To the Editor:
Although it has been accepted for decades that most cases of idiopathic thrombocytopenic purpura (ITP) are caused by autoantibodies against platelets,1 the clinical utility of serologic testing in ITP never has been established.2 A recent report by Brighton et al3 analyzed predictive characteristics of direct and indirect tests for platelet-associated IgG (PAIgG) and an antigen capture test (MAIPA) designed to detect autoantibodies specific for platelet glycoproteins IIb/IIIa or Ib/IX. It was concluded that the MAIPA was better than tests for PAIgG in discriminating immune from nonimmune thrombocytopenia.3
We recently analyzed the clinical utility in ITP of a commercially available serologic test for PAIgG, the Capture-P (Immucor, Inc, Norcross, GA). This is a solid-phase red blood cell adherence assay that is used to detect alloantibodies for platelet crossmatching and also has been reported to have high sensitivity and specificity in ITP.4 In this test, microtiter wells are coated with an agent that binds platelets from autologous (direct method) or allogeneic (indirect method) platelet-rich plasma. Adherent platelets create a substrate for capture of antiplatelet IgG from test plasma. Platelet-bound IgG is detected by subsequent adherence of indicator red blood cells coated with anti-human IgG.
A search of the University of Iowa Hospitals' medical record department computer data base retrieved 359 records that contained results of Capture-P tests performed between August 1989 and July 1994. Of these, 94 records that contained ICD-9 coded primary diagnoses of primary thrombocytopenia (287.3) or thrombocytopenia, unspecified (287.5), were reviewed. Patients were retrospectively classified into three groups: (1) ITP (platelet count <150,000/μL, normal or increased numbers of bone marrow megakaryocytes, and no other known cause of thrombocytopenia) (n = 46); (2) thrombocytopenia of other established cause (n = 15); or (3) thrombocytopenia of undetermined cause (incomplete data or criteria for ITP not met, and no other cause of thrombocytopenia established) (n = 33). Group 3 was excluded from analysis.
Predictive Characteristics of Serologic Tests for Platelet Autoantibodies
| Test . | Method . | Sensitivity . | Specificity . | Likelihood Ratio* . |
|---|---|---|---|---|
| Capture-P | Direct | 17/46 (37%) | 10/15 (67%) | 1.1 |
| Capture-P | Indirect | 20/46 (43%) | 9/15 (60%) | 1.1 |
| CELIA† | Direct | 60/81 (74%) | 12/46 (26%) | 1.0 |
| ELISA‡ | Indirect | 30/88 (34%) | 41/53 (77%) | 1.5 |
| MAIPAρ | Modified | 33/71 (47%) | 34/40 (85%) | 3.1 |
| Test . | Method . | Sensitivity . | Specificity . | Likelihood Ratio* . |
|---|---|---|---|---|
| Capture-P | Direct | 17/46 (37%) | 10/15 (67%) | 1.1 |
| Capture-P | Indirect | 20/46 (43%) | 9/15 (60%) | 1.1 |
| CELIA† | Direct | 60/81 (74%) | 12/46 (26%) | 1.0 |
| ELISA‡ | Indirect | 30/88 (34%) | 41/53 (77%) | 1.5 |
| MAIPAρ | Modified | 33/71 (47%) | 34/40 (85%) | 3.1 |
The sensitivity and specificity of the Capture-P for the clinical diagnosis of ITP were 37% and 67% for the direct method, and 43% and 60% for the indirect method (Table 1). The likelihood ratio (LR) was 1.1 for both the direct and indirect Capture-P (Table 1), which indicates that a positive test result does not meaningfully increase the a priori odds ratio for the clinical diagnosis of ITP. The predictive characteristics of the Capture-P were very similar to those of the direct and indirect tests for PAIgG reported by Brighton et al, which produced LR values of 1.0 and 1.5, respectively (Table 1). The performance of the MAIPA in the Brighton study was better, with an LR value of 3.1 (Table 1).
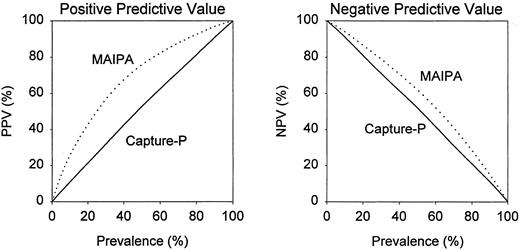
An instructive way to assess clinical utility of serological tests is to calculate positive and negative predictive values, which are dependent on the prevalence of a disease in the population to be tested. For the Capture-P, the positive and negative predictive values differed little from prevalence of ITP at any range (Fig 1), which indicates that the Capture-P has essentially no diagnostic predictive value in ITP. In the study of Brighton et al, the negative predictive values of the MAIPA differed only slightly from prevalence (Fig 1). The positive predictive values of the MAIPA differed more distinctly from prevalence, especially when the prevalence of ITP was between 20% and 60% (Fig 1).
Dependence of positive predictive value (PPV) and negative predictive value (NPV) on prevalence of ITP in the population to be tested. ( — ), Direct capture-P; (⋅⋅⋅⋅⋅), modified MAIPA from Brighton et al.3
Dependence of positive predictive value (PPV) and negative predictive value (NPV) on prevalence of ITP in the population to be tested. ( — ), Direct capture-P; (⋅⋅⋅⋅⋅), modified MAIPA from Brighton et al.3
This analysis suggests that the Capture-P, like other serologic tests for PAIgG,2 is not useful for establishing or excluding the diagnosis of ITP. Although tests for PAIgG differ in sensitivity and specificity, they all have low LR values and weak positive and negative predictive values, possibly because they detect IgG in platelet granules rather than true platelet autoantibodies.5 These findings are in keeping with the recent American Society of Hematology practice guideline, which concludes that tests for PAIgG are neither necessary nor appropriate in the evaluation of childhood or adult ITP.6
That better laboratory tests for the diagnosis of ITP are needed is evidenced by the large number of records excluded from our retrospective analysis because of ambiguous clinical findings. In the Brighton study, the MAIPA had a higher LR and better positive predictive values than tests for PAIgG, but almost equally low negative predictive values. Thus, the MAIPA appears to have modest clinical utility in supporting the diagnosis of ITP, but has little utility in excluding it. For example, based on the data of Brighton et al, if the true prevalence of ITP in the test population was 50%, then 76% of patients with a positive MAIPA result and 38% of patients with a negative MAIPA result would have ITP. Clearly, the diagnosis of ITP in most patients must be based on clinical criteria.
The study by Brighton et al is exemplary for its prospective design and use of rigorous clinical criteria for classification of thrombocytopenic patients. As newer antigen-specific tests for antiplatelet antibodies become available, it will be important to subject them to rigorous clinical validation to avoid inappropriate widespread use of uninformative tests.
Response
Dr Raife and colleagues have recently analyzed the clinical usefulness of a commercially available test for platelet-associated IgG (PAIgG), the Capture-P assay (Immucor, Norcoss, GA) in the diagnosis of idiopathic thrombocytopenic purpura (ITP). In agreement with our previously published results, they found that the test for PAIgG to have poor positive and negative predictive values and conclude that the Capture-P, like other PAIgG tests, is not useful for the diagnosis of ITP. They further emphasize that it is important that evaluation of tests for ITP be carried out prospectively as we did in our study. We fully agree with Dr Raife and colleagues on those two points.
In addition, we would strongly endorse their view that any new tests for platelet antibodies should be subjected to vigorous clinical evaluation prospectively to avoid widespread use of tests that have little or no clinical utility.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal