Abstract
Neutrophil (PMN) activation is associated with increased surface expression of several membrane proteins that are translocated from intracellular pools. Indirect evidence suggests that the intracellular storage pools of complement receptor type 1 (CR1) in resting PMN are distinct from traditional granules and may be the secretory vesicles in which albumin is also stored, but it is not known if this compartment is homogeneous or heterogeneous. To isolate and characterize the CR1-containing vesicles, we used antibodies against unique sequences in the cytoplasmic tail of CR1. Affinity-purified IgG was used to adsorb CR1 storage vesicles from the light membrane fraction (γ-band) of nitrogen cavitates of resting PMN. The immunoadsorbent could quantitatively remove the CR1-containing vesicles, whereas control adsorbents with nonimmune IgG showed no specific binding of CR1. Immunoblots of specifically isolated vesicles also showed enrichment of albumin, decay-accelerating factor, FcγRIII, and CR3; whereas HLA class I was not detectable in these vesicles. Enzyme assay of specifically isolated vesicles after treatment with Triton X-100 showed that these vesicles contained most of the cells' latent alkaline phosphatase. An additional population of vesicles containing albumin, but not CR1, and that did not bind to anti-CR1 adsorbent was also identified. Immunoelectron microscopy showed that the specifically isolated vesicles had mean diameters of 0.086 to 0.1 μm and stained positive for CR1 and albumin. These results indicate that CR1 storage vesicles can be isolated with antibodies against the cytoplasmic tail of CR1 and show that these vesicles also contain albumin as well as glycosyl-phosphatidyl inositol-anchored proteins. These results are most compatible with the hypothesis that CR1-containing vesicles have arisen by endocytic retrieval of proteins that had been on the plasma membrane.
HUMAN NEUTROPHILS (PMN) acquire increased functional capabilities upon exposure to chemoattractants.1,2 This is primarily due to a marked increase in the plasma membrane expression of several functionally important proteins, which are rapidly translocated from preformed intracellular storage pools during PMN activation.3-10 These intracellular storage compartments represent a readily mobilizable pool of proteins that are needed early in PMN activation and that appear on the plasma membrane before significant degranulation occurs. Proteins that are translocated to the surface with similar rapid kinetics belong to several different families and have diverse structures, including traditional membrane-spanning proteins as well as phosphatidyl inositol-anchored proteins. In addition, secretion of soluble proteins also occurs.11-14 The number and identity of the structures in which these proteins are stored remains unknown and they have not been completely characterized.
Electron microscopic studies suggest that complement receptor type 1 (CR1; the complement C3b/C4b receptor, CD35) is stored in resting PMN in intracellular vesicles that are distinct from primary and specific granules.8 In initial attempts at subcellular fractionation of resting neutrophils using sucrose gradients, CR1 was found in fractions separate from the traditional granules, but these fractions also contained the plasma membranes.15 In more recent studies using Percoll density gradients and free-flow electrophoresis, CR1 was found in fractions enriched in secretory proteins that were distinct from the fractions containing markers for the plasma membrane or traditional granules.11 However, these putative secretory vesicles have only been partially purified, and it is thus far still not clear whether all of the different proteins present in the secretory vesicle fraction are actually stored in the same structures or whether different types of proteins are stored in different structures having similar densities. We therefore sought to purify CR1 storage vesicles by immunoisolation techniques using antibodies against the cytoplasmic tail of CR1, because the receptor should be stored with its extracellular domain inside these vesicles and its cytoplasmic tail protruding outwards and available for binding to immobilized antibody. Magnetic beads were coated with antibodies generated against synthetic peptides corresponding to unique sequences in the tail of CR1. These antibodies bound to native CR1 in detergent lysates of PMN and were used to isolate the CR1-containing vesicles. This immunoisolation technique has allowed direct characterization of the specifically isolated vesicles by immunochemical and enzymatic methods and direct visualization by electron microscopy. The results of these analyses have important implications for hypotheses about the formation of this unique neutrophil secretory compartment and may allow in vitro studies of the mechanisms of its selective translocation during PMN activation.
MATERIALS AND METHODS
Antibodies to cytoplasmic tail of CR1.The predicted amino acid sequence of CR1 has been previously reported by Klickstein et al.16 The sequence of the cytoplasmic tail [(1495) KHRKGNNAHENPKEVAIHLHSQGGSSVHPRTLQTNEENSRVLP (1537, C-terminal)] contains two stretches of unique sequence (underlined) that were selected to maximize the specificity for CR1. The first peptide is a 19-mer corresponding to residues 1500-1518, and the second is an 11-mer beginning after a region homologous to the epidermal growth factor receptor phosphorylation site (residue 1527) and extending to include the C-terminus (residue 1537). The peptides were custom synthesized by Multiple Peptide Systems (San Diego, CA), with cysteines added to the C-terminus of the 19-mer and to the N-terminus of the 11-mer to facilitate conjugation. The peptides were coupled through the terminal cysteine thiol to keyhole limpet hemocyanin (KLH) with the heterobifunctional cross-linking agent maleimidobenzoyl-N-hydroxysuccinimide ester, in a ratio of 1 part peptide to 1 part KLH (wt/wt).
For development of polyclonal antisera, the peptide-KLH conjugates were suspended in Dulbecco's phosphate-buffered saline (PBS) at 3.1 mg/mL and emulsified by mixing with an equal volume of complete Freund's adjuvant. A total volume of 0.6 mL containing 1 mg conjugate was injected into five to six subcutaneous sites in the back of NZW rabbits for the initial immunization. Subsequent boosters consisted of similar doses of conjugate and were administered with complete Freund's adjuvant on day 21 after the initial immunization and on days 42 and 63 using incomplete Freund's adjuvant. Rabbits were bled on days 52, 73, and 80; and sera were separated and frozen at −80°C. The antibody titers were determined against the original immunizing conjugate as well as unconjugated peptides using an enzyme-linked immunosorbent assay (ELISA).
Affinity purification of peptide-specific antibodies.Rabbit antisera were immunoaffinity purified using immobilized peptide columns. Ten milligrams of purified synthetic peptide was coupled to 2 mL of cyanogen bromide-activated agarose gel (Multiple Peptide Systems). After deactivation of the remaining activated sites, the gel was equilibrated in 0.1 mol/L MOPS (3-[N-Morpholino]propanesulfonic acid; Sigma Chemical Co, St Louis, MO) buffer (pH 7.5) at room temperature and poured into a column. The rabbit serum was diluted with an equal volume of MOPS buffer and passed through the column. The column was then washed with an excess of MOPS buffer and the bound antibody was eluted with a pH 3.0 elution buffer (Bio-Rad Laboratories, Richmond, CA). The eluates were extensively dialyzed against PBS (pH 7.4) and protein concentrations were measured using the micro BCA method (Pierce, Rockford, IL).
Preparation of PMN lysates.Polymorphonuclear leukocytes (PMN) were isolated from heparinized peripheral blood drawn from healthy adult donors using Percoll density gradients as previously described.4,8 Contaminating erythrocytes were removed by lysis with hypotonic saline, and PMN were washed in Hanks' Balanced Salt Solution without Ca2+ or Mg2+ and held at 0°C in this solution until beginning the lysis or the N2 cavitation process. Cells prepared in this way are routinely greater than 95% pure by microscopic examination and greater than 95% viable as determined by trypan blue dye exclusion. Lysates were prepared from 1 × 108 PMN/mL by suspension in a lysis buffer consisting of PBS (pH 7.2) with 1% NP-40, 10 mmol/L EDTA, 0.2% sodium dodecyl sulfate (SDS), and the following protease inhibitors: 10 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 100 μmol/L Nα-p-tosyl-L-lysine chloromethyl ketone, 100 μmol/L N-tosyl-L-phenylalanine chloromethyl ketone, 100 μmol/L leupeptin, and 1 μmol/L pepstatin.11 The mixture was held on ice for 1 hour with occasional vortexing, the intact cells and cell debris were separated with a microfuge, and the supernatant was collected and frozen at −80°C until further analysis.
ELISA to detect binding of antipeptide sera to CR1 in PMN lysates.In initial experiments, the previously described capture ELISA for the detection of CR1 in PMN lysates11 was modified for testing the reactivity of antisera raised against synthetic CR1 peptides. Intact CR1 in detergent lysates of PMN was captured on F(ab′)2 of a mouse monoclonal antibody (MoAb; 3D9) to the extracellular domain of CR1 that was coated onto the plate. After washing, rabbit antisera to the cytoplasmic tail peptides were added, incubated, and washed. Binding of rabbit antibody was then detected with peroxidase-conjugated goat antirabbit IgG. In later experiments, purified rabbit antipeptide IgG was used as the coating antibody at a concentration of 10 μg/mL. After the wells were blocked with 5% nonfat dry milk in PBS-Tween (PBST; 0.5% Tween-20; PBST-milk), they were incubated with or without PMN lysates and the captured CR1 was detected with biotinylated MoAb to its extracellular domain, followed by the addition of streptavidin-peroxidase conjugate. After additional washing, the reaction was developed with o-phenylene diamine substrate and plates were read in an automated ELISA reader. Normal rabbit IgG was included as a control in all plates.
Isolation of CR1 storage vesicles from nitrogen cavitates of resting PMN using antipeptide IgG conjugated to magnetic beads.As a first approach to immunoisolation of CR1-containing vesicles, we used a modification of the above-described assay. Instead of PMN lysates prepared with detergent, nitrogen cavitates, in which the vesicles should remain intact, were used and Tween-20 was omitted from all washing buffers. Resting PMN were held at 0°C to prevent the spontaneous upregulation of plasma membrane CR1 expression that may accompany rewarming of isolated PMN.4 The cells were suspended in relaxation buffer5 [100 mmol/L KCl, 3 mmol/L NaCl, 1 mmol/L ATP(Na)2 , 3.5 mmol/L MgCl2 , 10 mmol/L PIPES; pH 7.3] containing protease inhibitors as described above and were subjected to nitrogen cavitation at 4°C as described by Borregaard et al.5 Nuclei and unbroken cells were removed from the crude PMN cavitate by centrifugation at 500g for 10 minutes at 4°C. The resulting postnuclear supernatant (PNS) was layered over a 20-mL Percoll gradient (density = 1.065 g/mL), with a 1-mL Percoll cushion (1.12 g/mL) at the bottom.11 When the gradient was centrifuged for 30 minutes at 37,000g, a light membrane fraction containing the secretory vesicles (γ-band) was found as a distinct band in the upper part of the gradient, whereas the traditional granules formed a broader band at the bottom of the gradient tube. The γ-band and the material just above the band containing the granules was aspirated. The Percoll from the γ-band was pelletted by ultracentrifugation at 180,000g for 1 hour.5 The supernatant was collected and used for the isolation of vesicles containing CR1.
Magnetic beads with covalently bound sheep antirabbit IgG (Dynabeads M-280; Dynal, Inc, Lake Success, NY) were saturated with purified immune or control rabbit IgG at 5 to 10 μg/mg beads at 4°C overnight and then washed with PBS as described earlier.17,18 The beads were then incubated with PBS containing 5% nonfat dry milk and 1% bovine serum albumin (BSA) for 1 hour at 4°C and washed with PBS. Based on results in ELISA assays and other systems, the following steps were used to prevent nonspecific binding. The γ-band prepared as above was first precleared two times in wells of Immulon IV microtiter plates (Dynatech Laboratories, Chantilly, VA) coated with 10 μg/mL of nonimmune rabbit IgG, each time by incubating for 45 minutes at 4°C. The γ-band from these wells was then further precleared by incubation with magnetic beads saturated with nonimmune rabbit IgG for 45 minutes at 4°C. The beads were removed from the γ-band by applying a magnet to the wall of the test tube for about 5 minutes at 4°C. This precleared γ-band was then incubated with beads saturated with purified immune or control nonimmune rabbit IgG overnight at 4°C. After washing with the relaxation buffer containing the protease inhibitors described above, beads were either directly processed for electron microscopic studies or were resuspended in PBS with 1% Triton X-100 or hot 2% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer,19 both containing protease inhibitors, for analysis by enzyme assay or SDS-PAGE and Western blots, respectively.
To analyze the different proteins present in the bound vesicles using both immunoblots and ELISAs, in preliminary experiments we studied the use of SDS sample buffer versus PBS-Triton X-100 for solubilizing the proteins. The results from these experiments indicated that, whereas SDS in the sample buffer readily solubilized CR1, an integral membrane protein found in the membranes of the vesicles, this concentration of SDS interfered with subsequent ELISA assays. On the other hand, Triton X-100 failed to release all of the CR1 present in the vesicles bound to the adsorbent. However, the soluble protein albumin was readily released from these vesicles by Triton X-100 and could then be quantitated by ELISA. This detergent was also satisfactory for assay of latent alkaline phosphatase, which is not accessible in the absence of detergent.14 Therefore, we used aliquots solubilized with SDS sample buffer for immunoblot analysis of CR1, albumin, FcγRIII, decay-accelerating factor (DAF ), α-chain of CR3, and HLA class I; whereas we used samples solubilized in PBS–Triton X-100 for analysis of the total contents of albumin by ELISA and alkaline phosphatase by enzyme assay.
Immunoblotting.Protein samples were run on either 7% or 10% SDS-PAGE under nonreducing conditions in a Mini Protean II Dual Slab Cell (Bio-Rad). In 7% gels, the following prestained molecular weight (MW) markers (Bio-Rad) were used: myosin (205 kD), β-galactosidase (117 kD), BSA (89 kD), and ovalbumin (47 kD). In 10% gels, phosphorylase B (101 kD), BSA (83 kD), ovalbumin (50.6 kD), carbonic anhydrase (35 kD), soybean trypsin inhibitor (29.1 kD), and lysozyme (20.9 kD) were used. Separated proteins on SDS gels from the vesicle isolations were then transferred onto nitrocellulose membranes in 192 mmol/L glycine, 25 mmol/L Tris (pH 8.3), and 20% (vol/vol) methanol, using a Mini transblot electrophoretic transfer cell (Bio-Rad). The membrane was blocked with PBST-milk for 1 hour at room temperature and then reacted with MoAb against CR1, human albumin (Accurate Chemical and Scientific Corp, Westbury, NY), DAF (CD55; Wako Chemicals USA, Inc, Richmond, VA), FcγRIII (CD16b; clone 3G8), HLA class I (clone W6/32), or α-chain of CR3 (CD11b, Dako-C3bi-R; Dako A/S, Glostrup, Denmark) at 1 μg/mL in PBST-milk and incubated for 2 hours at room temperature. This was followed by incubation with 1:1,000 dilution of F(ab′)2 of goat antimouse IgG conjugated to alkaline phosphatase (Organon Teknika Corp, West Chester, PA) for 1 hour and then by three additional washes with PBST. The reaction was developed with 5-bromo-4-chloro-3-indolylphosphate-toluidine and nitroblue tetrazolium chloride (Sigma Chemical Co) and was stopped by washing extensively with distilled water.
ELISA to detect human albumin.Albumin was determined in 1% Triton X-100 extracts of isolated vesicles using an ELISA assay. To determine whether albumin was free and could be detected in the absence of detergent, isolated vesicles and unbound fractions were also resuspended in PBS without Triton X-100 but containing protease inhibitors. All washes in these steps were made with PBS containing protease inhibitors but without any detergent to prevent the premature lysis of intact vesicles. An IgG fraction from rabbit polyclonal antihuman albumin (Boehringer Mannheim Biochemicals, Indianapolis, IN) was used as the capture antibody at 1:5,000 dilution. The unreacted sites in the ELISA plate wells were blocked with PBST buffer containing 5% nonfat dry milk and 1% BSA. After incubation with the samples and washes as before, the plates were incubated with 1:1,000 dilution of mouse antihuman albumin MoAb (Accurate Chemical and Scientific Corp) and washed. The binding of this antibody was detected with peroxidase-conjugated goat antimouse IgG (Sigma Chemical Co). After additional washing, the reaction was developed and read as described above.
Assay for alkaline phosphatase.Latent alkaline phosphatase activity was measured in samples of isolated vesicles after they were permeabilized with PBS containing Triton X-100 as described by Borregaard et al.14 The samples were diluted in sodium-barbital buffer, pH 10.5,20 and were incubated with PNP substrate (1 mg/mL; Sigma Chemical Co) for 90 minutes at 37°C. At the end of the incubation the reaction was stopped with ice-cold sodium barbital buffer and the optical density was immediately measured at 405 nm using a Thermomax reader (Molecular Devices, Sunnyvale, CA). Human placental alkaline phosphatase (P-3895; Sigma Chemical Co) was used as a standard to assure linearity of the assays in the microplate reader. Enzyme activity in isolated fractions was expressed as conventional units: 1 μmol/L substrate converted per minute using 18.6 mmol/L−1 cm−1 as the extinction coefficient for p-nitrophenol.21 Alkaline phosphatase activity was also determined in vesicles resuspended in PBS without Triton X-100 but containing protease inhibitors. As with assays for albumin release, all washes in these assays were made with PBS containing the protease inhibitors described above, but without any detergent to prevent the premature lysis of intact vesicles.
Immunoelectron microscopy.For localization of CR1 or albumin, vesicles isolated on magnetic beads were fixed by the addition of 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4, for 30 minutes at room temperature followed by 8% paraformaldehyde also for 30 minutes at room temperature. Beads were embedded in 10% gelatin, which was then infused overnight at 4°C with polyvinyl pyrrolidone-sucrose.22 The blocks were then mounted on specimen holders and stored in liquid N2 . Ultrathin cryosections were cut on a Reichert Ultracut FC E (Reichert Optische Werke AG, Vienna, Austria) at −130°C, placed on Formvar-coated copper grids (Electron Microscopy Sciences, Fort Washington, PA), and then immunostained as previously described.22 Briefly, sections were blocked using 1% ovalbumin in Tris-buffered saline (TBS; pH 7.6), containing 10−4 mol/L PMSF for 60 minutes. Sections were stained with anti-CR1 MoAbs 3D9 and C543 or anti-albumin MoAb (Accurate) or with nonimmune mouse IgG1 (MOPC 21; Sigma Chemical Co) in TBS containing 1% ovalbumin, 0.2% cold water fish gelatin (Sigma Chemical Co), and 10−4 mol/L PMSF for 90 minutes and were washed four times in TBS-PMSF. Sections were then stained with 5-nm gold-conjugated goat antimouse IgG for 90 minutes, washed again with TBS-PMSF, and washed four additional times with distilled water. The sections were postembedded in a 9:1 mixture of methylcellulose (Sigma Chemical Co) and 3% uranyl acetate and were examined with a JEOL 100 CX transmission electron microscope (JEOL Ltd, Tokyo, Japan).
RESULTS
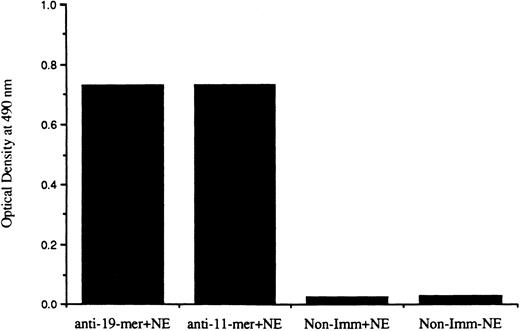
Reactivity of antipeptide antibodies with intact CR1.To specifically isolate the vesicles in which CR1 is stored in resting PMN, we desired to use antibodies against the cytoplasmic tail of CR1, which should be exposed on the outside of these vesicles. We therefore developed antibodies against KLH conjugates of synthetic peptides corresponding to unique sequences within the tail of CR1 and confirmed their reactivity with these peptides. Because reactivity of antisera with synthetic peptides does not necessarily indicate that these antibodies do in fact recognize their determinants in the native molecule, we tested these antibodies against CR1 in a capture ELISA system. The results indicated that the antisera to both peptides reacted with native CR1 in detergent lysates of PMN (Fig 1). We then affinity-purified the specific IgG from the antisera using peptides bound to agarose. We used a sandwich ELISA in which the purified IgG preparations were applied to ELISA plates to test their ability to capture intact CR1 from PMN lysates. Captured CR1 was then detected with biotinylated MoAb to its extracellular domain and peroxidase-streptavidin conjugates. The purified IgG preparations against both peptides clearly bound CR1. In contrast, purified normal rabbit IgG showed no reactivity with CR1. This confirmed that the antipeptide rabbit antibodies bound specifically to the cytoplasmic tail of native CR1. We then attempted to use these antibodies to isolate CR1 storage vesicles directly on magnetic beads.
Reactivity of rabbit antisera against the 19-mer and 11-mer peptides of cytoplasmic tail of CR1. Rabbit sera were tested at a dilution of 1:100 in an ELISA system in which native CR1 was captured from detergent neutrophil extracts (NE) onto wells precoated with F(ab′)2 of mouse MoAb to the extracellular domain of CR1. Binding of rabbit IgG to the captured CR1 was detected with peroxidase-conjugated goat antirabbit antibody and o-phenylene diamine. The sera were tested in parallel in wells that received neutrophil extract containing CR1 (+NE) but nonimmune rabbit IgG and in wells that did not receive CR1 (−NE). The reactivity in wells with immune sera that had not received NE was equal to or less than the values obtained for nonimmune sera with NE. The results represent the mean of duplicate determinations from two separate experiments.
Reactivity of rabbit antisera against the 19-mer and 11-mer peptides of cytoplasmic tail of CR1. Rabbit sera were tested at a dilution of 1:100 in an ELISA system in which native CR1 was captured from detergent neutrophil extracts (NE) onto wells precoated with F(ab′)2 of mouse MoAb to the extracellular domain of CR1. Binding of rabbit IgG to the captured CR1 was detected with peroxidase-conjugated goat antirabbit antibody and o-phenylene diamine. The sera were tested in parallel in wells that received neutrophil extract containing CR1 (+NE) but nonimmune rabbit IgG and in wells that did not receive CR1 (−NE). The reactivity in wells with immune sera that had not received NE was equal to or less than the values obtained for nonimmune sera with NE. The results represent the mean of duplicate determinations from two separate experiments.
Isolation of CR1 storage vesicles using magnetic beads conjugated with antipeptide antibodies.For isolation of intact CR1 storage vesicles, we initially used the whole PNS from the N2 cavitate in preliminary experiments. Because the results from these experiments showed excessive nonspecific binding, we further fractionated the PNS using Percoll density gradients.11 We next prepared the light membrane fraction or γ-band11 from the PNS of nitrogen cavitates of resting PMN. Tween and other detergents were omitted from all washing buffers so that the vesicles would remain intact and could be isolated without lysis. To preserve the integrity of CR1, six different protease inhibitors were incorporated in solutions used in every step of the process. To minimize nonspecific binding, unreacted sites on the beads were blocked with 5% nonfat dry milk and 1% BSA and preclearing steps in which the γ-band was incubated in ELISA wells coated with normal rabbit IgG and then on magnetic beads conjugated with normal rabbit IgG were included (see the Materials and Methods for details). The amount of CR1 present in PNS was 37.0 ± 10.08 ng/mL (mean ± SEM, n = 3) as determined by the indirect ELISA using soluble CR1 (T-cell Sciences, Cambridge, MA) as the standard. The amount of CR1 present in γ-band from the Percoll gradients was more than 85% of that found in PNS, in agreement with previous results,11 and during the preclearing steps there was a less than 5% loss of CR1.
Affinity-purified antibodies against the 11 and 19 residue peptides were combined and bound to sheep antirabbit IgG that was covalently attached to magnetic beads, which then served as an adsorbent to capture CR1 vesicles from the precleared γ-band. The material unbound to the beads after the overnight incubation was collected to determine the efficiency of capturing the CR1-containing vesicles present in the γ-band. After washing the beads with relaxation buffer containing protease inhibitors, the vesicles bound to the beads were either lysed by the addition of hot SDS-PAGE sample buffer for analysis on SDS-PAGE and Western blots or were resuspended in PBS containing Triton X-100 for analysis by ELISA.
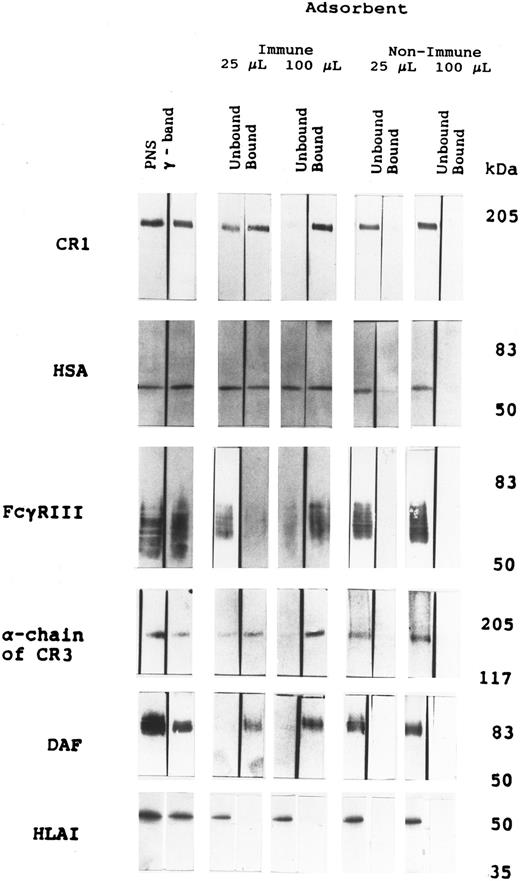
Western blots for CR1 and other proteins.The proteins present in the SDS eluates of the beads were separated on gels that were subsequently transferred onto nitrocellulose membranes and were used to determine the presence of CR1 and other proteins of specific interest in the isolated vesicular structures. When probed with MoAb 3D9 against the extracellular domain of CR1, these blots showed enrichment of the ∼205-kD band corresponding to CR1 in the specifically isolated vesicles (Fig 2). We used two different amounts of beads to optimize recovery of CR1 vesicles while minimizing nonspecific binding. As shown in Fig 2, although a large proportion of the CR1 was bound to 25 μL of beads, a considerable amount of the CR1 remained unbound. When 100 μL of beads were used, the CR1 was totally adsorbed from the γ-band. In contrast, neither 25 nor 100 μL of control beads bearing nonimmune rabbit IgG showed any appreciable binding of CR1. These results indicate that the immunoadsorbent with antibodies against the cytoplasmic tail could quantitatively capture CR1-containing vesicles present in the γ-band.
Western blot of PNS, γ-band, and fractions from vesicle isolation experiments using immune or nonimmune beads. For the detection of CR1 (MW = ∼205 kD) and α-chain of CR3 (MW = 165 kD), samples were run on a 7% SDS-PAGE and transferred onto nitrocellulose filters. CR1 and CR3 in the figure are shown on portion of blot above 117 kD, as determined by the marker β-galactosidase. For the detection of FcγRIII (MW = 50 to 70 kD), albumin (HSA; MW = 67 kD), DAF (MW = 70 kD), and HLA class I (MW = 46 kD), samples were run on a 10% SDS-PAGE and transferred onto nitrocelluose filters (refer to the Materials and Methods for details). FcγRIII, albumin, and DAF in the figure represent the portion of the blot between 101 kD and 35 kD, as determined by the markers phosphorylase B and carbonic anhydrase. HLA class I in the figure represents the portion of the blot between 50 kD and 35 kD, as determined by the markers ovalbumin and carbonic anhydrase. The volume of each sample was adjusted to equal the volume of γ-band applied to allow direct comparisons.
Western blot of PNS, γ-band, and fractions from vesicle isolation experiments using immune or nonimmune beads. For the detection of CR1 (MW = ∼205 kD) and α-chain of CR3 (MW = 165 kD), samples were run on a 7% SDS-PAGE and transferred onto nitrocellulose filters. CR1 and CR3 in the figure are shown on portion of blot above 117 kD, as determined by the marker β-galactosidase. For the detection of FcγRIII (MW = 50 to 70 kD), albumin (HSA; MW = 67 kD), DAF (MW = 70 kD), and HLA class I (MW = 46 kD), samples were run on a 10% SDS-PAGE and transferred onto nitrocelluose filters (refer to the Materials and Methods for details). FcγRIII, albumin, and DAF in the figure represent the portion of the blot between 101 kD and 35 kD, as determined by the markers phosphorylase B and carbonic anhydrase. HLA class I in the figure represents the portion of the blot between 50 kD and 35 kD, as determined by the markers ovalbumin and carbonic anhydrase. The volume of each sample was adjusted to equal the volume of γ-band applied to allow direct comparisons.
Identical blots probed with antialbumin MoAb showed a distinct band at 67 kD (Fig 2). However, the results of the blots in material unbound to 100 μL of immune beads suggest that an equal amount of albumin was still present in the unbound material, whereas the CR1 had been completely removed from this fraction. The proportion of albumin bound to both 25 and 100 μL of anti-CR1 (immune) beads is much greater than the very small amounts bound to equivalent aliquots of nonimmune beads. Taken together, these results suggest that the CR1 vesicles contain albumin, but that there is also albumin in structures not bearing CR1. This conclusion was studied in more detail using ELISAs, as described below.
Additional blots that were run in parallel were reacted with antibodies against FcγRIII, DAF, CD11b (the α-chain of CR3), and HLA class I (Fig 2). A comparison of the amount of each protein present in PNS in relation to γ-band indicates that most of the FcγRIII and HLA class I present in the whole PNS seems to be present in the γ-band, whereas only some of the CR3 and DAF are found in the γ-band. Previous studies have shown that a substantial amount of the FcγRIII in PMN is present in intracellular vesicles that morphologically resemble those in which CR1 is stored.23 In density gradients of cavitates of resting neutrophils, most of the CR3 localizes in fractions containing specific granules and gelatinase granules, whereas only a small fraction of the CR3 sediments with the light membranes11 and/or the plasma membrane-enriched fractions.24-26 Thus, the amount of CR3 in the γ-band that we used for isolation of these vesicles is only a small fraction of the cells' total content of this protein. Apart from kinetic studies of its mobilization,7 there are few data regarding the localization of DAF in PMN. The results of the blots for FcγRIII, α-chain of CR3, and DAF in material bound to 100 μL immune beads show that, of the amount present in the γ-band, most of these proteins are contained in structures adsorbed by the anti-CR1 bearing beads. In contrast, control beads bearing nonimmune rabbit IgG showed very little or no binding of these proteins. These results indicate that the efficiency of isolation of these proteins was similar to that for CR1, suggesting that they are packaged together. Subcellular fractionation studies by Bjerrum and Borregaard27 have shown that a majority of HLA class I is localized on the plasma membrane and therefore it has been used as a plasma membrane marker in human neutrophils. The results of the blots prepared from the vesicles isolated by anti-CR1 bearing beads show that HLA class I is not present in these vesicles (Fig 2). This shows that the binding of vesicles to beads bearing antibodies to the tail of CR1 is specific and that there is no contamination with plasma membrane fragments.
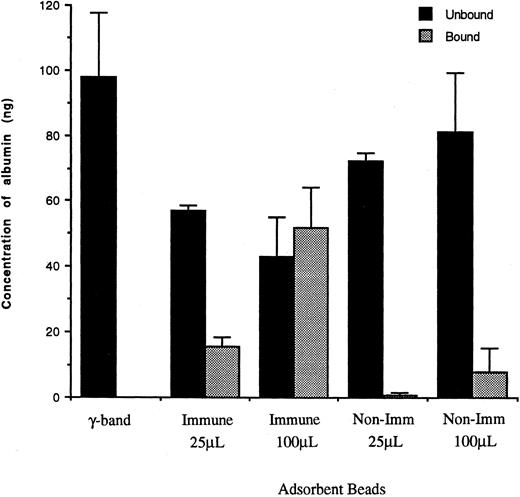
Fractionation of human albumin during adsorption of CR1-containing vesicles. Bound vesicles on beads and unbound supernatants were resuspended in PBS containing 1% Triton X-100 and ELISA was performed as described in the Materials and Methods. () The material bound to either 25 μL or 100 μL beads bearing anti-CR1 peptide IgG (Immune) or to 25 μL or 100 μL beads bearing nonimmune rabbit IgG (Non-Imm). γ-Band and material unbound (▪) to beads were also included to compare the efficiency of isolation. The volume of each sample was adjusted back to the same volume as the initially applied γ-band to allow direct comparison. The results shown are the mean ± SEM of duplicate determinations from each of three separate experiments.
Fractionation of human albumin during adsorption of CR1-containing vesicles. Bound vesicles on beads and unbound supernatants were resuspended in PBS containing 1% Triton X-100 and ELISA was performed as described in the Materials and Methods. () The material bound to either 25 μL or 100 μL beads bearing anti-CR1 peptide IgG (Immune) or to 25 μL or 100 μL beads bearing nonimmune rabbit IgG (Non-Imm). γ-Band and material unbound (▪) to beads were also included to compare the efficiency of isolation. The volume of each sample was adjusted back to the same volume as the initially applied γ-band to allow direct comparison. The results shown are the mean ± SEM of duplicate determinations from each of three separate experiments.
ELISA for albumin and assay for alkaline phosphatase.To better quantitate the albumin in the immunoisolated vesicles, they were permeabilized with PBS containing 1% Triton X-100 and their content of human albumin was quantitated by ELISA. A comparison of the material bound and unbound to 100 μL of immune beads indicates that about 45% of the applied albumin did not bind to anti-CR1 bearing beads (Fig 3, middle set of bars). These results are comparable to those obtained by immunoblotting (Fig 2), which showed that there was a considerable amount of albumin in the unbound fraction, even though there was no CR1 detectable in this fraction of material not bound to 100 μL of immune beads. There was minimal binding of albumin to beads bearing nonimmune IgG (Fig 3, right most set of bars). These results imply that, although the CR1 vesicles contain albumin, there is also a substantial fraction of albumin present in a pool distinct from CR1-containing vesicles.
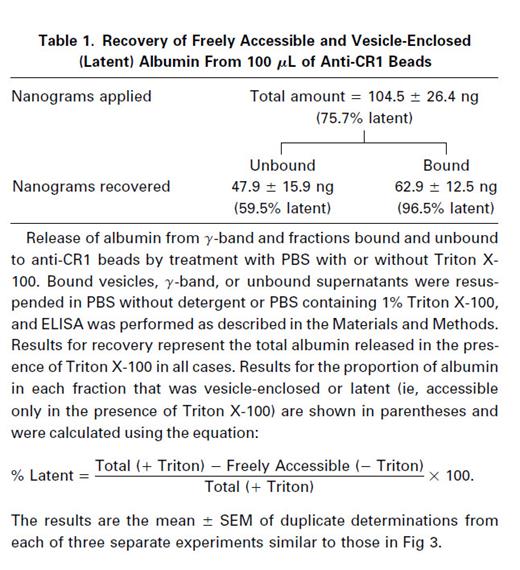
To determine whether the albumin in the various fractions was free or contained within vesicles, release of albumin from the isolated CR1 vesicles and other fractions was tested in the absence and presence of Triton X-100 (Table 1). Of the total amount of albumin detected in the γ-band in the presence of Triton, only ∼25% was freely accessible in the absence of Triton, thus indicating that ∼75% was contained within intact vesicles (latent). The results also show that only about 3.5% of the albumin present in the vesicles bound to anti-CR1 bearing beads was detectable in the absence of Triton X-100, indicating that 96.5% was latent. In the fraction that was unbound to anti-CR1 bearing beads, about 40% of the albumin was freely accessible, whereas approximately 60% was detectable only in the presence of Triton X-100. Nonimmune beads showed very little binding of albumin (11.1 ± 9.4 ng; ≤10% of total applied).
Thus, in the fraction unbound to 100 μL of immune beads, a significant amount of the albumin is still stored inside vesicles, because 60% of it can only be detected in the presence of detergent. However, because the immunoblot for CR1 (Fig 2) shows that this fraction contains little or no CR1, this must be a subset of vesicles that do not contain CR1. This suggests that there is heterogeneity in the secretory vesicle compartment. Because the majority of the albumin contained in the unbound fractions as well as in the bound fractions is only accessible in the presence of the detergent and hence is contained in intact vesicles, there seems to have been relatively little damage to or breakage of vesicles during the isolation procedures. These observations strongly suggest that the vesicles adsorbed to the beads are intact and that the isolation technique we have used is specific. Hence, the release of bound albumin might be adaptable as a useful tool for vesicle-membrane fusion studies.
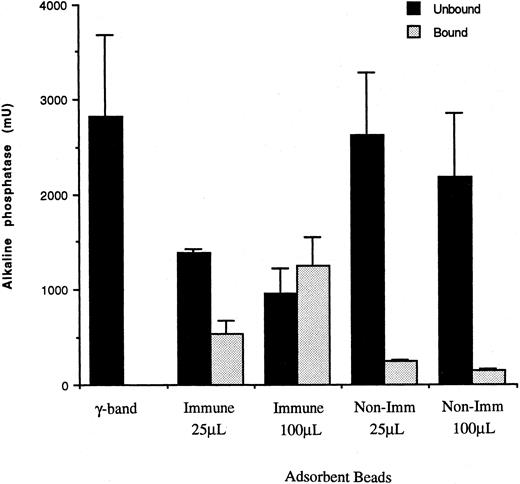
The total recovery of alkaline phosphatase activity in the vesicles was determined in samples that were permeabilized with PBS containing Triton X-100, as described earlier by Borregaard et al.14 The results in Fig 4 show that alkaline phosphatase is specifically enriched in CR1 containing vesicles because the amount of enzyme bound to 100 μL of immune beads (Fig 4, stippled bar, middle pair) is much greater than the minimal amount bound to nonimmune beads (Fig 4, stippled bar, right most pair). It should be noted that the latter did not contain any CR1 (Fig 2). Furthermore, Fig 4 also shows that about ≤40% of the alkaline phosphatase applied to 100 μL of immune beads failed to bind (solid bar, middle pair). This contrasts with Fig 2, which showed that this fraction did not contain any CR1.
Fractionation of alkaline phosphatase during adsoption of CR1-containing vesicles. Bound vesicles and unbound supernatants were resuspended in PBS containing 1% Triton X-100 and assayed for alkaline phosphatase using PNP substrate in sodium-barbital buffer, pH 10.5, as described in the Materials and Methods. () The material bound to either 25 μL or 100 μL beads conjugated to anti-CR1 peptide IgG (Immune) or to 25 μL or 100 μL beads conjugated to nonimmune rabbit IgG (Non-Imm). (▪) γ-Band and unbound material. All volumes were adjusted to equal the volume of the γ-band originally applied to allow direct comparisons. The results shown are the mean ± SEM of duplicate determinations from each of three separate experiments.
Fractionation of alkaline phosphatase during adsoption of CR1-containing vesicles. Bound vesicles and unbound supernatants were resuspended in PBS containing 1% Triton X-100 and assayed for alkaline phosphatase using PNP substrate in sodium-barbital buffer, pH 10.5, as described in the Materials and Methods. () The material bound to either 25 μL or 100 μL beads conjugated to anti-CR1 peptide IgG (Immune) or to 25 μL or 100 μL beads conjugated to nonimmune rabbit IgG (Non-Imm). (▪) γ-Band and unbound material. All volumes were adjusted to equal the volume of the γ-band originally applied to allow direct comparisons. The results shown are the mean ± SEM of duplicate determinations from each of three separate experiments.
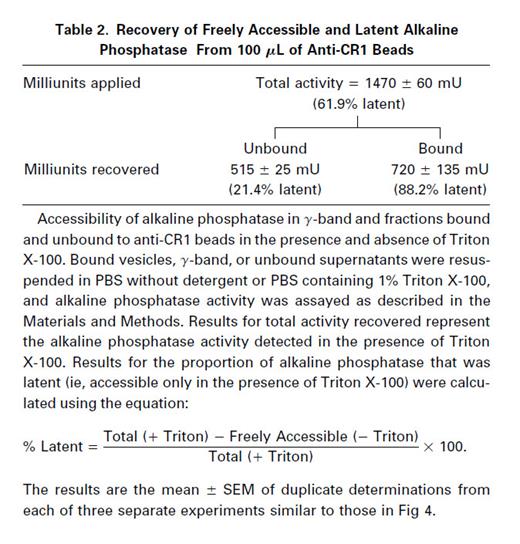
Determination of whether the alkaline phosphatase in the various fractions was freely accessible or enclosed in vesicles (latent) was determined by comparing samples not containing detergent with samples permeabilized with PBS containing Triton X-100, respectively.14 The results in Table 2 show that more than 60% of the alkaline phosphatase present in the γ-band applied to the immunoadsorbent was latent (detectable only in the presence of Triton X-100), whereas less than 40% was readily accessible (nonlatent). In the vesicles that were bound to immune beads, more than 88% of the detectable alkaline phosphatase activity was latent, thus suggesting that latent alkaline phosphatase is stored inside CR1 containing vesicles. In the fraction that was unbound to immune beads, about 21% of the alkaline phosphatase activity was latent, whereas 79% was freely accessible. These results suggest that most of the alkaline phosphatase detected in the unbound fraction represents fragments of or vesicles derived from the plasma membrane, in which the active site of the enzyme is exposed externally. Because only 21% of the alkaline phosphatase activity in the fraction not bound to immune beads was latent and this fraction contained ∼40% of the total alkaline phosphatase recovered, we conclude that less than 10% of the latent alkaline phosphatase in the cell is present in vesicles that do not contain CR1.
Nonimmune beads bound very little of either latent or freely accessible alkaline phosphatase activity. Of the small amount of alkaline phosphatase bound to the nonimmune beads, there was no enrichment of vesicle-enclosed enzyme, because the proportion that was latent (∼50%, not shown) was no higher than that in the material applied (Table 2, total activity).
The lack of binding of HLA class I to anti-CR1 beads as indicated in Fig 2, the very small amount of freely accessible alkaline phosphatase bound to the anti-CR1 bearing beads (Fig 4 and Table 2), and the minimal nonspecific binding of latent alkaline phosphatase to nonimmune beads all suggest that there is very little nonspecific binding of intracellular or plasma membrane vesicles onto the anti-CR1 beads and, thus, that the technique we have adopted for the isolation of CR1 vesicles is very specific.
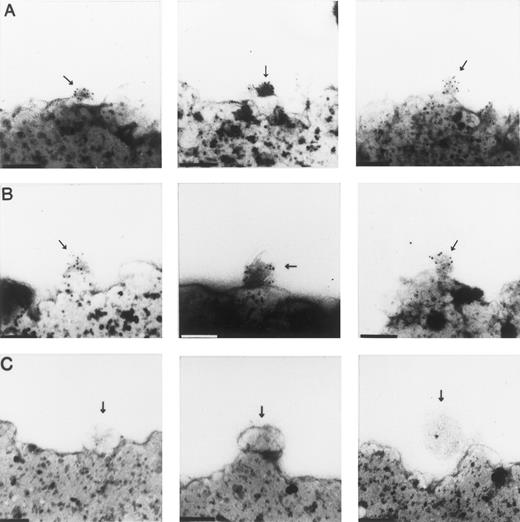
Electron microscopy for CR1 and albumin.An advantage of the use of magnetic beads as the immunoadsorbent is that these particles can be processed directly for EM studies,18,28 thus obviating any losses or alterations in the vesicles that might occur in trying to elute them from other adsorbents. Cryosections of vesicles bound to magnetic beads bearing antibodies against the CR1 tail peptides were probed with a mixture of MoAbs 3D9 and C543 against the extracellular domain of CR1, and the latter antibodies were identified with gold-conjugated goat antimouse IgG. The vesicles isolated on magnetic beads conjugated with antipeptide rabbit IgG stained positively for CR1 (Fig 5A). These structures appear as small vesicles with irregular borders with mean diameters of 0.086 to 0.1 μm, consistent with the size and shape of the CR1-containing structures in sections of intact resting cells.8 The somewhat variable appearance of the vesicles bound to the beads is compatible with results in other systems in which immunomagnetic beads have been used to isolate small secretory or synaptic vesicles.29 These vesicles also stained positively with MoAb against albumin (Fig 5B). In contrast, cryosections of vesicles probed with nonimmune mouse IgG (MOPC 21) did not bind any gold particles (Fig 5C). Cryosections from beads conjugated with nonimmune IgG did not show any bound vesicles, and none of the beads showed any typical appearing primary or secondary granules.
Immunoelectron microscopy of vesicles isolated on magnetic beads coated with antibodies to CR1 peptides. (A) Vesicles stained with a mixture of MoAbs 3D9 and C543 to the extracellular domains of CR1. (B) Vesicles stained with antihuman albumin MoAb. (C) Vesicles stained with control nonimmune antibody MOPC 21. Arrows show vesicles specifically bound to beads bearing antibodies to CR1 tail peptides. (A) and (B) show staining with 5-nm gold-conjugated goat antimouse IgG. (C) fails to show staining with the same gold-conjugated goat antimouse IgG. Bars = 0.1 μm.
Immunoelectron microscopy of vesicles isolated on magnetic beads coated with antibodies to CR1 peptides. (A) Vesicles stained with a mixture of MoAbs 3D9 and C543 to the extracellular domains of CR1. (B) Vesicles stained with antihuman albumin MoAb. (C) Vesicles stained with control nonimmune antibody MOPC 21. Arrows show vesicles specifically bound to beads bearing antibodies to CR1 tail peptides. (A) and (B) show staining with 5-nm gold-conjugated goat antimouse IgG. (C) fails to show staining with the same gold-conjugated goat antimouse IgG. Bars = 0.1 μm.
DISCUSSION
In this study we used antibodies against synthetic peptides from the cytoplasmic tail of CR1 to isolate CR1-containing storage vesicles from resting PMN. Previous studies from our laboratory showed that the sites of intracellular storage of CR1 in resting neutrophils are structurally unique and morphologically distinguishable from traditional neutrophil granules.8 More recent density gradient and free-flow electrophoresis studies have suggested that CR1 may be stored in secretory vesicles that also contain albumin and latent alkaline phosphatase.11 Several lines of indirect evidence, including kinetic and pharmacological studies, suggest that DAF may also be stored in these vesicles, because the kinetics of upregulation of DAF and CR1 expression in response to different stimuli are similar and also resemble those for translocation of alkaline phosphatase to the plasma membrane and for secretion of albumin.7,11,12,14 CR1, alkaline phosphatase, and some but not all of the cell's CR3 are considered readily mobilizable because they can clearly be translocated to the PMN surface without exocytosis of traditional granule constituents such as myeloperoxidase, lactoferrin, or B12 binding protein.11-14,30 Also, immunochemistry and double-labeling immunoelectron microscopy studies with antibodies against cytochrome b558 and CR3 has shown that these proteins colocalized with albumin in the same vesicles.24 However, the movement of an additional portion of the CR3 can be differentiated from that of CR1 pharmacologically and kinetically11,31 and its storage pool is physically distinct.11 15 It thus seems clear that CR1 is stored separately from the traditional granules and most of the CR3. However, despite these results, there has been no direct evidence on whether the different readily mobilizable proteins (CR1, alkaline phosphatase, DAF, and albumin) are stored together in a single type of secretory vesicle or whether they are stored in distinct structures that share some physical properties.
Earlier studies attempting to identify the distinct intracellular pools of CR1 and CR3 by density gradient centrifugation failed to resolve the CR1 storage compartment from the plasma membrane-containing fractions.15 Although high-voltage free-flow electrophoresis did result in separation of secretory vesicles from neutrophil plasma membrane fragments,11 it was not clear that this technique resulted in the isolation of a single population of biochemically homogeneous vesicles.
To approach this problem of heterogeneity of vesicular structures in PMN, we used a specific immunoaffinity technique. Similar immunoisolation techniques using antibodies to exposed cytoplasmic tails of other receptors and transporters have been successfully used in the isolation and characterization of a variety of vesicular structures from other types of cells.29,32-39 The specific enrichment we achieved using antibodies to the cytoplasmic tail of CR1 is indicated in the Western blot of vesicles isolated using immune rabbit IgG as opposed to normal IgG (Fig 2). The lack of binding of CR1-containing vesicles to beads bearing nonimmune IgG and the lack of binding of the plasma membrane marker HLA class I to the beads bearing anti-CR1 show the specificity of this immuno-affinity technique. Electron micrographs of the isolated CR1 containing structures bound to their adsorbent showed somewhat irregularly shaped vesicles that correspond in size to the CR1-containing vesicles demonstrated earlier in fixed, intact PMN.8 These vesicles stained positively with MoAb to the extracellular domain of CR1 as well as with MoAb to albumin.
Normal human neutrophils have been shown to contain intracellular albumin in small, membrane-limited structures that have been referred to as secretory vesicles because albumin is readily secreted after stimulation by chemoattractants.11,13 Previous studies from our laboratory have shown that, in fMLP-stimulated PMN, albumin was endocytosed into multivesicular bodies and that the membranes of these structures contained CR1 that originated from the plasma membrane and that was initially reinternalized in small endocytic vesicles along with the endocytosed albumin tracer.40 Our present study provides positive confirmation that CR1-containing vesicles in resting cells also contain albumin by both the immunoblotting studies as well as the immunoelectron microscopy of specifically isolated vesicles. Because earlier observations indicate that PMN do not synthesize albumin,13 its presence in CR1 vesicles is consistent with previous results, suggesting it gains access to these vesicles as they form during the endocytic process.13,40 The presence of endocytosed plasma proteins in the CR1 storage vesicles suggests that they may be analogous to the α-granules in which endocytosed plasma proteins are stored in platelets.41
It has been previously shown that secretory vesicles can be separated from plasma membrane vesicles by using free-flow electrophoresis and that their markers appear on the plasma membrane after mild PMN stimulation.13,42 Our results show that the CR1-containing vesicles also contain the GPI-linked proteins DAF, FcγRIII, and alkaline phosphatase in addition to some CR3 and about half of the albumin, which support earlier kinetic studies and other indirect observations.7,9,11,23-26,31 Previous electron microscopic observations have indicated that some FcγRIII in human neutrophils is present in small vesicles23 and that stimulation by chemoattractants causes a rapid translocation of intracellular FcγRIII to the PMN surface.9 Those results are thus also consistent with our current studies, which suggest that a major fraction of the intracellular FcγRIII is stored in the CR1 vesicles. Our observations that the GPI-linked proteins DAF, alkaline phosphatase, and FcγRIII are stored in the CR1 vesicles suggest that the cytoplasmic tail of CR1, or the lack of any cytoplasmic tail at all by the GPI-linked proteins, may not be the major determinant of the intracellular packaging of these proteins. However, it is not known how these structurally different proteins are sorted into the same storage compartment. We speculate that mixing of proteins initially packaged in different compartments may take place on the plasma membrane, perhaps after gradual exocytosis of different types of vesicles and granules in resting cells as they circulate or after initial constitutive plasma membrane expression. Reinternalization of these membrane proteins along with endocytosed soluble plasma proteins13 probably accounts for the content of diverse types of membrane proteins and soluble constituents in the CR1 vesicles. However, there must be some specificity of membrane internalization or recycling, because HLA class I antigens are not included in the same structures as CR1 and these other proteins, consistent with the previous separation of secretory vesicles from plasma membrane fragments.11 27
Our immunoblotting studies indicate that CR1 was completely removed from the γ-band by the immunoadsorbent. However, our results also show that a significant amount of albumin is found separately from the CR1 vesicles. The fact that the majority of the albumin in the fractions that did not bind to the anti-CR1 adsorbent was detectable only in the presence of Triton X-100 indicates that these fractions also contain intact vesicles. These data thus indicate that there are populations of albumin-containing vesicles that are distinct from CR1 vesicles, suggesting that they may have arisen by endocytosis of membrane domains free from CR1. Although about 40% of the alkaline phosphatase did not bind to the anti-CR1 beads, only ∼21% of this activity was latent, as compared with 88% latency of the activity bound to the anti-CR1 containing beads. Thus, greater than 90% of the latent alkaline phosphatase activity in the cell is stored in the CR1 vesicles, in agreement with previous results from Borregaard's laboratory.11 Most of the alkaline phosphatase not bound to the anti-CR1 adsorbent is thus likely to be in plasma membrane fragments or right-side out vesicles.
Our results suggest that CR1, DAF, alkaline phosphatase, and FcγRIII are stored in the same structures. These vesicles also contain albumin and a small amount of the cell's CR3, but they are distinct from and not contaminated with plasma membranes. Using these isolated CR1 storage vesicles and the knowledge that they contain releasable soluble products (albumin) as well as latent enzyme activity, we may now be able to study the mechanisms of their fusion with plasma membranes in vitro. This should allow us to determine how the exocytosis of readily mobilizable compartments such as the CR1 storage vesicles differs from that of the traditional primary and secondary granules and should greatly advance our understanding of the sequence of events during early neutrophil activation.
ACKNOWLEDGMENT
We are grateful to Drs Alan Tartakoff and Carolina Jost for advice and helpful discussions.
Supported by National Institutes of Health Grant No. AI22687.
Address reprint requests to Melvin Berger, MD, PhD, Department of Pediatrics, Rainbow Babies and Children's Hospital, 2101 Adelbert Rd, Cleveland, OH 44106.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal