Abstract
Phosphatidylinositol 3-kinase (PI3-kinase) is a cytosolic enzyme that plays key roles in mediating signaling through many receptors. The heterodimeric form of PI3-kinase is made up of a regulatory subunit, p85, and a catalytic subunit, p110. Although granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to activate PI3-kinase, the mechanisms by which this activation is mediated and regulated are incompletely understood. Here we show that treatment of human neutrophils with GM-CSF induced both time- and concentration-dependent increases in the level of tyrosine phosphorylation of p85. The ability of GM-CSF to activate PI3-kinase was abolished by pretreating the cells with erbstatin, a tyrosine kinase inhibitor. The simultaneous treatment of the cells with GM-CSF and phorbol esters such as phorbol 12-myristate 13-acetate (PMA) and phorbol 12,13-dibutyrate (PDBu) significantly inhibited both the tyrosine phosphorylation of p85 and the activation of PI3-kinase. The inhibitory effects of phorbol esters were not induced by their inactive analogues and they were selective to the stimulation of tyrosine phosphorylation of p85 since phorbol esters did not alter the enhancement of the pattern of tyrosine phosphorylation of other cellular proteins, including that of Jak2 induced by GM-CSF. However, PMA significantly inhibited the in situ tyrosine phosphorylation and the activation of lyn observed in response to GM-CSF. The results suggest that the activation of PI3-kinase by GM-CSF is mediated by the tyrosine phosphorylation of p85 and that this activation is downregulated by PKC possibly via the inhibition of lyn.
GRANULOCYTE-MACROPHAGE colony-stimulating factor (GM-CSF) is a glycoprotein of 22 kD released by several activated cell types that promotes the growth and differentiation of granulocyte and monocyte progenitors.1-3 The effects of GM-CSF extend to the regulation of the functional responsiveness of mature human neutrophils. Preincubation of neutrophils with GM-CSF enhances (primes) their ability to respond to subsequent stimulation by a number of stimuli, such as chemotactic factors and phagocytic particles.4,5 The biologic activities known to be primed by GM-CSF include superoxide production,3,4,6 phagocytosis,5 calcium mobilization,7 phospholipase D (PLD),8,9 and arachidonic acid release and synthesis of leukotriene B4 (LTB4).10 In addition, GM-CSF exerts a number of direct effects on neutrophils. These include the stimulation of tyrosine phosphorylation,11 synthesis of interleukin-1 (IL-1) and IL-1-receptor antagonist (IL-1-Ra),5 surface expression of adhesion molecules of the β2-integrin family,12 modulation of the number as well as the affinity of fMLP receptors,3,5 and induction of cytosolic alkalinization.13 14 Although most of these activities are well documented, the intracellular mechanisms by which they are mediated are poorly or incompletely understood.
GM-CSF binds to a high-affinity receptor made up of two subunits, α and β.15 The α subunit, which is unique to the GM-CSF receptor, has a short cytoplasmic tail and is thought to serve mainly as a binding site for GM-CSF.15,16 However, recent evidence suggests that it may also play a signaling role.16 The β subunit, on the other hand, has a large intracytoplasmic tail and is believed to be critically involved in GM-CSF–mediated signaling.17 Neither subunit of the GM-CSF receptor has an intrinsic kinase domain nor are they apparently directly linked to a G-protein.15
Several lines of evidence suggest that the transduction of the GM-CSF signal in human neutrophils is mediated, at least in part, by cytoplasmic tyrosine kinases. GM-CSF increases the tyrosine phosphorylation of a number of mostly unidentified substrates,11 and tyrosine kinase inhibitors inhibit the increase in c-fos expression and the priming of calcium mobilization induced by GM-CSF.11 In addition, the stimulation of the activity of lyn18 and of fes19 20 and the increased tyrosine phosphorylation of Jak219,21 have recently been reported upon the stimulation of human neutrophils by GM-CSF. The functional significance of these events remains to be established.
Another effector system that is activated by GM-CSF is that mediated by phosphatidylinositol 3-kinase (PI3-kinase).22 PI3-kinase phosphorylates the third hydroxyl position of the inositol ring of phosphatidylinositol (PtdIns), phosphatidylinositol-4-phosphate (PtdIns4P), and phosphatidylinositol 4,5 bisphosphate (PtdIns4,5P2), leading to the production of PtdIns3P, PtdIns3,4P2, and PtdIns3,4,5P3, respectively.23-25 Two families of PI3-kinase have so far been described. The best characterized is composed of a tyrosine phosphorylation-dependent p85 regulatory subunit and a catalytic domain p110 subunit of which two isozymes have been described, p110α and p110β.26 More recently, a distinct, G-protein–activated, p85-independent isotype of p110 (p110γ) has been biochemically characterized27 and cloned.28 PI3-kinase has been implicated in the regulation of cell proliferation,29 protein secretion,30 and membrane ruffling31 in various cellular systems. In addition, the products of PI3-kinase, namely, PtdIns3,4P2 and PtdIns3,4,5P3 can activate several calcium-insensitive protein kinase C (PKC) isotypes, including PKCε32 and PKCζ.33 Although the role that PI3-kinase plays in GM-CSF–induced signaling pathways is presently unknown, its involvement is likely, as increased PI3-kinase activity was recovered in antiphosphotyrosine precipitates from GM-CSF–stimulated human neutrophils.22 However, it is unclear at present whether PI3 kinase (or p85 in particular) is directly tyrosine phosphorylated in neutrophils in response to chemotactic factors or cytokines.34-36
The present study was initiated to examine further the relationship between the PI3-kinase and the signaling pathways activated by GM-CSF in human neutrophils. The results obtained demonstrate that treatment of human neutrophils with GM-CSF induces a time-, as well as concentration-dependent, increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase. The stimulated tyrosine phosphorylation of p85 is essential for the increased activity of PI3-kinase, as both were abolished by erbstatin, a tyrosine kinase inhibitor. It was also observed that the simultaneous activation of PKC by phorbol esters significantly inhibited the activation of PI3-kinase and the tyrosine phosphorylation of p85 induced by GM-CSF. PMA was also found to inhibit the in situ phosphorylation of lyn and its kinase activity without affecting the tyrosine phosphorylation of Jak2.
MATERIALS AND METHODS
Materials.GM-CSF and erbstatin were generously provided by the Genetics Institute (Cambridge, MA) and Dr K. Umezawa (Keio University, Yokohama, Japan), respectively. Phorbol 12-myristate 13-acetate (PMA), 4αPMA, phorbol 12,13-dibutyrate (PDBu), 4αPDBu, Nonidet P-40 (NP-40), low-endotoxin dimethyl sulfoxide (DMSO), and O-phospho-L-tyrosine were obtained from Sigma Chemical (St Louis, MO). Sephadex G-10, protein A, Dextran T-500, and Ficoll-Paque were purchased from Pharmacia Biotech (Dorval, Quebec, Canada). The monoclonal antiphosphotyrosine antibody UB 05-321 was purchased from UBI (Lake Placid, NY). Polyclonal anti–PI3-kinase antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and from UBI. The polyclonal anti-lyn and anti-Jak2 antibodies were obtained from Santa Cruz Biotechnology. Src substrate was purchased from GIBCO-BRL (Burlington, Ontario, Canada). The enhanced chemiluminescence (ECL) Western blotting system was obtained from Amersham (Oakville, Ontario, Canada).
Neutrophil preparation.Blood was collected from healthy adult volunteers into heparinized tubes. The cells were centrifuged for 10 minutes at 1,000 rpm to remove platelet-rich plasma. After 2% dextran sedimentation of erythrocytes for 30 minutes, neutrophils were purified under sterile conditions by centrifugation on Ficoll-Paque cushions. Contaminating erythrocytes were removed by hypotonic lysis and the cells were suspended in magnesium-free Hanks' balanced salt solution (HBSS) that contained 1.6 mmol/L CaCl2 at a final count of 40 × 106 cells/mL. The entire procedure was performed at room temperature.11
Cell stimulation and lysis.Neutrophil suspensions (1 mL of 40 × 106 cells/mL) were either stimulated with the indicated agonists or treated with the same volume of the appropriate diluents for the indicated periods of time at 37°C. For the determination of the tyrosine phosphorylation patterns in whole cells, 100 μL of the cell suspensions was directly transferred to microtubes that contained an equal volume of 2× Laemmli's sample buffer (1× is 62.5 mmol/L Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate [SDS], 5% β-mercaptoethanol, 8.5% glycerol, 2.5 mmol/L orthovanadate [NaVO4], 10 mmol/L paranitro-phenylphosphate [NaP2O4], 10 μg/mL leupeptin, 10 μg/mL aprotinin, 0.025% bromophenol blue) preheated to 100°C. Another 500-μL aliquot was added to an equal amount of lysis buffer (2×) as described later for the immunoprecipitation protocols.
For nondenaturing lysis, buffers containing Tris-HCl 20 mmol/L, pH 7.4, NaCl 137 mmol/L, EDTA 5 mmol/L, EGTA 5 mmol/L, glycerol 10%, sodium fluoride (NaF) 50 mmol/L, NaP2O4 20 mmol/L, NaVO4 1 mmol/L, pepstatin 1 μmol/L, aprotinin 10 μg/mL, leupeptin 10 μg/mL, phenylmethylsulfonylfluoride (PMSF) 1 mmol/L, and 1% NP-40 (final concentrations) were used. The lysed cells were incubated on ice for 10 minutes before centrifugation at 12,000 rpm for 20 minutes. A preclearing with protein A–Sepharose for 30 minutes at 4°C was performed. The lysates were carefully transferred to new tubes and used for immunoprecipitation.37
For lysates prepared under reducing conditions, 500 μL of the cell suspensions was added to equal amounts of denaturing buffer A containing 50 mmol/L Tris-HCl pH 8, 150 mmol/L NaCl, 2 mmol/L EDTA, 50 mmol/L NaF, 2 mmol/L NaVO4, 20 mmol/L NaP2O4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μmol/L pepstatin, 1 mmol/L N-ethylmaleimide (NEM), 1 mmol/L PMSF, 1% SDS, and 0.6% β-mercaptoethanol (final concentrations) preheated to 100°C and incubated for 10 minutes. The lysates were centrifuged at 12,000 rpm for 10 minutes at room temperature. The supernatants were then filtered through sephadex G-10 columns to remove the denaturing agents. To prepare the columns, 3 g of sephadex per sample was suspended in 10 mL of buffer that contained 20 mmol/L Tris HCl, pH 8.0, 5 mmol/L EDTA, 5 mmol/L EGTA, and 137 mmol/L NaCl (final concentrations) for at least 3 hours at room temperature with occasional shaking before use. NP-40 (1% final) and 5 μL of bovine serum albumin (BSA; 0.01%, wt/vol) were added to the eluates, which were subsequently used for immunoprecipitation.
Immunoprecipitation of tyrosine phosphorylated proteins.Lysates (1 mL) obtained as described earlier were incubated either with 10 μg of agarose-conjugated antiphosphotyrosine antibodies for 6 hours or with 5 μg of free antibodies for 5 hours at 4°C on a rotator platform. In the latter case, this was followed by incubation with 20 μg of protein A–Sepharose for 1 hour at 4°C. The beads were collected and were washed twice with modified buffer A containing 1% NP-40, but no SDS or β-mercaptoethanol, and twice with LiCl buffer (LiCl 0.5 mol/L, HEPES 20 mmol/L, pH 7.4). The supernatants were removed carefully, 45 μL of 2× boiling Laemmli's buffer was added, and the samples were incubated at 100°C for 10 minutes. The specificity of the immunoprecipitation with the antiphosphotyrosine antibodies was determined by incubating the agarose-conjugated antiphosphotyrosine antibodies with 10 mmol/L O-phospho-L-tyrosine for 1 hour at 37°C before their use in immunoprecipitation.
Lyn tyrosine kinase activity in vitro.Anti-lyn polyclonal antibodies (5 μg/1 mL of lysates) were added to nondenatured precleared lysates prepared as described earlier and left at 4°C for 2 hours. The antibodies were pelleted after incubation with 20 μg of protein A–Sepharose for 1 hour (4°C) by a 1-minute centrifugation at 12,000 rpm. The immunoprecipitates were washed once with the lysis buffer, and once with LiCl 0.5 mol/L, Tris-HCl 100 mmol/L, pH 7.4. This was followed by resuspension in 250 μL of kinase buffer without adenosine triphosphate (ATP) or substrate (Tris-HCl 20 mmol/L, pH 7.5, MnCl2 5 mmol/L, MgCl2 5 mmol/L). The immunoprecipitates were divided into two, centrifuged (12,000 rpm, 1 minute), and suspended in 30 μL of kinase buffer containing 32P-ATP (10 μCi) and either the substrate or the control peptide as indicated by the manufacturer. The reactions were allowed to proceed for 8 minutes and were stopped by centrifugation. The supernatants were transferred to new tubes, 21 μL of glacial acetic acid and 91 μL of trichloroacetic acid were added, and the tubes were left on ice for 10 minutes. The tubes were centrifuged and the supernatants were again transferred to clean tubes. Ten microliters of each sample was added to individual phosphocellulose disks, which were then washed with 1% phosphoric acid. The levels of radioactivity bound to the phosphocellulose disks were measured by liquid scintillation counting.
In vitro measurement of PI3-kinase activity.The assay was conducted as previously described.34 Briefly, PI3-kinase was immunoprecipitated by adding 4 μg of anti-p85 antibodies to 900 μL of nondenatured precleared lysates and left at 4°C for 2 hours. The antibodies were collected following a 1-hour incubation at 4°C with protein A–Sepharose. The immunoprecipitates were washed once with each of the following: lysis buffer (LiCl 0.5 mol/L, Tris-HCl 100 mmol/L, pH 7.4; EDTA 1 mmol/L, NaCl 100 mmol/L, Tris-HCl 10 mmol/L, pH 7.4), and kinase buffer without ATP (Tris-HCl 20 mmol/L, pH 7.5, MnCl2 5 mmol/L, MgCl2 5 mmol/L). The immunoprecipitates were suspended in 50 μL of kinase buffer containing phosphatidylinositol substrate (300 μg/mL) and 32P-ATP (10 μCi). The reactions were allowed to proceed for 90 seconds before being quenched with 80 μL of HCl (1N). The lipids were extracted by the addition of 200 μL chloroform/methanol (1:1 vol/vol ratio) and separated on thin-layer chromatography (TLC) plates precoated with ammonium oxalate. The migration solution contained 2-n-propanol and acetic acid (2 mol/L) at a 2:1 ratio (vol/vol). The phosphorylated lipids were revealed by autoradiography. The specificity of PI3-kinase activity was confirmed by its inhibition in the presence of either wortmannin (20 nmol/L) or 2.5% NP-40.
Electrophoresis and immunoblotting.Samples were electrophoresed on 7.5% to 20% SDS-polyacrylamide gradient gels.
Electrophoretic transfer cells (Hoeffer Scientific Instruments, Pharmacia Biotech, Dorval, Québec, Canada) were used to transfer proteins from the gels to Immobilon PVDF membranes (Millipore, Bedford, MA). Western blotting was performed as previously described.11 Briefly, nonspecific sites were blocked using 2% gelatin in TBS-Tween (25 mmol/L Tris-HCl, pH 7.8, 190 mmol/L NaCl, 0.15% Tween 20) for 1 hour at 37°C. The first antibody, either monoclonal antiphosphotyrosine antibodies or polyclonal anti-lyn or anti-Jak2 antibodies, was then incubated with the membranes for 1 hour at 37°C at a final dilution of 1/4,000 and 1/1,000 respectively, in fresh blocking solution. The membranes were washed three times at room temperature in TBS-Tween for a total duration of 30 minutes, and then incubated with horseradish peroxidase-labeled sheep antimouse or donkey antirabbit IgG (Amersham, Oakville, Ontario, Canada) for 1 hour at 37°C at a final dilution of 1/20,000 in fresh blocking solution. The membranes were washed three times with TBS-Tween and the protein bands were revealed using the ECL Western blotting detection system following the manufacturer's directions.
RESULTS
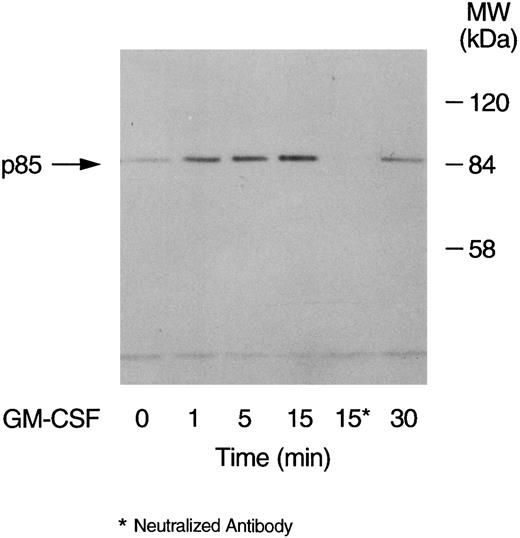
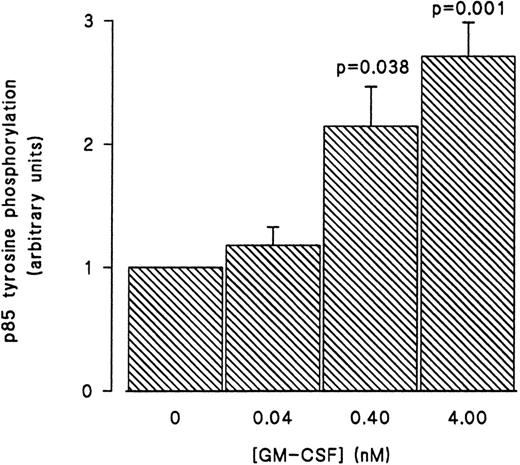
GM-CSF–induced tyrosine phosphorylation of the p85 subunit of PI3-kinase in human neutrophils.The first set of experiments was initiated to examine the ability of GM-CSF to affect the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase in human neutrophils. The cells were incubated at 37°C with GM-CSF (4 nmol/L) or an equal volume of diluent (0.01% BSA) for various times. The reactions were stopped by adding aliquots of the cells to equal volumes of boiling lysis buffer containing SDS and β-mercaptoethanol (lysis under reducing conditions). After removing the denaturing agents, as described in Materials and Methods, the tyrosine-phosphorylated proteins were immunoprecipitated with the agarose-conjugated antiphosphotyrosine antibodies and the amounts of p85 in the precipitates was determined by blotting with anti-p85 antibodies. A representative blot from these experiments is shown in Fig 1. As compared with unstimulated cells (lane 1), a significant increase in the level of p85 in antiphosphotyrosine immunoprecipitates was evident after 1 minute of treatment with GM-CSF (P = .041). The amounts of p85 in antiphosphotyrosine immunoprecipitates continued to increase until they reached their maximum after 15 minutes of stimulation (lane 4). The levels of p85 in the antiphosphotyrosine immunoprecipitates then decreased until, at 30 minutes, they stopped being significantly (P = .21) different from those in control cells (lane 6). The specificity of the immunoprecipitation was demonstrated by the absence of p85 in immunoprecipitates performed with preneutralized antiphosphotyrosine antibodies (lane 5). Similar results were obtained using the reverse protocol, ie, immunoprecipitation with anti-p85 antibodies and detection with antiphosphotyrosine antibodies (data not shown). The increase in the tyrosine phosphorylation of p85 was not reproducibly observed in lysates prepared in nondenaturing buffer as described in Materials and Methods (data not shown). To examine the dependence of the tyrosine phosphorylation of p85 on the concentration of GM-CSF, cells were treated with 0.04 nmol/L, 0.4 nmol/L, and 4 nmol/L GM-CSF or an equal volume of diluent (0.01% BSA) for 15 minutes at 37°C. Lysis under reducing conditions, immunoprecipitation and detection were conducted as described in Materials and Methods. As summarized in Fig 2, no significant stimulation of tyrosine phosphorylation of p85 was detected with 0.04 nmol/L of GM-CSF. On the other hand, significant increases in the amounts of p85 in antiphosphotyrosine immunoprecipitates were observed at both 0.4 and 4 nmol/L GM-CSF (P = .038 and P = .001 respectively).
Time-dependent increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase in GM-CSF–treated neutrophils. The cells were stimulated with GM-CSF (4 nmol/L) for 1, 5, 15, and 30 minutes at 37°C. At the indicated times, the cells were lysed under denaturing conditions and the immunoprecipitations were performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit of PI3-kinase was revealed by blotting with specific anti-p85 antibodies. The antibodies used in lane 5 were preneutralized by 10 mmol/L O-phospho-L-tyrosine. The data are representative of three experiments, which yielded similar results.
Time-dependent increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase in GM-CSF–treated neutrophils. The cells were stimulated with GM-CSF (4 nmol/L) for 1, 5, 15, and 30 minutes at 37°C. At the indicated times, the cells were lysed under denaturing conditions and the immunoprecipitations were performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit of PI3-kinase was revealed by blotting with specific anti-p85 antibodies. The antibodies used in lane 5 were preneutralized by 10 mmol/L O-phospho-L-tyrosine. The data are representative of three experiments, which yielded similar results.
Concentration-dependent increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase in GM-CSF–treated neutrophils. The cells were stimulated with 0.04, 0.4, and 4 nmol/L of GM-CSF for 15 minutes at 37°C, lysed under denaturing conditions, and the immunoprecipitations were performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit of PI3-kinase was revealed by blotting with specific anti-p85 antibodies. The densities of the bands were quantified by densitometry. Statistical significance of the difference from control, unstimulated, cells was tested using unpaired Student's t-test. Mean ± SEM of three independent experiments.
Concentration-dependent increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase in GM-CSF–treated neutrophils. The cells were stimulated with 0.04, 0.4, and 4 nmol/L of GM-CSF for 15 minutes at 37°C, lysed under denaturing conditions, and the immunoprecipitations were performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit of PI3-kinase was revealed by blotting with specific anti-p85 antibodies. The densities of the bands were quantified by densitometry. Statistical significance of the difference from control, unstimulated, cells was tested using unpaired Student's t-test. Mean ± SEM of three independent experiments.
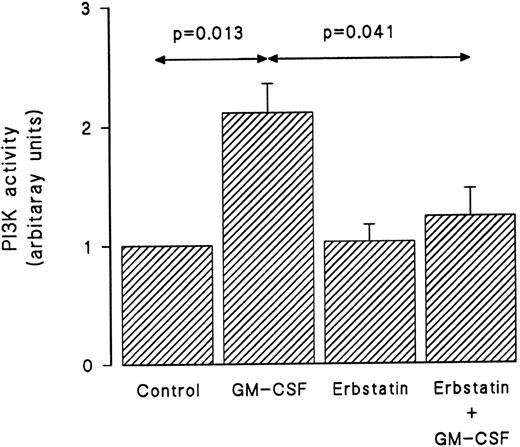
Erbstatin, a specific tyrosine kinase inhibitor,38 was used next to investigate the role that tyrosine kinases play in the activation of PI3-kinase by GM-CSF in human neutrophils. In these experiments, cells were suspended at 5 × 106/mL and pretreated with erbstatin (10 μg/mL) or diluent for 1 hour at 37°C. The viability of the cells following incubation with erbstatin has previously been demonstrated.38 The cells were then washed and suspended at 30 × 106/mL and stimulated with GM-CSF (4 nmol/L) or diluent (BSA) for 15 minutes before lysis under nondenaturing conditions. Immunoprecipitation was conducted using anti–PI3-kinase antibodies, and an in vitro PI3-kinase assay was performed using phosphatidylinositol as substrate as described in Materials and Methods. The in vitro reaction was allowed to proceed for 90 seconds. The lipid products were separated by TLC and phosphorylated reaction products were revealed by autoradiography and identified using internal standards. As shown in Fig 3, GM-CSF induced a significant increase in the PI3-kinase in vitro activity. While erbstatin treatment did not affect the basal level of PI3-kinase activity, it abolished the ability of GM-CSF to augment in vitro PI3-kinase activity.
GM-CSF–induced activation of PI3-kinase is dependent on tyrosine kinase activity. Neutrophils were pretreated with erbstatin (10 μg/mL) or diluent (0.1% DMSO) for 1 hour at 37°C. This was followed by treatment with GM-CSF or diluent (0.01% BSA) for 15 minutes at 37°C. Lysis was conducted under nondenaturing conditions and immunoprecipitation was performed using anti-p85 polyclonal antibodies. An in vitro kinase assay was conducted using phosphatidylinositol as substrate. Lipids were separated by TLC and revealed by autoradiography and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
GM-CSF–induced activation of PI3-kinase is dependent on tyrosine kinase activity. Neutrophils were pretreated with erbstatin (10 μg/mL) or diluent (0.1% DMSO) for 1 hour at 37°C. This was followed by treatment with GM-CSF or diluent (0.01% BSA) for 15 minutes at 37°C. Lysis was conducted under nondenaturing conditions and immunoprecipitation was performed using anti-p85 polyclonal antibodies. An in vitro kinase assay was conducted using phosphatidylinositol as substrate. Lipids were separated by TLC and revealed by autoradiography and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
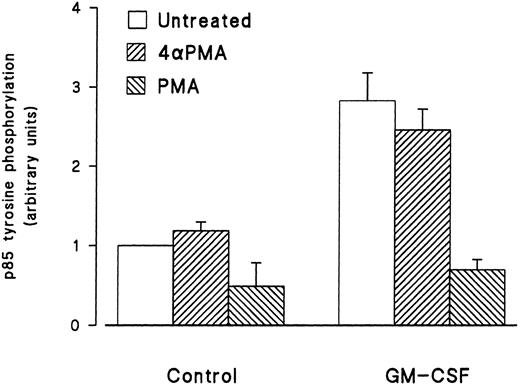
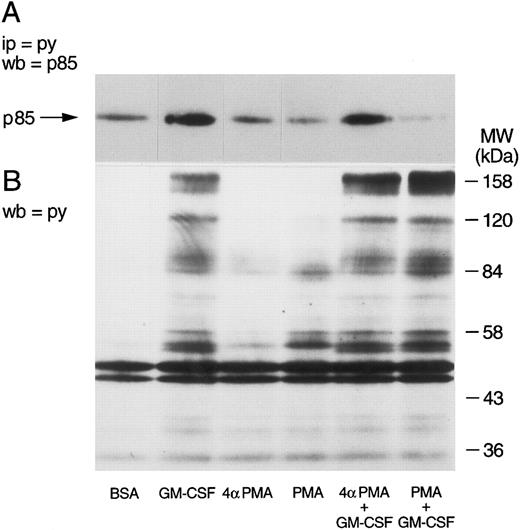
Effect of phorbol esters on p85 tyrosine phosphorylation and PI3-kinase activity in GM-CSF–stimulated cells.The effects of phorbol esters on the ability of GM-CSF to increase the tyrosine phosphorylation of p85 in human neutrophils were investigated next. The phorbol esters used were PMA and PDBu, and their inactive analog 4αPMA and 4αPDBu. The cells were treated with the phorbol esters (90 nmol/L) either alone or simultaneously with GM-CSF (4 nmol/L). After 15 minutes of stimulation, the cells were lysed under denaturing conditions and immunoprecipitation with antiphosphotyrosine antibodies, and Western blotting for the p85 subunit of PI3-kinase was conducted as described earlier. As shown in Fig 4, PMA did not stimulate the tyrosine phosphorylation of p85. However, the simultaneous treatment of neutrophils with PMA and GM-CSF significantly (P = .05) inhibited the ability of the latter to increase the level of tyrosine phosphorylation of p85. The inhibitory effects of PMA were not shared by 4αPMA, its inactive analog, which had no significant effect on the increases of the level of p85 tyrosine phosphorylation induced by GM-CSF. Similar results were obtained with PDBu and 4αPDBu (data not shown). Equivalent amounts of tyrosine phosphorylated proteins were loaded in each lane as estimated by immunoblotting with antiphosphotyrosine antibodies.
Effects of PMA on the activation of the tyrosine phosphorylation of the p85 subunit of PI3-kinase induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. Neutrophils were lysed under denaturing conditions and the immunoprecipitation was performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit was revealed by blotting using specific anti-p85 antibodies and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
Effects of PMA on the activation of the tyrosine phosphorylation of the p85 subunit of PI3-kinase induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. Neutrophils were lysed under denaturing conditions and the immunoprecipitation was performed using agarose-conjugated antiphosphotyrosine antibodies. The p85 subunit was revealed by blotting using specific anti-p85 antibodies and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
The selectivity of the inhibitory effects of PMA was verified by determining first whether PMA also inhibited the tyrosine phosphorylation of other cellular proteins. Whole cells aliquots were taken, dissolved in boiling Laemmli's sample buffer, and tested for the level of tyrosine phosphorylation by Western blot. The tyrosine phosphorylation of p85 was verified in parallel. The results of these experiments are illustrated in Fig 5. As described earlier, GM-CSF increased the tyrosine phosphorylation of p85 (Fig 5A). While neither phorbol esters had any effects of their own, PMA, but not 4αPMA, significantly inhibited the phosphorylation of p85 induced by GM-CSF. The cells stimulated with GM-CSF alone showed the characteristic pattern of tyrosine phosphorylation,39 namely, increased phosphorylation of proteins at 150 to 170, 120, 80 to 90, 55, and 42 kD (Fig 5B). PMA increased (as previously reported39 ) the tyrosine phosphorylation of a number of proteins (lane 4), yet with a different pattern than that of GM-CSF. The simultaneous treatment with the two agonists caused the appearance of a mixed pattern of tyrosine phosphorylation, with no apparent inhibitory effects on the level of tyrosine phosphorylation of the proteins characteristically observed in GM-CSF–treated neutrophils. 4αPMA, on the other hand, did not stimulate tyrosine phosphorylation or have an effect on that induced by GM-CSF.
Effects of phorbol esters on the tyrosine phosphorylation of p85 and on the pattern of tyrosine phosphorylation in human neutrophils. Neutrophils were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. (A) Following denaturing lysis, immunoprecipitation was performed with agarose-conjugated antiphosphotyrosine antibodies and p85 was revealed by blotting with anti-p85 antibodies. (B) Cells were lysed in boiling sample buffer and blotting was conducted using antiphosphotyrosine antibodies. The samples in (A) and (B) were derived from the same experiment, which was repeated four times with similar results.
Effects of phorbol esters on the tyrosine phosphorylation of p85 and on the pattern of tyrosine phosphorylation in human neutrophils. Neutrophils were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. (A) Following denaturing lysis, immunoprecipitation was performed with agarose-conjugated antiphosphotyrosine antibodies and p85 was revealed by blotting with anti-p85 antibodies. (B) Cells were lysed in boiling sample buffer and blotting was conducted using antiphosphotyrosine antibodies. The samples in (A) and (B) were derived from the same experiment, which was repeated four times with similar results.
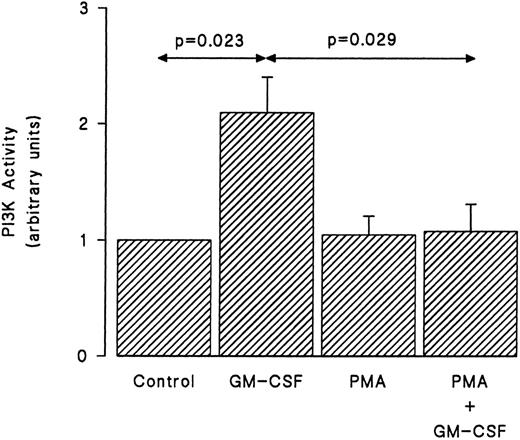
We examined next whether the inhibition by PMA of the tyrosine phosphorylation of p85 was reflected in a similar reduction of the ability of GM-CSF to stimulate the activity of PI3-kinase. Cells were treated with GM-CSF and/or PMA as earlier, and the in vitro PI3-kinase activity was monitored as described in Materials and Methods. As shown in Fig 6, GM-CSF increased the in vitro PI3-kinase activity. By itself, PMA had no effect on the lipid kinase activity recovered in p85 immunoprecipitates. However, the simultaneous stimulation of human neutrophils with PMA and GM-CSF essentially abrogated the ability of the latter agonist to stimulate in vitro PI3-kinase activity.
Effects of PMA on the activation of PI3-kinase induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. Lysis was conducted under nondenaturing conditions and immunoprecipitation was performed using anti-85 polyclonal antibodies. An in vitro kinase assay was conducted using phosphatidylinositol as substrate. Lipids were separated by TLC, revealed by autoradiography, and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of five independent experiments.
Effects of PMA on the activation of PI3-kinase induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. Lysis was conducted under nondenaturing conditions and immunoprecipitation was performed using anti-85 polyclonal antibodies. An in vitro kinase assay was conducted using phosphatidylinositol as substrate. Lipids were separated by TLC, revealed by autoradiography, and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of five independent experiments.
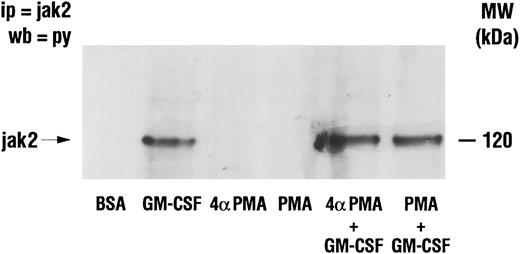
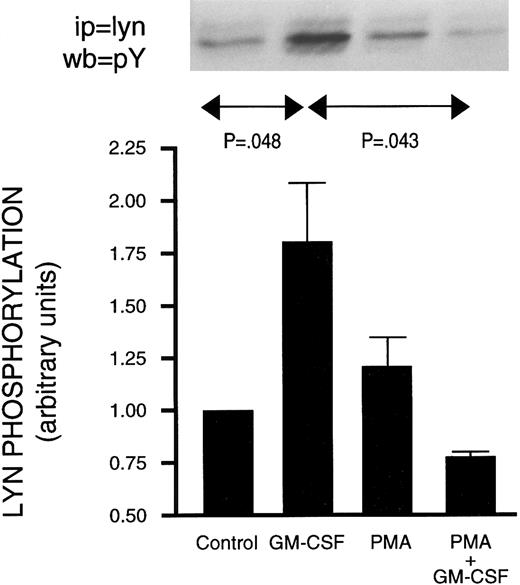
Effect of PMA on GM-CSF–induced activation of Jak2 and lyn.The activation of Jak2 and of lyn has been linked to the mechanism of action of GM-CSF.18,19 Furthermore, increased PI3-kinase activity has been recovered in lyn immunoprecipitates from GM-CSF–stimulated human neutrophils.22 The possibility that PMA inhibited GM-CSF–induced p85 tyrosine phosphorylation (and PI3-kinase activity) by interfering with signaling between GM-CSF receptors and the above two tyrosine kinases was therefore investigated. Neutrophils were treated as described earlier with GM-CSF and/or phorbol esters, and lysed under denaturing conditions. Immunoprecipitation was conducted using anti-Jak2 or anti-lyn antibodies and the level of tyrosine phosphorylation of the kinases was revealed by Western blotting as shown in Figs 7 and 8. Jak2 immunoprecipitated from unstimulated cells or from cells treated with phorbol esters showed no detectable tyrosine phosphorylation (Fig 7). However, increased tyrosine phosphorylation of Jak2 was observed in immunoprecipitates derived from GM-CSF–stimulated cells. Neither PMA nor 4αPMA had any effect on the tyrosine phosphorylation of Jak2 stimulated by GM-CSF. The level of in situ tyrosine phosphorylation of lyn was examined next (Fig 8). As previously reported,37 lyn recovered from GM-CSF–treated cells showed increased tyrosine phosphorylation. While PMA had no effect on its own, it, on the other hand, completely inhibited the increases in the levels of tyrosine phosphorylation of lyn induced by GM-CSF.
Effect of PMA on the stimulation of the tyrosine phosphorylation of Jak2 induced by GM-CSF in human neutrophils . The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. Neutrophils were lysed under denaturing conditions and the immunoprecipitation was performed using anti-Jak2 antibodies. The level of tyrosine phosphorylation was revealed by blotting with anti-p85 antibodies. The data illustrated are representative of similar results obtained in four other independent experiments. Ip, immunoprecipitation; wb, Western blot.
Effect of PMA on the stimulation of the tyrosine phosphorylation of Jak2 induced by GM-CSF in human neutrophils . The cells were simultaneously treated with BSA (0.01%), GM-CSF (4 nmol/L), and/or phorbol esters (90 nmol/L) for 15 minutes at 37°C. Neutrophils were lysed under denaturing conditions and the immunoprecipitation was performed using anti-Jak2 antibodies. The level of tyrosine phosphorylation was revealed by blotting with anti-p85 antibodies. The data illustrated are representative of similar results obtained in four other independent experiments. Ip, immunoprecipitation; wb, Western blot.
Effect of PMA on the stimulation of the in situ tyrosine phosphorylation of lyn induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.0.1%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. (Top) In situ tyrosine phosphorylation of lyn was determined by immunoprecipitating lyn under denaturing conditions from control or stimulated cells and blotting using antiphosphotyrosine antibodies. (Bottom) Mean ± SEM of five independent experiments such as presented in the top panel and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test.
Effect of PMA on the stimulation of the in situ tyrosine phosphorylation of lyn induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.0.1%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. (Top) In situ tyrosine phosphorylation of lyn was determined by immunoprecipitating lyn under denaturing conditions from control or stimulated cells and blotting using antiphosphotyrosine antibodies. (Bottom) Mean ± SEM of five independent experiments such as presented in the top panel and quantified by densitometry. Statistical significance was evaluated using unpaired Student's t-test.
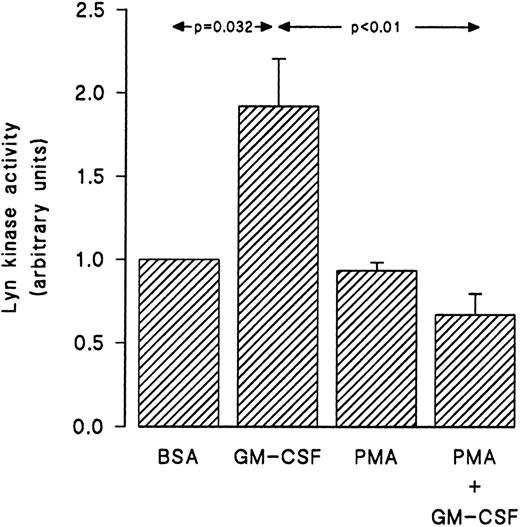
As the tyrosine phosphorylation of src-like kinases may be reflected in increased or decreased kinase activity,40 41 the ability of lyn derived from GM-CSF and/or PMA-treated cells to phosphorylate an exogenous substrate was determined. To this effect, lyn was immunoprecipitated from cells treated with PMA and/or GM-CSF and an in vitro kinase assay was conducted using a specific src-peptide as described in Materials and Methods. The results presented in Fig 9 show that GM-CSF stimulated the activity of lyn, which resulted in increased tyrosine phosphorylation of the src-derived peptide. In addition, the results of these experiments also demonstrate that PMA markedly inhibited the increase in lyn kinase activity induced by GM-CSF.
Effect of PMA on the stimulation of the activity of lyn induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.0.1%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. The cells were then lysed under nondenaturing conditions and lyn was immunoprecipitated as described in Materials and Methods. An in vitro kinase assay using a specific synthetic substrate was conducted as detailed in Materials and Methods. The level of the phosphorylation of the peptide was monitored by liquid scintillation counting. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
Effect of PMA on the stimulation of the activity of lyn induced by GM-CSF in human neutrophils. The cells were simultaneously treated with BSA (0.0.1%), GM-CSF (4 nmol/L), and/or PMA (90 nmol/L) for 15 minutes at 37°C. The cells were then lysed under nondenaturing conditions and lyn was immunoprecipitated as described in Materials and Methods. An in vitro kinase assay using a specific synthetic substrate was conducted as detailed in Materials and Methods. The level of the phosphorylation of the peptide was monitored by liquid scintillation counting. Statistical significance was evaluated using unpaired Student's t-test. Mean ± SEM of four independent experiments.
DISCUSSION
GM-CSF is one of the key cytokines that regulate the biologic activities of hematopoietic cells, including mature neutrophils.1,2 GM-CSF binds to a high-affinity receptor made up of two subunits, α and β, neither of which possesses enzymatic activity or associates directly with a G-protein. Instead, GM-CSF receptors make use of cytoplasmic tyrosine kinases. Accordingly, one of the prominent effects of GM-CSF in neutrophils is an increase in the level of tyrosine phosphorylation of a number of cellular proteins and the activation of several tyrosine kinases, including lyn,18 Jak2,19 and fes.19
Previous work in neutrophils has shown an increase in PI3-kinase activity upon treatment with GM-CSF.22 However, the role of tyrosine phosphorylation in the mediation of this activation is not clear. The phosphorylation of the p85 subunit of PI3-kinase on serine and tyrosine residues is thought to play key roles in regulating PI3-kinase activity.42 While serine phosphorylation seems to have an inhibitory effect, it is unclear whether p85 is itself tyrosine phosphorylated and what the role of this phosphorylation may be. The evidence for the tyrosine phosphorylation of PI3-kinase is based on an increase in PI3-kinase activity in antiphosphotyrosine immunoprecipitates after stimulation by GM-CSF.22 This increase may be due to the tyrosine phosphorylation of PI3-kinase or to an association with a tyrosine-phosphorylated protein. Furthermore, several reports failed to show a direct tyrosine phosphorylation of p85 in neutrophils.34,35 This led to the conclusion that the tyrosine phosphorylation of p85 may not be essential for the activation of PI3-kinase.34 36 The experiments, the results of which are described here, were designed to directly address these issues in human neutrophils stimulated by GM-CSF.
The first obstacle that had to be overcome was to ensure adequate preservation and sensitive detection of tyrosine-phosphorylated proteins in neutrophil lysates. This problem is particularly acute in neutrophils because of their unusual complement of proteases and phosphatases, which easily escape from control once the cells are lysed. On the basis of preliminary experiments (manuscript in preparation), all of the experiments in which the levels of tyrosine phosphorylation were measured were conducted using lysates prepared under denaturing conditions as described in Materials and Methods. This allowed the consistent preservation of the levels of tyrosine phosphorylation of various substrates, including p85. This procedure also ensured that most, if not all, protein-protein interactions are disrupted, thus effectively eliminating the possibility of coimmunoprecipitations. The results obtained showed a significant increase in the level of tyrosine phosphorylation of the p85 subunit of PI3-kinase upon treatment of neutrophils with GM-CSF. This increase was time-, as well as concentration-dependent. Small amounts of p85 were detected in the antiphosphotyrosine immunoprecipitates prepared from unstimulated cells, indicating a constitutive basal level of tyrosine phosphorylation. The stimulation of the tyrosine phosphorylation of p85 was apparently critical to the activation of PI3-kinase as erbstatin, a tyrosine kinase inhibitor,38 inhibited equally the phosphorylation of p85 (data not shown) and the stimulation of the activity of PI3-kinase induced by GM-CSF. These results extend the previously observed inhibition by genistein of the stimulation of PI3-kinase by GM-CSF22,34 by showing a parallel inhibition of the tyrosine phosphorylation of p85. Additionally, wortmannin and ly294002, two unrelated compounds which have been previously shown to inhibit PI3-kinase,43-45 were also found to inhibit the shape changes induced by GM-CSF (data not shown), thereby implicating PI3-kinase in this response. It should also be noted that, as previously reported,36 fMLP did not induce a significant increase in the level of tyrosine phosphorylation of p85 (data not shown).
It remains to be established whether it is the extent of phosphorylation of p85 or the specific site of phosphorylation that is the critical event in the activation of PI3-kinase. The src family of tyrosine kinases, the activity of which is regulated by a balance in phosphorylation between two tyrosine residues,40 provides a precedent for the latter possibility. Previous work has suggested the involvement of lyn in the activation of PI3-kinase by GM-CSF.22 This conclusion was supported by the observation of an increase in the level of PI3-kinase activity in lyn immunoprecipitates after stimulation of human neutrophils with GM-CSF. These interactions may be mediated through the SH3 domain of lyn and the proline rich sequences of p85. Binding of p85 to the SH3 domains of lyn and fyn has been observed in vitro using GST-SH3 fusion proteins.46 Whether a direct interaction between lyn and p85 takes place in situ in human neutrophils, and whether GM-CSF modulates it, remains to be directly tested.
Interplays (“cross talk”) among signaling pathways have been repeatedly observed in the last few years, and the PI3-kinase pathway is no exception. For example, specific members of the PKC family (PKCε and PKCζ32,33) are directly activated by the products of PI3-kinase activation. On the other hand, direct stimulation of PKC by phorbol esters can result in enhanced or diminished responses (activation of PLC and mobilization of calcium) in various cells, including neutrophils, depending on the concentration used and the length of the pre-incubation period.47-50 With respect to GM-CSF, preincubation with PMA has previously been shown to inhibit GM-CSF binding,51 as well as the stimulation of tyrosine phosphorylation by this growth factor.39 These considerations led us to examine the potential effects of the activation of PKC by phorbol esters on the stimulation of PI3-kinase by GM-CSF in human neutrophils.
The results obtained indicate that phorbol esters did not stimulate the activity of PI3-kinase or increase the tyrosine phosphorylation of p85. However, the ability of GM-CSF to induce both effects was significantly inhibited in the presence of phorbol esters. Similar results were obtained with PMA and PDBu (data not shown), but not with their respective inactive analogs. The simultaneous stimulation with the phorbol esters and GM-CSF did not eliminate the presence of functional GM-CSF receptors on the neutrophil membrane following phorbol esters pretreatment.51 This is evidenced by the normal, or additive, patterns of tyrosine phosphorylation observed upon stimulation with GM-CSF and PMA. These latter results also suggest that the tyrosine phosphorylation of the p85 subunit of PI3-kinase only represents a minor component of the proteins in the 80- to 90-kD range that are tyrosine-phosphorylated by GM-CSF. The lack of effect of PMA on the stimulation of the tyrosine phosphorylation of Jak2 by GM-CSF further indicates that the cells are capable of responding to GM-CSF under these conditions. The observations also demonstrate that the inhibitory effect of PMA monitored here is selectively directed towards the tyrosine phosphorylation of p85.
These results identify a new target for the negative regulation of cellular responsiveness by PKC, namely, the tyrosine phosphorylation of p85. It had been shown that PKC (mostly members of the c-PKC family52 ) is activated upon the stimulation of the PLC signaling pathway, which it, in turn, helps inhibit, possibly by phosphorylating the heterotrimeric G-proteins involved.53-55 Products of PI3-kinase such as PtdIns3,4,5P3 are similarly known to act as cofactors for the activation of a subset of PKC (n-PKC).32,33 The inhibition of the stimulation of PI3-kinase by phorbol esters establishes therefore a negative feedback regulation functionally equivalent to that observed in the PLC pathway. Evidence was obtained implicating interference by PMA (PKC) at the level of the activation of lyn as the mechanism of inhibition of the stimulation of PI3-kinase. An initial set of experiments established that PMA did not affect the stimulation of tyrosine phosphorylation of Jak2, one of the kinases thought to mediate GM-CSF-induced signaling.19,21 The formation of a complex between lyn and PI3-kinase has previously been reported in human neutrophils stimulated by GM-CSF.22 Furthermore, the results of preliminary experiments suggest that purified lyn can phosphorylate immunoprecipitated p85 (data not shown). Our data indicate that the stimulation of the in situ tyrosine phosphorylation of lyn and of its kinase activity by GM-CSF are both inhibited by phorbol esters. It is likely that this represents a significant and relevant element of the mechanism of inhibition of the activation of PI3-kinase by PKC activators. The potential involvement of phosphatases has not been examined in this investigation, and cannot therefore be evaluated at this stage.
Another question that still needs to be answered concerns the identity of the PKC involved in the downregulation of PI3-kinase. PKC family is divided into two major subfamilies, the Ca2+-dependent or the conventional PKC (cPKC) and the Ca2+-independent or the novel PKCs (nPKC).56 GM-CSF does not directly stimulate a calcium mobilization7 or activate PLC.8,57 Thus, the effects of GM-CSF are more likely to be linked to the nPKC than the cPKC family. Accordingly PKCε, a member of the nPKC subfamily, has been shown to be activated by GM-CSF.58 Similarly, the products of PI3-kinase were reported to activate members of the same family of PKC, including PKCε.32 Thus, while it is likely that PKCε may play an important role in mediating the downregulation mechanisms, the data available to date cannot confirm or deny these possibilities.
In conclusion, the data presented here indicate that the activation of PI3-kinase by GM-CSF is mediated by the tyrosine phosphorylation of its p85 subunit. This activation is subject to down regulation by presently unidentified members of the PKC family via a mechanism that involves an inhibition of the stimulated phosphorylation of p85, and inactivation of PI3-kinase, that is likely to be mediated by interference with the activation of lyn.
Supported in part by grants and fellowships from the Medical Research Council of Canada, the National Cancer Institute of Canada, the Arthritis Society of Canada, and the Fonds de la Recherche en Santé du Québec.
Address reprint requests to Paul H. Naccache, PhD, CHUL, 2705 Boulevard Laurier, Ste-Foy, Québec, Canada, G1V4G2.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal