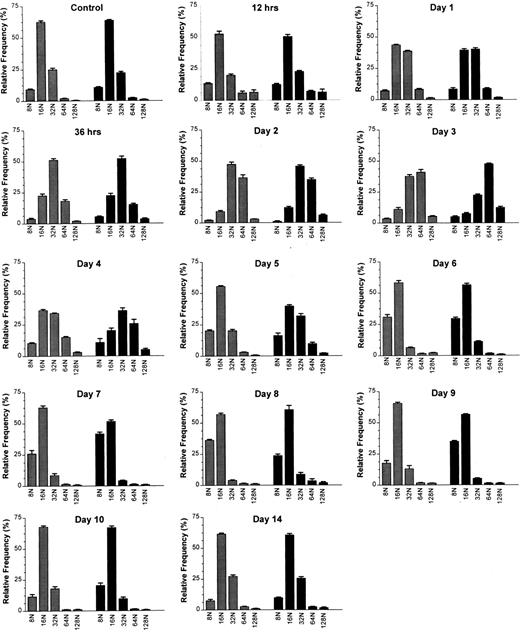
Megakaryocyte ploidy.The DNA ploidy classes were well resolved by the flow cytometric method at all time points after MGDF administration. This is illustrated in Fig 1, where representative DNA content distributions are shown for the two extremes of response (maximal stimulation and downregulation) to MGDF. Mean histograms derived from the results of individual flow cytometric profiles of DNA content are presented in Fig 2.
Representative megakaryocyte DNA distributions from the BM of individual mice given 1% mouse serum (A), or treated with 25 μg/kg rmMGDF and killed on day 3 (B) or day 6 (C).
Representative megakaryocyte DNA distributions from the BM of individual mice given 1% mouse serum (A), or treated with 25 μg/kg rmMGDF and killed on day 3 (B) or day 6 (C).
Effects of a single intravenous injection of rmMGDF on megakaryocyte ploidy distributions in mice treated with 25 μg/kg () or 250 μg/kg (▪) MGDF. Megakaryocyte ploidy distributions were significantly different from the control distribution from 12 hours to day 9 in mice treated with 25 μg/kg of MGDF, and from 12 hours to day 10 in mice treated with 250 μg/kg of MGDF. From 387 to 1,136 (median 871) 4A5 positive cells with DNA contents ≥ 8N were analyzed per mouse. Each distribution shows the mean ± SEM of each megakaryocyte ploidy class; for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls; for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Controls received 0.1 mL/10 g body weight of 1% NMS in PBS.
Effects of a single intravenous injection of rmMGDF on megakaryocyte ploidy distributions in mice treated with 25 μg/kg () or 250 μg/kg (▪) MGDF. Megakaryocyte ploidy distributions were significantly different from the control distribution from 12 hours to day 9 in mice treated with 25 μg/kg of MGDF, and from 12 hours to day 10 in mice treated with 250 μg/kg of MGDF. From 387 to 1,136 (median 871) 4A5 positive cells with DNA contents ≥ 8N were analyzed per mouse. Each distribution shows the mean ± SEM of each megakaryocyte ploidy class; for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls; for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Controls received 0.1 mL/10 g body weight of 1% NMS in PBS.
The modal polyploid DNA class for control C57Bl/6J mouse megakaryocytes was 16N (Fig 2); approximately two thirds of the polyploid megakaryocyte population were 16N, with 10% 8N, and 20% 32N. Less than 3% of cells had a DNA content of 64N or greater (Fig 2). A significantly altered polyploid distribution was observed from 12 hours until day 9 (25 μg/kg) or day 10 (250 μg/kg), following administration of a single injection of MGDF. An increase in megakaryocyte ploidy was detected as early as 12 hours, and the response was evident with both doses of MGDF (P ≤ .002 for both doses, based on permutation/Hotelling T2 test) (Fig 2). The combined relative frequency of 64N and 128N cells at 12 hours had increased from 3.0% to 12.0% (both doses), with a corresponding decrease of 16N cells, whereas the 32N frequency remained relatively unchanged. By 24 hours, the modal ploidy class was increased to 32N in mice administered 250 μg/kg of MGDF. 16N remained modal with the lower dose of MGDF; however, the relative frequency of 32N cells was approximately doubled from control values. By 36 hours, the modal megakaryocyte ploidy class had increased to 32N, in mice treated with either dose of MGDF, when the 32N cells represented over 50% of the polyploid population. The 64N and 128N cells comprised 20%, and the 8N and 16N cells less than 30%. The ploidy of the megakaryocytes continued to increase over the next 36 hours, and peaked at day 3, with 64N as the modal ploidy class at both MGDF doses. The relative frequency of 64N cells was slightly higher for the 250-μg/kg dose (48%) than for the 25-μg/kg dose (41%) and the frequency of 128N cells with the higher MGDF dose was double (13%) that of the lower dose (5%). By day 4, megakaryocyte ploidy began to shift back toward normal, where 32N was the modal ploidy with the 250-μg/kg dose and 16N was the modal for the 25-μg/kg dose. By day 5, the modal ploidy class had returned to 16N in mice treated with either dose of MGDF; however, the ploidy distribution remained significantly different from control (P < .0001 for both doses, permutation/Hotelling T2 test) due to the notable increase in the relative frequency of 8N cells. On day 6, in mice administered 25 μg/kg of MGDF, 8N cells were three times, whereas 32N cells were one fifth, the relative frequency found in control BM (P < .0001, permutation/Hotelling T2 test). On day 7, in mice administered 250 μg/kg of MGDF, 8N cells were four times and 32N cells were one fifth the relative frequency found in control marrow (P < .0001, permutation/Hotelling T2 test). 16N cells in both cases were only marginally below control values. These data suggest that megakaryocyte endomitosis was less stimulated in these mice and the majority of cells were not able to undergo more than three endomitotic cycles. By day 9, ploidy began to normalize in mice treated with 25 μg/kg of MGDF, and by day 10 the relative frequency in each ploidy class was no different from that of megakaryocytes from control BM (P = .006, permutation/Hotelling T2 test). Megakaryocyte ploidy of mice receiving the higher MGDF dose showed a similar pattern, but normalization of the ploidy distribution did not occur until day 14 (P = .408, permutation/Hotelling T2 test).
Megakaryocyte frequency.We chose to separate the megakaryocytes into two populations for frequency analysis: those with a presumed capacity for cell division, cells of 2N/4N DNA content, and those cells that had ceased dividing and were undergoing or had completed polyploidization (8N, 16N, 32N, 64N, and 128N cells). We realize that this division of cells has its limitations and is unable to discriminate the 2N cells not in cell cycle, or the 4N population that is truly polyploid, but it provides an estimate of the proliferating (2N/4N cells) versus the nonproliferating (8N-128N) megakaryocyte population.
Previous morphometric studies have estimated the frequency of morphologic recognizable megakaryocytes in normal BM to be 0.05%.36 Our current study, using flow cytometric analysis, estimated that the frequency of 4A5-positive polyploid cells (presumably the morphologically recognizable megakaryocytes) to be approximately 0.08%. The combined frequency of 4A5-positive 2N and 4N cells was approximately 0.035%.
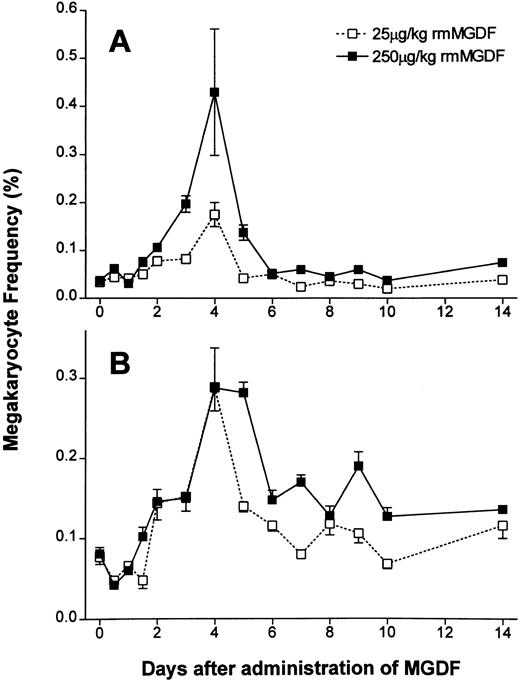
Administration of either 25 μg/kg or 250 μg/kg of rmMGDF had a significant effect on the frequency of 2N/4N cells (P < .0001, Kruskal-Wallis, both doses) (Fig 3A). The frequency of the 2N/4N cells in mice administered 25 μg/kg of MGDF remained constant for 36 hours after cytokine administration (Fig 3A). At day 2, the frequency of 2N/4N cells started to increase (P = .001, Mann-Whitney); by day 4, the 2N/4N cell frequency had peaked at 0.175%, a fivefold increase from baseline values (P < .0001, Mann-Whitney). By day 5, 2N/4N frequency promptly returned to baseline values (P = .13, Mann-Whitney) and remained not significantly different from controls through to day 14. Mice administered 250 μg/kg of MGDF showed similar kinetics in the 2N/4N frequency. The first increase was again detected on day 2 (P < .0001, Mann-Whitney), and the frequency increased to a maximum of 0.430% on day 4 (P < .0001, Mann-Whitney), representing a 10-fold increase over control values, or twice the increase observed with the 25-μg/kg dose. 2N/4N frequency sharply decreased to 0.125% on day 5, but in contrast to mice treated with the lower dose of MGDF, 2N/4N frequency was still significantly increased from baseline (P < .0001, Mann-Whitney). By day 6, 2N/4N frequency had normalized (P = .11, Mann-Whitney), and remained normal through to day 14.
Effect of a single intravenous injection of rmMGDF on the frequency of (A) 2N-4N megakaryocytes and (B) 8N-128N polyploid megakaryocytes. The frequency of 2N/4N cells was significantly increased from days 2 to 4 in mice treated with 25 μg/kg of MGDF and from days 2 to 5 in mice treated with 250 μg/kg ofMGDF (A). The frequency of 8N-128N cells was significantly increased from days 3 to 5 in mice treated with 25 μg/kg MGDF and from days 2 to 14 in mice treated with 250 μg/kg MGDF (B). At 12 hours, the frequency of 8N-128N cells was significantly decreased with the 250-μg/kg dose of MGDF (B). Each data point represents the mean ± SEM of megakaryocyte frequency; for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls and for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
Effect of a single intravenous injection of rmMGDF on the frequency of (A) 2N-4N megakaryocytes and (B) 8N-128N polyploid megakaryocytes. The frequency of 2N/4N cells was significantly increased from days 2 to 4 in mice treated with 25 μg/kg of MGDF and from days 2 to 5 in mice treated with 250 μg/kg ofMGDF (A). The frequency of 8N-128N cells was significantly increased from days 3 to 5 in mice treated with 25 μg/kg MGDF and from days 2 to 14 in mice treated with 250 μg/kg MGDF (B). At 12 hours, the frequency of 8N-128N cells was significantly decreased with the 250-μg/kg dose of MGDF (B). Each data point represents the mean ± SEM of megakaryocyte frequency; for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls and for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
Administration of either 25 μg/kg or 250 μg/kg rmMGDF also had a significant effect on the frequency of polyploid megakaryocytes (P < .0001, Kruskal-Wallis, both doses) (Fig 3B). The frequency of polyploid megakaryocytes, 12 hours after administration of either 25 or 250 μg/kg of MGDF, appeared to decrease, but this decrease was only found to be significant with the 250 μg/kg dose (P = .002 for 250 μg/kg; P = .06 for 25 μg/kg, Mann-Whitney). By day 3, the frequency of polyploid megakaryocytes was twofold of normal in mice treated with 25 μg/kg of MGDF (P = .003, Mann-Whitney), and continued to increase, reaching a maximal frequency of 0.289% (fourfold of normal) on day 4 (P < .0001, Mann-Whitney). After this time, the frequency of polyploid megakaryocytes decreased, and although still twofold of normal by day 5 (P = .003, Mann-Whitney), it was not significantly different from control values from day 6 onward. In contrast, mice treated with 250 μg/kg MGDF had an earlier and sustained increase in the frequency of polyploid megakaryocytes. By day 2, the frequency of polyploid megakaryocytes was increased (P = .0009, Mann-Whitney) and continued to rise to a maxima of 0.288% (fourfold of normal) on day 4 (P < .0001, Mann-Whitney). On day 5, the frequency of polyploid megakaryocytes in mice treated with 250 μg/kg of MGDF remained at fourfold of control (P < .0001, Mann-Whitney), decreased to twofold of normal by day 6 (P = .002, Mann-Whitney), and was sustained at a significantly elevated level through to day 14 (P ≤ .003, Mann-Whitney).
Megakaryocyte size.Flow cytometric measurement of FSC is an index of cell size. In our studies, we analyzed the average FSC of polyploid megakaryocytes to assess the effect of MGDF on megakaryocyte size. The megakaryocytes sized were the same cells analyzed for ploidy and frequency.
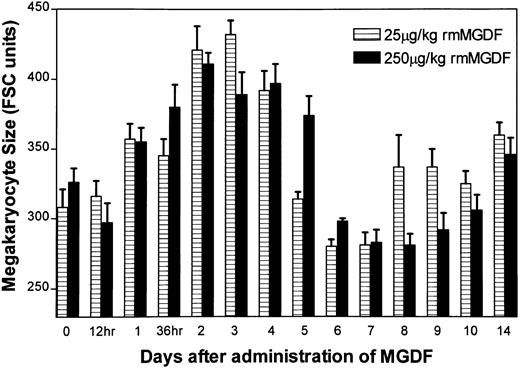
Administration of either 25 μg/kg or 250 μg/kg rmMGDF significantly altered mean megakaryocyte size of 8N-128N cells (P < .0001, Kruskal-Wallis, both doses) (Fig 4), with similar changes observed in both treatment groups. No change in mean megakaryocyte size was apparent until day 2, when average size increased to approximately 130% of normal with both MGDF doses (P = .001, 25 μg/kg; P = .0004, 250 μg/kg, Mann-Whitney). Mean megakaryocyte size remained elevated on day 3 (P = .001, 25 μg/kg; P = .003, 250 μg/kg, Mann-Whitney), but by day 5 megakaryocyte size had returned to control values. Although average sizes tended to be smaller than control values on days 6-7 in mice treated with 25 μg/kg MGDF, and on days 6 through 9 in mice treated with 250 μg/kg MGDF (P ≥ .02, Mann-Whitney, both doses), these values were associated with a P value > .004, which we selected for our significance level.
Effect of a single intravenous injection of rmMGDF on average size of polyploid megakaryocytes. The mean megakaryocyte size of 8N-128N cells was significantly increased from days 2 to 4 in mice treated with 25 μg/kg of MGDF, and on days 2 and 3 in mice treated with 250 μg/kg of MGDF. Each data point represents the mean ± SEM of megakaryocyte size (8N-128N cells); for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls and for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
Effect of a single intravenous injection of rmMGDF on average size of polyploid megakaryocytes. The mean megakaryocyte size of 8N-128N cells was significantly increased from days 2 to 4 in mice treated with 25 μg/kg of MGDF, and on days 2 and 3 in mice treated with 250 μg/kg of MGDF. Each data point represents the mean ± SEM of megakaryocyte size (8N-128N cells); for the 25-μg/kg dose, n = 4-10 mice per MGDF group and n = 13 for controls and for the 250-μg/kg dose, n = 5-9 mice per MGDF group and n = 20 for controls. Data from control mice were pooled to represent the day 0 data point.
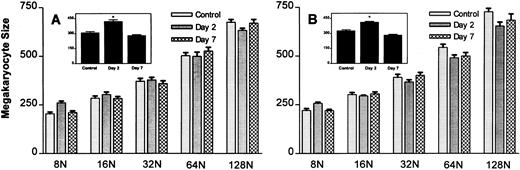
To determine if the increase in mean megakaryocyte size was related to the increase in megakaryocyte ploidy, megakaryocyte size was measured within each ploidy group (Fig 5). As shown, the average size of megakaryocytes increased, with increasing ploidy (Fig 5A and B). Although mean megakaryocyte size was elevated on day 2 with both doses of MGDF (see inset of Fig 5A and B), there was no change in the size of the megakaryocytes within each ploidy group; rather, the increase in mean megakaryocyte size observed on day 2 is a reflection of the modal ploidy shift from 16N to 32N observed with both doses of MGDF (Fig 2). As the modal ploidy returned to 16N on day 7, mean megakaryocyte size also returned to control values.
Effect of rmMGDF on megakaryocyte size within individual ploidy classes in mice treated with 25-μg/kg (A) or 250-μg/kg (B) dose. MGDF causes a significant increase in mean megakaryocyte size (see inset) on day 2, with injection of 25 μg/kg (P = .001, Mann-Whitney) or 250 μg/kg (P = .0004, Mann-Whitney) MGDF, with little change in the size of megakaryocytes within individual ploidy classes. The increase in mean megakaryocyte size results from an increase in the relative frequency of large polyploid cells. On day 2, 32N is the modal DNA class, while by day 7 16N is once again the normal modal class. Each data point represents the mean ± SEM of megakaryocyte size. For the 25-μg/kg dose, n = 13 for the control and n = 5 on days 2 and 7. For the 250-μg/kg dose, n = 20 for the control, and n = 5 on day 2 and n = 8 on day 7.
Effect of rmMGDF on megakaryocyte size within individual ploidy classes in mice treated with 25-μg/kg (A) or 250-μg/kg (B) dose. MGDF causes a significant increase in mean megakaryocyte size (see inset) on day 2, with injection of 25 μg/kg (P = .001, Mann-Whitney) or 250 μg/kg (P = .0004, Mann-Whitney) MGDF, with little change in the size of megakaryocytes within individual ploidy classes. The increase in mean megakaryocyte size results from an increase in the relative frequency of large polyploid cells. On day 2, 32N is the modal DNA class, while by day 7 16N is once again the normal modal class. Each data point represents the mean ± SEM of megakaryocyte size. For the 25-μg/kg dose, n = 13 for the control and n = 5 on days 2 and 7. For the 250-μg/kg dose, n = 20 for the control, and n = 5 on day 2 and n = 8 on day 7.
Megakaryocyte ultrastructure.Megakaryocyte ultrastructure was examined on day 4 (Fig 6), at the peak of the increased frequency of polyploid megakaryocytes (Fig 3B). BM from mice administered 1% NMS (Fig 6a), 25 μg/kg MGDF (not shown), or 250 μg/kg MGDF (Fig 6b) was examined by transmission electron microscopy. The ultrastructure of the megakaryocyte cytoplasm was normal in both groups of MGDF-treated mice. Although the increase in megakaryocyte size of MGDF-treated animals was apparent on day 4 (Fig 6b), the cytoplasmic distribution of the demarcation membrane system (DMS), α-granules, and other organelles was similar to that of the NMS-treated animals (Fig 6a).
Transmission electron micrographs of BM megakaryocytes from a mouse injected with 1% NMS (a) and a mouse injected with 250 μg/kg rmMGDF (b). Although considerably larger in size than cells from control animals, the megakaryocytes from MGDF-treated animals contain normally distributed organelles within their cytoplasm, including the demarcation membrane system. Nu, nucleus; dms, demarcation membrane system. Original magnification × 4,000.
Transmission electron micrographs of BM megakaryocytes from a mouse injected with 1% NMS (a) and a mouse injected with 250 μg/kg rmMGDF (b). Although considerably larger in size than cells from control animals, the megakaryocytes from MGDF-treated animals contain normally distributed organelles within their cytoplasm, including the demarcation membrane system. Nu, nucleus; dms, demarcation membrane system. Original magnification × 4,000.
DISCUSSION
Preclinical studies6-8,20 and early data from phase I trials49-51 clearly indicate that recombinant thrombopoietin will prove useful in the clinic to ameliorate thrombocytopenia, especially that associated with chemotherapy.6,20,52 In our preliminary experiments53 we noted that a single intravenous injection of PEG-rmMGDF was just as effective at elevating the platelet count threefold to fivefold of normal, as the multiple injection schedules used in these preclinical studies.6-8 Therefore, the aim of the present study was to determine the mechanism by which c-mpl ligand alters megakaryocytopoiesis to induce an increase in platelet production. To achieve this aim, we analyzed BM frequency of both the megakaryocyte precursor and the polyploid population, megakaryocyte DNA content, and megakaryocyte size at frequent intervals for 2 weeks after a single injection of MGDF to mice.
Our results show that a single injection of PEG-rmMGDF (25 μg/kg or 250 μg/kg) is able to substantially elevate megakaryocyte frequency and cause a marked shift in modal DNA content, which is accompanied by an increase in mean megakaryocyte size. The implication of these findings is that MGDF is capable of influencing both megakaryocytic proliferation and differentiation, a property that the classically defined thrombopoietin was thought to lack.54 55
Our data clearly indicate that MGDF acts on a proliferative megakaryocytic 2N precursor, and the degree of expansion of this compartment is dose dependent. Although 25 μg/kg of MGDF was able to expand the 2N/4N population fivefold, the higher dose of MGDF increased this population to 10-fold of normal. The greater expansion observed with 250 μg/kg of MGDF translated to a sustained increase in the frequency of polyploid megakaryocytes from days 3 to 14. In contrast, increased frequency of polyploid megakaryocytes was of only 3 days duration with the 25 μg/kg dose (days 3-5).
The exact nature of the 2N precursor population stimulated by MGDF in our studies is unknown. The antigen to which the 4A5 antibody is directed has been characterized as a thrombin-sensitive 74-kD glycoprotein present on murine megakaryocytes and platelets.56 Whether this antibody is capable of binding and labeling the CFU-Meg, or is restricted to only the transitional small acetylcholinesterase positive (SACHE+) cells,57 has not been determined. Our studies indicate that 48 hours were required to see an increase in the frequency of the 2N population, and the timing was independent of the dose given. However, the 2N population must have a short transition to the endomitotic compartment, as the increase in frequency of polyploid cells was observed only 24 hours later, on day 3. Therefore, the data suggest that a relatively late megakaryocyte progenitor (CFU-Meg) and the population of SACHE+ cells capable of cell division57 are a target for MGDF. Similar conclusions were reached from in vitro studies using isolated human marrow progenitors,15,58 showing that a subpopulation of megakaryocyte progenitors (CD34+, CD41+ cells) expressed the receptor, c-mpl,58 and may be the main target cells for thrombopoietin.15 These cells were shown to have limited capacity for cell division, usually generating colonies of small numbers of megakaryocytes.15 Furthermore, mice genetically engineered to be deficient in the thrombopoietin receptor (c-mpl−/−), showed a greater deficiency in the more mature committed CFU-Meg of limited proliferative potential than in less mature megakaryocyte progenitors.59 All these data suggest that relatively late megakaryocyte precursors are acutely responsive to changes in thrombopoietin levels and may play a pivotal role in accelerating megakaryocytopoiesis when there is an increased platelet demand.
A second major effect of MGDF is the profound stimulation of megakaryocyte endomitosis. The kinetics, although perhaps not the magnitude of stimulation, were similar to that observed in animals made acutely thrombocytopenic.60,61 As early as 12 hours after MGDF administration, a marked increase in the proportion of 64N and 128N cells was seen. Interestingly, the relative frequency of 32N cells, remained essentially unchanged. The increase in frequency of 64N and 128N cells must have resulted from stimulation of lower ploidy megakaryocytes (8N, 16N, 32N) to undergo a least one to two extra rounds of endomitosis within 12 hours. Given the steady-state cell-cycle time for rodent megakaryocytes of 10 hours,62 the data suggest that MGDF was able to shorten the cell cycle time for endomitotic megakaryocytes. An accelerated rate of megakaryocyte maturation has been reported in animals recovering from acute thrombocytopenia, where endogenous levels of thrombopoietin would be expected to be high.63 The maximal shift in megakaryocyte ploidy was observed at 3 days, where 64N was the modal polyploid class and nearly 50% of all megakaryocytes had a ploidy value of 64N or greater, compared with only 3% in control marrow.
Accompanying the increase in megakaryocyte ploidy was an increase in mean megakaryocyte size. There was no evidence of any increase in megakaryocyte size within a specific ploidy class. Rather, the increase in frequency of high ploidy megakaryocytes (which were always larger in size) produced the increase in mean megakaryocyte size. Mean megakaryocyte size was increased on days 2 and 3, when the modal megakaryocyte ploidy was either 32N or 64N. By day 5, when the modal ploidy had returned to the normal value of 16N, megakaryocyte size also returned to control values.
Our electron microscopic analysis of megakaryocyte morphology showed that MGDF promoted the development of ultrastructurally normal and mature megakaryocytes. We did not observe the maturational defects reported for megakaryocytes generated in culture with thrombopoietin.15 The most likely explanation for this aberrant megakaryocyte morphology in vitro is an insufficient microenvironment. Variation in exposure and clearance of MGDF may also contribute to the morphologic differences in megakaryocytes observed in vitro versus in vivo.
It has long been hypothesized that the number of platelets in circulation exercise feedback control on megakaryocytopoiesis, including regulation of megakaryocyte DNA content and size.60,61,64-66 Our data show that polyploidization was reduced between days 6 and 9 with 25 μg/kg of MGDF and days 6 and 10 with 250 μg/kg of MGDF. In studies reported elsewhere, we showed that a single injection of 25 μg/kg or 250 μg/kg MGDF raised the platelet count to threefold or fivefold on day 5.53 Therefore, the longer duration of lowered ploidy in mice treated with 250 μg/kg of MGDF was associated with the more pronounced thrombocytosis, and could be interpreted as the role of platelets in this regulatory process. However, at the same time, megakaryocyte number was also higher in mice treated with 250 μg/kg MGDF and increased megakaryocyte frequency may alternatively downregulate polyploidy formation.
A mechanism whereby circulating platelets may affect feedback regulation of megakaryocytopoiesis recently has been proposed.4,41,67 In vivo production of thrombopoietin by the liver (primary site) and the kidney (secondarily) is hypothesized to be constitutive. In this proposed model, changes in the endogenous level of circulating thrombopoietin reflect the degree of binding of thrombopoietin to platelets.41 Megakaryocytes also bind thrombopoietin,68 and therefore megakaryocyte mass also may be important in modulating the circulating thrombopoietin levels.69 Elevation of either platelet or megakaryocyte mass would be expected to decrease plasma thrombopoietin below the level necessary to sustain basal megakaryocyte differentiation. Therefore, the decrease in polyploidization we observed after the increase in megakaryocyte and platelet mass was most likely the result of lowered endogenous thrombopoietin levels in these mice. Support for this interpretation is provided by studies in mice deficient in thrombopoietin (TPO−/−), where the modal ploidy class is decreased to 8N and the megakaryocytes are smaller in size.70
In contrast to megakaryocyte maturation, no evidence for downregulation of megakaryocyte frequency is apparent after MGDF administration. This result is reminiscent of the response observed with 5-fluorouracil administration, where megakaryocyte ploidy and size were subject to different feedback regulatory mechanisms than proliferation.35 These findings imply that megakaryocyte maturation may be more dependent than megakaryocyte precursor proliferation on thrombopoietin.
In our studies we found that a 25-μg/kg dose of MGDF was sufficient to maximally stimulate megakaryocyte ploidization and size. In contrast, stimulation of megakaryocyte proliferation was dependent on the dose we administered; the higher dose of MGDF gave a greater expansion of the 2N/4N megakaryocytic population. One interpretation is that less thrombopoietin is needed to induce ploidization and increase cell size than to stimulate proliferation by megakaryocyte precursors. However, we also observed that when less circulating thrombopoietin was presumably available (because of the increase in megakaryocyte and platelet mass), ploidization, but not frequency, was reduced. This paradox may be explained by location of megakaryocytes versus their precursors in the BM, and their accessibility to thrombopoietin. Mature megakaryocytes are known to lie adjacent to the BM sinus endothelium71-73 and presumably have the most immediate access to circulating thrombopoietin. Thus, even at the lower dose of MGDF, megakaryocytes obviously had sufficient thrombopoietin binding to maximally stimulate ploidy and size. However, megakaryocyte precursors73 are located distal to the sinus endothelium, and the effect of injected MGDF may be limited by its rate of diffusion from the vascular sinusoid to their distal location. Hence, a higher dose of MGDF was needed to penetrate the marrow and induce the greater degree of precursor proliferation. Therefore, the parasinusoidal location of mature megakaryocytes72 makes them more susceptible to variations in circulating thrombopoietin levels than megakaryocyte precursors. Megakaryocyte number, unlike ploidy, did not decrease below control values on days 6 to 10 (Figs 2 and 3), when the thrombopoietin levels in the circulation were expected to be low. Because the megakaryocyte precursors are less reliant on circulating levels of thrombopoietin, they may be sustained by the low constitutive thrombopoietin production by marrow stromal cells.19 74
In summary, a single injection of PEG-rmMGDF significantly augments the proliferation of late megakaryocyte precursors and markedly stimulates megakaryocyte maturation. Although expansion of the proliferating 2N/4N cells appeared dependent on the dose of MGDF we administered, both 25-μg/kg and 250-μg/kg doses of MGDF were able to maximally enhance ploidization and cell size.
ACKNOWLEDGMENT
The authors acknowledge the technical expertise provided by Dr Richard Ashmun and Sam Lucas for flow cytometric analysis of megakaryocyte DNA distributions. We also thank Amgen Inc for generously supplying PEG-rmMGDF.
Supported in part by P30CA21765 Cancer Center Support Grant and P01CA201180 from the National Cancer Institute, Public Health Service, Department of Health and Human Services, and by American Lebanese Syrian Associated Charities.
Address reprint requests to Carl W. Jackson, PhD, Division of Experimental Hematology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal