Abstract
The Revised European-American Lymphoma (REAL) classification has been criticized for its emphasis on the unproven clinical relevance of immunophenotype. A worse prognosis for peripheral T-cell non-Hodgkin's lymphomas (PTCLs) has been inconsistently reported in part because the definition of PTCL has been imprecise (eg, T-cell–rich B-cell non-Hodgkin's lymphomas [TCRBCLs] have been misdiagnosed as PTCLs in the past) and because its correlation with other known prognostic factors has not been studied by multivariate analysis. We analyzed six protocols from 1984 to 1995 with Working Formulation intermediate grade and immunoblastic lymphomas (exclusive of mantle cell) and selected only those cases in which immunophenotyping was performed and was conclusive. Of a total of 560 evaluable patients, 68 were PTCLs (12%) and the remaining 492 (88%) were B-cell non-Hodgkin's lymphomas, including 16 TCRBCLs (3% of total). The 5-year failure-free survival (FFS) for PTCLs and B-cell large-cell lymphomas (BCLCLs) is 38% and 55%, respectively (P < .0001) and the 5-year overall survival (OS) is 39% and 262%, respectively (P < .001). The M.D. Anderson prognostic tumor score (MDATS) and International Prognostic Index (IPI) for all patients was calculated. With MDATS of less than 3 (good prognosis), the 5-year FFS for PTCL and BCLCL is 56% and 69%, respectively (P = .01), and the 5-year OS is 64% and 77%, respectively (P = .06). With MDATS of greater than 2 (poor prognosis), 5-year FFS for PTCL and BCLCL is 26% and 38%, respectively (P = .03), and the 5-year OS is 24% and 41%, respectively (P = .02). With an IPI of less than 3 (good prognosis), the 5-year FFS for PTCL and BCLCL is 49% and 64%, respectively (P = .001), and the 5-year OS is 55% and 71%, respectively (P = .013). With an IPI greater than 2 (poor prognosis), the 5-year FFS for PTCL and BCLCL is 11% and 35%, respectively (P = .044), and the 5-year OS is 10% and 40%, respectively (P = .011). Multivariate analysis shows that MDATS, IPI, and T-cell phenotype are totally independent and are the most significant predictors of FFS and OS. The 68 PTCLs include 45 PTCLs unspecified, 10 Ki-1 anaplastic (ALCL), 8 angioimmunoblastic, and 5 angiocentric lymphomas. Angiocentrics were usually refractory (1 of 5 remissions only). ALCL rarely relapsed late. We conclude that the immunophenotypic basis of the REAL classification is clinically relevant and that, although other prognostic features also influence outcome, the T-cell phenotype still remains an independent and significant prognostic factor.
T-CELL NON-HODGKIN'S lymphomas (NHLs) are uncommon types of lymphoma. As a result, the clinical relevance of identifying the T-cell origin of a particular lymphoma is still debated. To compound the problem, T-cell NHLs are a heterogenous group of tumors that have been difficult to classify. The Revised European-American Lymphoma (REAL) classification has first divided lymphomas on the basis of B- or T-cell immunophenotype and has then attempted to define certain subtypes of T-cell NHL on the basis of clinical and pathological criteria.1 The most distinctive division within the T-cell NHLs is between those with a mature immunophenotype from those with an immature immunophenotype. Phenotypically immature T-cell NHLs are generally classified as precursor T-cell lymphoblastic lymphoma. The REAL classification assigns the remaining mature T-cell NHLs to the peripheral T-cell lymphoma (PTCL) category. These postthymic T-cell NHLs have been more difficult to classify aside from a few clinically distinct categories such as mycosis fungoides/Sezary's syndrome and adult T-cell lymphoma/leukemia arising in human T-lymphotropic virus-1 (HTLV-1)–infected individuals. Although uncommon, the incidence of PTCL varies quite substantially from 8% to 30% in cohorts of aggressive NHLs, depending on the series.2-5 The recently described T-cell–rich B-cell NHL is important in this regard as it can easily be misdiagnosed as a PTCL causing false conclusions about the prognosis of the latter.6 PTCL has suffered from being uncommon, subclassified in a variety of different ways, and possibly misdiagnosed in some cases and, thus, to date has remained poorly understood.
The study and treatment of aggressive NHLs have benefitted from the application of recently defined prognostic models.7 These models have not studied the influence of immunophenotype as it relates to response and survival. In those studies that have tried to determine whether PTCL has a worse prognosis than B-cell large-cell lymphomas (BCLCL), the results have been inconsistent.2-5,8-18 The comparison of B- and T-cell lymphomas has not to date been stratified for differences in other established prognostic factors. On the other hand, the comparison would be simple, but most studies have confirmed the findings that PTCL occurs more frequently in the elderly, with more advanced stage, frequent extranodal disease, and constitutional symptoms.2,3,9,10,12,13,15 These recognized poor prognostic factors thus may confound a meaningful comparison of these disorders. Given these limitations, it is not surprising that several studies have found that PTCL has a worse prognosis than BCLCL,2-4,8,9,15,16 whereas others have not.5 18
In an attempt to correct for previous limitations in comparing PTCL with aggressive B-cell NHLs, we report a single institution retrospective analysis assessing the prognostic value of immunophenotype in aggressive NHLs with stratification for other prognostic factors using the International Prognostic Index (IPI) and M.D. Anderson prognostic tumor score (MDATS).7,19 20 We also report the applicability of these risk models to PTCL.
MATERIALS AND METHODS
We retrospectively reviewed six clinical trials conducted at M.D. Anderson Cancer Center between March 1984 and March 1995. Patient enrollment by March 1995 was used as the cutoff date for currently active trials. Table 1 shows the six trials included in this study, the specific regimen name, the dates of active patient accrual, and the number of patients with B-cell and T-cell immunophenotype included in our study. These six trials represent all front-line chemotherapy trials for aggressive NHLs without prior treatment conducted at M.D. Anderson during this period and reflect individually different therapeutic approaches to intermediate-grade NHLs dictated by risk stratification of patients using either the M.D. Anderson prognostic stage or the more recent prognostic tumor score (MDATS).19 20 They include patients with both favorable and unfavorable prognostic features.
Protocol and Patients
| Regimen . | Dates (from-to) . | Total No. of Patients Enrolled . | Confirmed Immunophenotype and Evaluable . | B-Cell . | T-Cell . |
|---|---|---|---|---|---|
| CHOPB/CMED (DM84-24) good prognosis | 3/84-4/88 | 143 | 80 | 72 | 8 |
| CHODB/DHAP (DM86-10) poor prognosis | 12/87-11/91 | 63 | 46 | 17 | 29 |
| CHOPB/OPEN/XRT (DM88-087) good prognosis | 8/88-1/93 | 114 | 72 | 69 | 3 |
| ATT (DM88-089) poor prognosis | 8/88-8/92 | 322 | 165 | 153 | 12 |
| Id ATT (DM92-054) poor prognosis | 8/92-3/95 | 152 | 107 | 98 | 9 |
| CHOPB/XRT (DM93-003) good prognosis | 1/93-3/95 | 112 | 90 | 83 | 7 |
| Total | 3/84-3/95 | 906 | 560 (62%) | 492 | 68 |
| Regimen . | Dates (from-to) . | Total No. of Patients Enrolled . | Confirmed Immunophenotype and Evaluable . | B-Cell . | T-Cell . |
|---|---|---|---|---|---|
| CHOPB/CMED (DM84-24) good prognosis | 3/84-4/88 | 143 | 80 | 72 | 8 |
| CHODB/DHAP (DM86-10) poor prognosis | 12/87-11/91 | 63 | 46 | 17 | 29 |
| CHOPB/OPEN/XRT (DM88-087) good prognosis | 8/88-1/93 | 114 | 72 | 69 | 3 |
| ATT (DM88-089) poor prognosis | 8/88-8/92 | 322 | 165 | 153 | 12 |
| Id ATT (DM92-054) poor prognosis | 8/92-3/95 | 152 | 107 | 98 | 9 |
| CHOPB/XRT (DM93-003) good prognosis | 1/93-3/95 | 112 | 90 | 83 | 7 |
| Total | 3/84-3/95 | 906 | 560 (62%) | 492 | 68 |
The six trials.The oldest trial, DM84-23, included all adults (>16 years of age) with any Ann Arbor stage (AA). AA stage I patients received 8 cycles of CHOPBleo (cyclophosphamide at 750 mg/m2 on day 1, adriamycin at 50 mg/m2 on day 1, vincristine at 2 mg on day 1, and bleomycin at 5 mg/m2 on day 1 intravenously [IV], and prednisone at 100 mg orally [PO] on days 1 through 5). They also received 4 cycles more of COP (CHOPBleo without the adriamycin and bleomycin) and involved field radiotherapy. AA stage II or greater received alternating courses of CHOPBleo and CMED (cyclophosphamide at 500 mg/m2 on day 1, etoposide at 100 mg/m2/d on days 2 through 4, and methotrexate at 1 g/m2 on day 1 with leucovorin rescue days 2 and 3 IV, as well as dexamethasone at 40 mg/d PO on days 1 through 5) for a total of 12 cycles with involved field radiation in stage II and III patients.
DM86-10 was designed for poor prognosis patients and included initially adults with intermediate-grade NHLs and AA stage III or IV disease, but because of preliminary encouraging results in PTCL, was amended in 1989 to accrue only PTCL from that point on. It consists of 8 total cycles of alternating CHODBleo (CHOPBleo with prednisone replaced by dexamethasone at 40 mg on days 1 through 4 PO) and DHAP (dexamethasone at 40 mg on days 1 through 4, cisplatin at 100 mg/m2 continuous infusion (CI) for 24 hours on day 1, and Ara-c at 2 g/m2 every 12 hours for two doses on day 2 IV).
DM88-087 was designed for the most favorable prognosis patients and included adults with M.D. Anderson stage A only.19 21 They received 3 cycles of CHOPBleo and involved field radiation if only a single nodal site less than 5 cm was involved; if more than one nodal site was involved or if the tumor measured 5 to 7 cm, then CHOPBleo alternating with OPEN (vincristine at 2 mg on day 1, etoposide at 100 mg/m2 on days 1 through 3, and mitoxantrone at 10 mg/m2 on day 1 IV, as well as prednisone at 100 mg on days 1 through 5 PO) was administered for 6 total cycles. Involved field radiation was used to consolidate the treatment to initial tumor sites 5 to 7 cm in diameter.
DM88-089 was designed for patients with a poor enough prognosis not to warrant inclusion in DM88-087. It consists of 12 total cycles of alternating triple therapy (ATT) with ASHAP (ara-c, adriamycin, and cisplatin), MBACOS (adriamycin, cyclophosphamide, vincristine, bleomycin, and solumedrol), and MINE (mesna, ifosfamide, mitoxantrone, and etoposide).22
DM92-054 was designed for poor prognosis patients and included adults with an MDATS greater than 2.20 It consists of 8 cycles of alternatively ASHAP or MBACOS with randomization to idarubicin (2 mg/m2 on days 1 through 3) instead of adriamycin in ASHAP (2.33 mg/m2 on days 1 through 3) in MBACOS. This is followed by consolidation with 3 cycles of MINE for complete responders.
DM93-003 was designed for good prognosis patients and included adults with an MDATS less than 3. It consists of 3 cycles of CHOPB and involved field radiation in those with a single nodal site involved or 6 cycles of alternating CHOPB/OPEN as well as involved field radiation for initial bulky sites greater than 7 cm.
Patient data.All pathology slides were reviewed by one or more members of the Hematopathology Section, M.D. Anderson Cancer Center. All patients who had a confirmed immunophenotype were included in our study. Of the total 906 patients enrolled in these studies, 560 had confirmed immunophenotype. All patients were negative for human immunodeficiency virus. All patients after 1988 were serologically tested for HTLV-1 and were excluded from our analysis if positive.
There were 492 patients who had a B-cell immunophenotype as assessed with either fresh or paraffin-embedded tissue by flow cytometry or immunohistochemistry for B-cell antigens. B-cell immunophenotype was determined by the expression of at least one pan-B antigen or by the expression of monotypic light chain combined with the negativity of at least one pan-T antigen. In frozen tissue samples antibodies versus κ and λ light chains, Leu-1 (CD5), Leu-4 (CD3), and Leu-14 (CD22) were used, whereas in paraffin-embedded tissues, antibodies versus L-26 (CD20), UCHL-1 (CD45RO), Leu-22 (CD43), Leu-M1 (CD15), and LCA (CD45) were used. The majority of samples were fresh. Included as B-cell phenotype were 16 cases of T-cell–rich B-cell NHL identified in a previous review of these trials.6 Patients were considered to have a T-cell immunophenotype if they expressed at least two T-cell antigens and lacked B-cell antigens. Most were tested with the same antibodies as in the BCLCL with additional staining with Ki-1 (CD30), Leu-2a or T8 (CD8), Leu-3a or T4 (CD4), Leu-9 (CD7), and/or Leu-19 (CD56), depending on the case. Again, the majority of samples were fresh tissue.
Initially, 72 patients were found to have a T-cell immunophenotype. All of these cases were reviewed again by a senior hematopathologist to confirm the diagnosis and classification of PTCL using currently accepted criteria.23 24 Four cases were excluded: 1 because the diagnosis was changed to Hodgkin's disease, 1 that was an atypical case of Sezary's syndrome, and 2 that were unclassifiable. The remaining 68 patients all met the histopathologic criteria for PTCL.
Classification of PTCL
| Real Classification . | +TCRβ . | CD30+ . | Age* . | AA 3+4 (%) . | CR Rate (%) . | OS† . |
|---|---|---|---|---|---|---|
| PTCL unspecified (N = 45) | 10/13 | 5/13 | 55 | 80 | 69 | 19 |
| ALCL (N = 10) | 2/2 | 9/10 | 44 | 60 | 80 | 23 |
| AIL (N = 8) | 3/5 | 1/1 | 70 | 88 | 63 | 24 |
| Angiocentric (N = 5) | 0/1 | 0 | 36 | 20 | 20 | 23 |
| Total (N = 68) | 15/21 | 15/24 | 54 | 78 | 65 |
| Real Classification . | +TCRβ . | CD30+ . | Age* . | AA 3+4 (%) . | CR Rate (%) . | OS† . |
|---|---|---|---|---|---|---|
| PTCL unspecified (N = 45) | 10/13 | 5/13 | 55 | 80 | 69 | 19 |
| ALCL (N = 10) | 2/2 | 9/10 | 44 | 60 | 80 | 23 |
| AIL (N = 8) | 3/5 | 1/1 | 70 | 88 | 63 | 24 |
| Angiocentric (N = 5) | 0/1 | 0 | 36 | 20 | 20 | 23 |
| Total (N = 68) | 15/21 | 15/24 | 54 | 78 | 65 |
Abbreviation: N, number of patients.
Median age in years.
Median OS in months.
Comparison of Clinical Features and Outcomes
| Factor . | BCLCL (N = 492) . | PTCL (N = 68) . | P Value . |
|---|---|---|---|
| Mean age (yr) | 54% | 52% | NS |
| Age >60 yr | 43% | 38% | NS |
| Stage 3 or 4 | 48% | 74% | <.001 |
| B symptoms | 28% | 56% | <.001 |
| Extranodal disease >1 | 15% | 16% | NS |
| PS >1 | 15% | 21% | NS |
| LDH elevated | 44% | 59% | .02 |
| β2M >3.0 mg/dL | 26% | 46% | .001 |
| IPI 0 or 1 | 46% | 34% | |
| IPI 2 | 25% | 35% | |
| IPI 3 | 18% | 13% | |
| IPI >3 | 11% | 18% | |
| MDATS 0 or 1 | 40% | 25% | |
| MDATS 2 | 15% | 18% | |
| MDATS 3 | 20% | 27% | |
| MDATS > 3 | 25% | 30% | |
| CR rate | 76% | 65% | .04 |
| 5-yr FFS | 56% | 38% | <.001 |
| 5-yr OS | 63% | 39% | <.001 |
| IPI >2 CR rate | 60% | 43% | .15 |
| IPI <3 CR rate | 83% | 75% | .15 |
| IPI >2 5-yr FFS | 35% | 11% | .044 |
| IPI <3 5-yr FFS | 64% | 49% | .001 |
| IPI >2 5-yr OS | 40% | 10% | .011 |
| IPI <3 5-yr OS | 71% | 55% | .013 |
| MDATS >2 CR rate | 61% | 53% | .33 |
| MDATS <3 CR rate | 88% | 80% | .22 |
| MDATS >2 5-yr FFS | 37% | 25% | .032 |
| MDATS <3 5-yr FFS | 70% | 56% | .007 |
| MDATS >2 5-yr OS | 41% | 24% | .026 |
| Factor . | BCLCL (N = 492) . | PTCL (N = 68) . | P Value . |
|---|---|---|---|
| Mean age (yr) | 54% | 52% | NS |
| Age >60 yr | 43% | 38% | NS |
| Stage 3 or 4 | 48% | 74% | <.001 |
| B symptoms | 28% | 56% | <.001 |
| Extranodal disease >1 | 15% | 16% | NS |
| PS >1 | 15% | 21% | NS |
| LDH elevated | 44% | 59% | .02 |
| β2M >3.0 mg/dL | 26% | 46% | .001 |
| IPI 0 or 1 | 46% | 34% | |
| IPI 2 | 25% | 35% | |
| IPI 3 | 18% | 13% | |
| IPI >3 | 11% | 18% | |
| MDATS 0 or 1 | 40% | 25% | |
| MDATS 2 | 15% | 18% | |
| MDATS 3 | 20% | 27% | |
| MDATS > 3 | 25% | 30% | |
| CR rate | 76% | 65% | .04 |
| 5-yr FFS | 56% | 38% | <.001 |
| 5-yr OS | 63% | 39% | <.001 |
| IPI >2 CR rate | 60% | 43% | .15 |
| IPI <3 CR rate | 83% | 75% | .15 |
| IPI >2 5-yr FFS | 35% | 11% | .044 |
| IPI <3 5-yr FFS | 64% | 49% | .001 |
| IPI >2 5-yr OS | 40% | 10% | .011 |
| IPI <3 5-yr OS | 71% | 55% | .013 |
| MDATS >2 CR rate | 61% | 53% | .33 |
| MDATS <3 CR rate | 88% | 80% | .22 |
| MDATS >2 5-yr FFS | 37% | 25% | .032 |
| MDATS <3 5-yr FFS | 70% | 56% | .007 |
| MDATS >2 5-yr OS | 41% | 24% | .026 |
Abbreviation: NS, not significant.
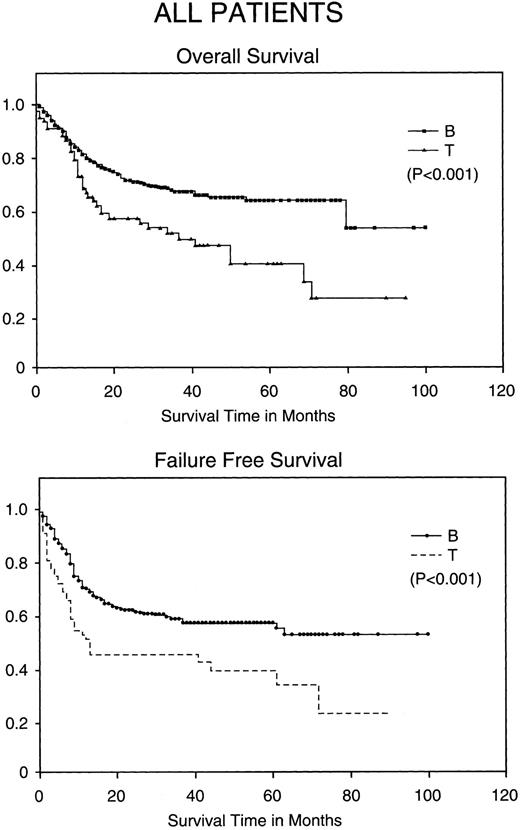
Kaplan-Meier analysis of OS and FFS in months comparing all patients with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences in OS and FFS were demonstrated (both P < .001) by the log-rank test.
Kaplan-Meier analysis of OS and FFS in months comparing all patients with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences in OS and FFS were demonstrated (both P < .001) by the log-rank test.
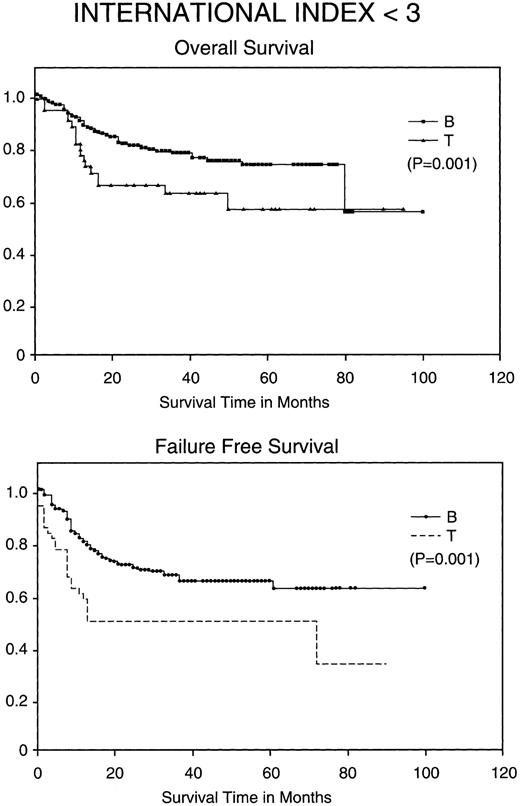
Kaplan-Meier analysis of OS and FFS comparing good prognosis patients (those with an IPI <3) with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences in OS and FFS were demonstrated (both P = .001) by the log-rank test.
Kaplan-Meier analysis of OS and FFS comparing good prognosis patients (those with an IPI <3) with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences in OS and FFS were demonstrated (both P = .001) by the log-rank test.
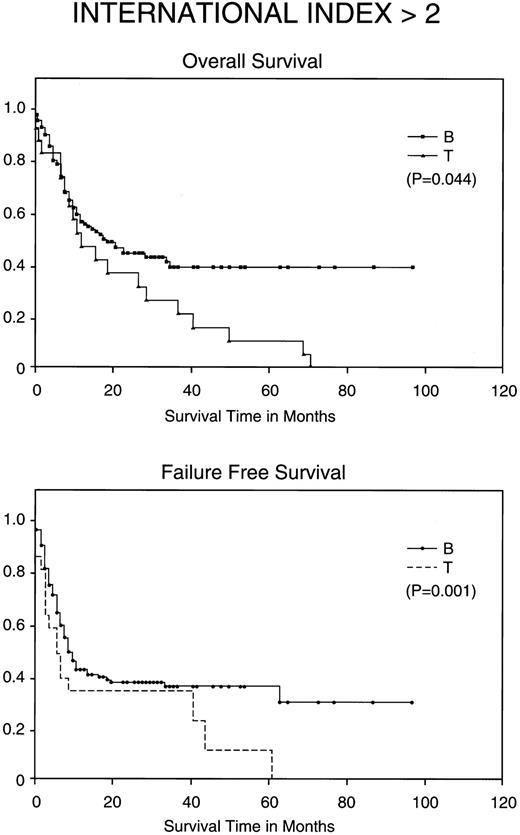
Kaplan-Meier analysis of OS and FFS in months comparing poor prognosis patients (those with an IPI <2) with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences for both FFS (P = .001) and OS (P = .044) were demonstrated by the log-rank test.
Kaplan-Meier analysis of OS and FFS in months comparing poor prognosis patients (those with an IPI <2) with BCLCL (B) versus PTCL (T) lymphomas. Statistically significant differences for both FFS (P = .001) and OS (P = .044) were demonstrated by the log-rank test.
Clinical data on each patient were obtained through a review of charts and protocol databases. Information obtained on each patient included protocol, age at start of therapy, performance status (Zubrod), B symptoms (>10% weight loss, fever >38.5°C, or night sweats), AA stage, sites of bulky disease (>7 cm diameter), number and sites of extranodal disease, serum lactate dehydrogenase (LDH) level, and serum β2 microglobulin level at presentation, and outcomes including maximal response, and failure-free survival (FFS) and overall survival (OS) in months. Finally, the MDATS and IPI were calculated using the available clinical data. Complete data were available on all patients except for 6 patients who lacked presenting β2 microglobulin levels (4 BCLCL and 2 PTCL). MDATS for these patients was calculated assuming a value of less than 3.0 mg/dL, because none of them had an MDATS of 2 in which the serum β2 microglobulin would have made a difference in classifying them as good prognosis (<3) or unfavorable (>2).
Statistics.The one-sided Student's t-test was used for continuous variable, whereas a χ2 test was used for discrete variables when comparing BCLCL with PTCL. FFS and OS were calculated using the Kaplan-Meier method. The log-rank test was used for comparing differences in FFS and OS between groups.
RESULTS
Classification of PTCL according to the REAL system and distinguishing features.Some of the 68 cases of PTCL fulfilled the histologic criteria for specific subtypes of PTCL and showed in some cases unique clinical features (Table 2). Most patients had unspecified PTCL. Eight patients had angioimmunoblastic lymphoma (AIL), 3 of which were confirmed to be monoclonal T-cell proliferations by TCRβ gene rearrangements. T-cell Ki-1+ anaplastic large-cell lymphomas (ALCLs) were present in 10 patients; 5 had angiocentric T-cell lymphomas, 3 of which presented with localized nasopharyngeal primaries. Among the 21 patients with TCRβ gene rearrangement studies, 6 failed to show a monoclonal rearrangement. All 6 also failed to show a clonal B-cell population by IgH gene rearrangement studies.
Although there were no statistically significant differences, some types of PTCL tended to present or behave in a clinically distinct fashion. Angiocentric lymphomas and ALCLs tended to occur in younger patients. Angiocentric lymphomas often failed to achieve a complete remission (CR), whereas, conversely, ALCLs were more likely to have a CR (Table 2). Overall, PTCL presents with advanced disease, with 78% having AA stage 3 or 4, frequent extranodal disease (56%), systemic or B symptoms (56%), elevated LDH level (57%), and elevated β2 microglobulin level (44%). Thirty-one percent of patients had an IPI greater than 2, and 56% had an MDATS greater than 2.
Comparison of clinical features and risk stratification between PTCL and BCLCL.PTCL appears to have a different spectrum of clinical features and to present with more advanced disease when compared with BCLCL. PTCLs were overwhelmingly more likely to present with AA stage 3 or 4 disease and B symptoms (Table 3). They were also more likely to have an elevated LDH or β2 microglobulin level. Age and extranodal disease was not significantly different. When comparing PTCL and BCLCL using the IPI and MDATS, PTCL on the whole presented with poorer prognostic features. This was most noticeable in the most favorable prognostic groups of both the IPI and MDATS, with only 34% of PTCLs compared to 46% of BCLCLs having an IPI of 0 or 1 and only 25%, compared with 40%, having an MDATS of 0 or 1.
Comparison of PTCL outcomes with BCLCL.Without stratification, PTCL has a worse CR rate, FFS, and OS when compared with BCLCL (Table 3). The CR rate is 76% in BCLCL, but 65% in PTCL (P = .036). The 5-year FFS for BCLCL is 56% (95% confidence interval [CI95%], 50% to 62%) and for PTCL is 38% (CI95%, 22% to 53%), respectively (P = .0001), and the 5-year OS for BCLCL and PTCL is 63% (CI95%, 53% to 73%) and 38% (CI95%, 235 to 54%), respectively (P = .001; Fig 1).
Most importantly, when stratified for IPI and MDATS, there is also a statistically significant difference between the 5-year FFS and OS of PTCL and BCLCL. With an IPI of greater than 2 or less than 3, the 5-year FFS and OS survival of PTCL is significantly worse as compared with BCLCL (Table 3 and Figs 2 and 3). Similarly, PTCL has a significantly worse prognosis when stratified for an MDATS greater than 2 or less than 3 (Table 3). Although not statistically significant, the CR rates for patients when stratified for either IPI or MDATS were consistently worse for PTCL.
In comparing treatments, the protocol-specific OS reflects the risk stratification used as outlined in the Materials and Methods (Table 4). Although within each protocol there were no statistically significant differences in OS between BCLCL and PTCL, with all treatments PTCL did worse than BCLCL.
Comparison of Regimens
| Regimen . | CR Rate . | 5-yr FFS . | 5-yr OS . |
|---|---|---|---|
| CHOPB/CMED DM84-24 | 74% | 55% | 52% |
| CHODB/DHAP DM86-10 | 59% | 38% | 32% |
| CHOPB/OPEN/XRT DM88-087 | 99% | 75% | 95+% |
| ATT DM88-089 | 75% | 55% | 59% |
| Id ATT DM92-054 | 56% | 34% (3 yr) | 48% (3 yr) |
| CHOPB/OPEN/XRT DM93-003 | 89% | 60% (2 yr) | 92% (2 yr) |
| Regimen . | CR Rate . | 5-yr FFS . | 5-yr OS . |
|---|---|---|---|
| CHOPB/CMED DM84-24 | 74% | 55% | 52% |
| CHODB/DHAP DM86-10 | 59% | 38% | 32% |
| CHOPB/OPEN/XRT DM88-087 | 99% | 75% | 95+% |
| ATT DM88-089 | 75% | 55% | 59% |
| Id ATT DM92-054 | 56% | 34% (3 yr) | 48% (3 yr) |
| CHOPB/OPEN/XRT DM93-003 | 89% | 60% (2 yr) | 92% (2 yr) |
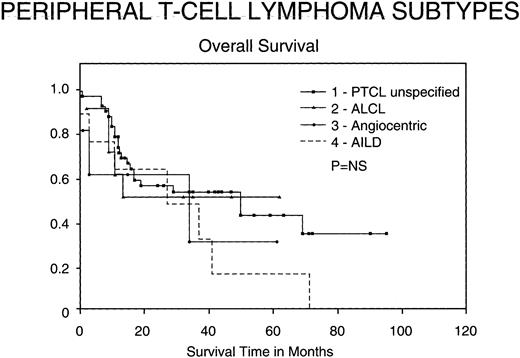
Outcomes for PTCL subtypes.Figure 4 shows the OS of the subtypes of PTCL included in our study according to the REAL classification. Although not statistically significant, certain trends appeared. ALCL seem to have a better OS than the other groups, whereas angiocentric lymphomas conversely have a worse survival. In the patients with unspecified PTCL, there continues to be a significant number of deaths greater than 3 years after treatment, many of which were due to late relapses.
Kaplan-Meier analysis of OS in months comparing various subtypes of PTCL: PTCL unspecified, Ki-1 ALCL, angiocentric lymphomas, and AIL disorders. Results were not statistically significant.
Kaplan-Meier analysis of OS in months comparing various subtypes of PTCL: PTCL unspecified, Ki-1 ALCL, angiocentric lymphomas, and AIL disorders. Results were not statistically significant.
DISCUSSION
This retrospective analysis comparing PTCL with BCLCL is the largest so far reported, involving 560 immunophenotyped and evaluable patients. Classification of patients as either T- or B-cell was rigorously performed in this review. T-cell–rich B-cell NHLs were carefully identified and considered to be BCLCL for the purpose of analysis. The FFS and OS of PTCL is worse than BCLCL, with and without risk stratification by IPI or MDATS. This was also independent of the therapy as administered in our various protocols. Thus, the immunophenotype is a significant and independent prognostic factor in the category of cases known as an intermediate grade and immunoblastic lymphomas (exclusive of mantle cell).
Our rigorous patient selection may account for our relatively low prevalence rate of PTCL (12%). Although prevalence rates might vary in those studies from the Far East, in which HTLV-1 and Epstein-Barr virus are thought to be etiologic factors, the rates in European or North American populations, in which almost all of the comparative studies have been performed, should be similar.15 However, the prevalence of PTCL varies between 8% and 30% in these comparative studies.2-5 This would suggest significant differences in patient referral, the histologic definition of PTCL, and in some cases possible misclassification of T-cell–rich B-cell NHLs as PTCL, or other factors.
Our study suggests that certain subtypes of PTCL may indeed have different natural histories. In this regard, the angiocentric lymphomas were perhaps our clearest category. Most of these patients were younger and had favorable prognostic features, yet failed to go into remission with combination chemotherapy. The poor survival of these patients who fail to achieve a CR has been described.25 The other subtype with apparent distinct features was ALCL. ALCL also tended to occur in younger patients, but conversely was associated with a slightly higher response rate, few late relapses, and a better survival. There is mounting evidence to suggest that these patients have improved response rates to more dose-intensive regimens such as were used in our protocols.26 AIL is recognized by the REAL classification but appears to be a heterogenous disorder. Frizzera et al27 have attempted to further classify AIL into those with or without clonal T-cell proliferations and those with aggressive or more indolent disease, respectively. We were only able by gene rearrangement studies to establish clonality in 3 of our 8 cases and failed to see a trend in clinical features or outcomes in these patients. Nevertheless, it is likely that certain subtypes of PTCL behave differently and could alter outcomes in studies.
Although only 62% of all patients in these trials were included in our study, there was no real difference in the proportion from each trial with immunophenotyping performed except from DM86-10, in which it was a stipulated criterion. Furthermore, by including all front-line protocols for untreated aggressive NHLs used over the last 11 years at M.D. Anderson in our analysis, we have tried to avoid a possible source of patient selection bias in our comparison. By using the IPI we can also compare the results of our treatments with the results of more standard regimens. The proportion of patients and overall 5-year OS of our patients when stratified by the IPI is comparable to that reported for those in the initial study establishing the IPI and confirms that BCLCL did no worse in our various protocols (data not shown).7
Table 5 summarizes the results of all trials with clear patient ascertainment comparing PTCL with aggressive B-cell NHLs, including ours.2,4,5,9-11,14 In most trials, PTCL had a worse outcome despite the use of an anthracycline-based combination chemotherapy. The exceptional results from the Stanford series can perhaps be explained by the favorable age and stage as well as histology in these patients.5 The Stanford study included only diffuse large-cell NHLs, some of which could have been Ki-1+ ALCLs. In the French trial, age and stage were no more favorable, but perhaps their failure to exclude T-cell–rich B-cell NHLs might have improved their outcomes.2 Inclusion of angiocentric lymphomas and AIL may also have worsened our results.
Prior Studies of PTCL
| Institution . | No. of Patients5-150 . | Median Age (yr) . | AA Stage 3 or 4 (%) . | B Symptom (%) . | CR Rate (%) . | 5-yr OS (%) . | Ref . |
|---|---|---|---|---|---|---|---|
| Vanderbilt | 33 | 64 | 79 | 67 | 245-151 | <10 | 14 |
| Arizona | 20 | 59 | 80 | 60 | 50 | 40 | 11 |
| Netherlands | 20 | 57 | 60 | — | — | 27 | 4 |
| Stanford | 21 | <50 | 18 | 29 | 95 | 78 | 5 |
| Austria | 38 | 54 | — | — | 37 | <40 | 10 |
| Nebraska | 80 | 57 | 72 | 57 | 50 | 40 | 9 |
| France | 108 | — | 77 | 58 | 72 | 45 | 2 |
| M.D. Anderson | 68 | 54 | 78 | 56 | 75 | 39 | |
| Total | 360 | 52-57 | 65-75 | 55-60 | 50-70 | 30-50 |
| Institution . | No. of Patients5-150 . | Median Age (yr) . | AA Stage 3 or 4 (%) . | B Symptom (%) . | CR Rate (%) . | 5-yr OS (%) . | Ref . |
|---|---|---|---|---|---|---|---|
| Vanderbilt | 33 | 64 | 79 | 67 | 245-151 | <10 | 14 |
| Arizona | 20 | 59 | 80 | 60 | 50 | 40 | 11 |
| Netherlands | 20 | 57 | 60 | — | — | 27 | 4 |
| Stanford | 21 | <50 | 18 | 29 | 95 | 78 | 5 |
| Austria | 38 | 54 | — | — | 37 | <40 | 10 |
| Nebraska | 80 | 57 | 72 | 57 | 50 | 40 | 9 |
| France | 108 | — | 77 | 58 | 72 | 45 | 2 |
| M.D. Anderson | 68 | 54 | 78 | 56 | 75 | 39 | |
| Total | 360 | 52-57 | 65-75 | 55-60 | 50-70 | 30-50 |
Patients with intermediate-grade histology and receiving combination chemotherapy only.
Not all had an anthracycline-containing regimen.
In terms of response, we observed that the CR rate was lower for PTCL as a group, with trends to statistical significance within stratified groups. The poor outcomes with PTCL may in large part then relate to their distinct tumor biology. In this regard, several observations may be clinically significant and deserve further study. First, our approach to treating patients using less intensive regimens for good risk disease is of uncertain benefit in PTCL. In the last trial, DM93-003, more than half of the good prognosis patients with PTCL have relapsed. Secondly, certain histologies also deserve attention when considering standard therapy. In particular, the angiocentric lymphomas in our study responded poorly to anthracycline-based combination chemotherapy. Finally, using the IPI or MDATS, we can identify a group of PTCL that have a 5-year OS of less than 25%. This poor survival suggests that newer therapeutic interventions such as autologous bone marrow transplantation should be tested.28
In conclusion, our analysis of the M.D. Anderson experience with aggressive NHLs over the last decade not only confirms the worse prognosis conferred by T-cell phenotype, but also establishes phenotype as having prognostic significance independent of other well-defined prognostic features included in the IPI and MDATS. We conclude that the use of phenotype in the REAL classification is not only biologically sound but also clinically relevant.
Address reprint requests to F. Cabanillas, MD, Department of Hematology, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal