Abstract
G28-5 sFv-PE40 is a single-chain immunotoxin targeted to CD40, which is highly expressed on human hematologic malignancies, including non-Hodgkin's lymphoma, B-lineage leukemias, multiple myeloma, and Hodgkin's disease, as well as certain carcinomas. In vitro analysis showed that this monovalent immunotoxin had a binding affinity of 3 nmol/L, within 15-fold of the bivalent parental monoclonal antibody. G28-5 sFv-PE40 was stable when incubated in mouse serum at 37°C for 6 hours and cleared from the circulation of mice with a half-life of 16.7 minutes. This immunotoxin was effective in treating human Burkitt's lymphoma xenografted SCID mice with complete responses, defined by an asymptomatic phenotype for greater than 120 days, obtained at doses of 0.13 to 0.26 mg/kg. The efficacy of treatment was dependent on the schedule used, with every three days for five injections being the most effective tested. The toxicity of G28-5 sFv-PE40 was examined in SCID mice, rats, and monkeys, with the maximum tolerated dose being 0.48, 1.0, and 1.67 mg/kg, respectively. Comparative immunohistology showed that the G28-5 specificity was qualitatively similar between human and monkey tissue. In summary, G28-5 sFv-PE40 was effective at inducing complete antitumor responses in lymphoma xenografted mice at doses that were well tolerated in mice, rats, and monkeys.
EACH YEAR IN THE United States there are approximately 40,000 new cases of non-Hodgkin's lymphoma (NHL) diagnosed, 90% of which are B-lineage in origin. Although combination chemotherapy is capable of inducing complete remission in the majority of patients suffering from NHL, most of these patients eventually relapse, with the total cure rate using conventional therapies being on the order of 30% to 40%.1-3 In addition, over the past 30 years, the incidence of NHL has been steadily increasing in part but not entirely due to the increase in acquired immunodeficiency syndrome-related lymphomas.1,4 5 Thus, new strategies are needed to improve the cure rate of NHL.
One approach for the treatment of NHL and other hematologic malignancies is the development of immunotoxins.6 Immunotoxins are bifunctional protein molecules consisting of a monoclonal antibody (MoAb) chemically conjugated or genetically fused to a protein toxin.7 Antibodies are selected for immunotoxins based on their reactivity towards cell surface receptors or antigens that are preferentially expressed on malignant cells. After MoAb-mediated internalization, the toxin portion of an immunotoxin traffics into the cytosol, where the enzymatic activity innate to the toxin catalytically inhibits protein synthesis, resulting in cell death. Several immunotoxin conjugates for the treatment of B-lineage NHL, targeting the surface antigens CD19 and CD22, have shown promise in early phase clinical trials.8-10
We have recently described a single-chain immunotoxin, G28-5 sFv-PE40, targeted to CD40 that specifically kills CD40+ cell lines derived from B-cell malignancies in vitro.11 G28-5 sFv-PE40 consists of the single-chain variable regions cloned from the antihuman CD40 MoAb G28-5 genetically fused to PE40, a binding defective truncated form of Pseudomonas exotoxin A. CD40 is a 45- to 50-kD integral membrane glycoprotein originally identified on the surface of B lymphocytes and some carcinomas.12,13 It is expressed early in B-cell development, after CD19 expression but before CD20 and CD22 expression.14
CD40 is highly expressed in B-cell malignancies, including B-lineage leukemias, NHL, Hodgkin's disease, and multiple myeloma.14-16 Several normal human tissues, including endothelial cells,17,18 dendritic cells,19 thymic epithelial cells,20 monocytes,21,22 and fibroblasts,23 also express CD40. We have shown that, whereas malignant B-cell lines are sensitive to G28-5 sFv-PE40 in vitro, primary endothelial and monocytic cells are completely insensitive to this immunotoxin under standard culture conditions, although activation with certain cytokines can sensitize these cells to the anti-CD40 immunotoxin.24
In this report, we have further characterized the in vitro properties of G28-5 sFv-PE40 and have examined both its in vivo efficacy and toxicity. The data show that this anti-CD40 immunotoxin is effective in treating SCID mice xenografted with the human Burkitt's lymphoma cell line Ramos as a disseminated disease at doses that are well tolerated in mice, rats, and monkeys.
MATERIALS AND METHODS
Reagents and cell culture.G28-5 sFv-PE40, with the sFv moiety in the VL -VH orientation, was produced and purified essentially as previously described.11 Protein concentration was determined by absorbance at 280 nm (A280 ) using an extinction coefficient of 1.3 mL × mg−1 × cm−1. Ramos human Burkitt's lymphoma cell line, purchased from American Type Culture Collection (Rockville, MD), was cultured in RPMI 1640 media containing 10% fetal bovine serum (FBS), 100 U/mL of penicillin G, and 100 μg/mL streptomycin. Media was purchased from GIBCO-BRL (Gaithersburg, MD). Female C.B.-17 SCID mice were purchased from Taconic (Germantown, NY) and were 6 to 8 weeks old at the initiation of all studies. Goat antimouse Ig (H + L) horseradish peroxidase conjugate for enzyme-linked immunosorbent assay (ELISA) analysis and fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 antimouse IgG for fluorescence activated cell sorting (FACS) analysis were purchased from Southern Biotechnology Association (Birmingham, AL).
BIAcore binding analysis.All binding studies were run on BIAcore 1000 and 2000 instruments (Pharmacia Biosensor, Uppsala, Sweden) at 25°C using phosphate-buffered saline, pH 7.4, containing 0.005% surfactant P20 (Pharmacia Biosensor) as the running buffer. The carboxymethylated dextran matrix was activated with 0.1 mol/L N-hydroxysuccinimide/0.4 mol/L N-ethyl-N′(3-dimethylaminopropyl)carbodiimide25 before the ligand, CD40-Ig, was immobilized. CD40-Ig consists of the extracellular domain of human CD40 fused to the Fc domain of human IgG1 .26 G28-5 sFv-PE40 or G28-5 MoAb was injected and the association and dissociation rates were determined by curve fitting using BIAevaluation 2.1 (Pharmacia Biosensor). The equilibrium constant, kd, was calculated by dividing the dissociation rate by the association rate.
Inhibition of protein synthesis.Inhibition of protein synthesis was analyzed as previously described.27 Briefly, 100 μL of immunotoxin samples diluted in leucine-free RPMI 1640 media containing 10% FBS, 100 U/mL of penicillin G, and 100 μg/mL streptomycin was plated in flat-bottom, 96-well cell culture plates. One hundred microliters per well of Ramos cells at 105 cells/mL in leucine-free media was subsequently added. After incubating for 24 hours at 37°C the plates were pulsed for 6 hours with 1 μCi/well 3H-leucine (Amersham, Arlington Heights, IL). The plates were then harvested using a Tomtec cell harvester (Orange, CT) and the incorporation of 3H-leucine into cellular protein was analyzed with an LKB Beta-Plate liquid scintillation counter (Wallac, Gaithersburg, MD).
Serum stability analysis.Blood was isolated and pooled from 7 SCID mice and allowed to clot at room temperature. The sample was then centrifuged at maximum speed in a microcentrifuge and the serum was removed from the pelleted clot. G28-5 sFv-PE40 was mixed with the serum at 20 μg/mL and incubated at 37°C. At the indicated times, samples were taken and frozen at −20°C. The samples were then analyzed for inhibition of protein synthesis as described above.
In vivo SCID mouse studies.For pharmacokinetic analysis, three naive mice per group were injected intravenously (IV) through the tail vein with 0.26 mg/kg G28-5 sFv-PE40. At the indicated times, mice were bled from the retroorbital capillary bed. All plastic and glassware used in these studies had been treated with heparin before use to prevent clotting. The blood samples were centrifuged and the plasma was removed and rapidly frozen. For determining the concentration of immunotoxin present in each sample, ELISA binding analysis was performed using immobilized CD40-Ig.11 The concentration of immunotoxin in plasma was determined by comparing the ELISA results of the samples with controls consisting of known amounts of immunotoxin diluted in naive mouse plasma. Total blood volume in the mice was estimated as 8% total body weight and plasma volume was estimated as 50% blood volume. The t1/2α and t1/2β were calculated with the use of the program MK Model (Biosoft, Cambridge, UK).
For immunotoxin therapy studies, SCID mice were injected IV through the tail vein with 1 × 106 Ramos cells on day 0. The mice were then randomly distributed into groups of five and received either no treatment, G28-5 sFv-PE40, or BR96 sFv-PE40,27 starting 24 hours after Ramos cell injection, at the doses and schedules indicated in the Results. Animals were killed at the onset of hind-leg paralysis. Complete antitumor responses were defined as animals that survived for greater than 120 days with no signs of paralysis.
FACS analysis.For FACS analysis of solid tumor samples, the tumors were dissected with a scalpel and passed through a small pore wire mesh to obtain single-cell suspensions. Tumor samples or Ramos cells were then diluted to 1 × 106 cells/mL in RPMI 1640 containing 10% FBS and were incubated on ice for 30 minutes. Samples were then incubated on ice for 1 hour with 5 μg/mL of anti-CD40 MoAb G28-5,13 anti-CD20 MoAb 1F5,28 anti-CD22 MoAb G28-7,29 or anti-CD19 MoAb B-C3 (BioSource, Camarillo, CA); washed twice with media; incubated on ice for 1 hours with FITC-conjugated goat F(ab′) antimouse Ig diluted 1:50 in media; and analyzed on a FACScan fluorescence activated cell sorter (Becton Dickinson, San Jose, CA).
Toxicity analysis in mice, rats, and monkeys.The toxicity of G28-5 sFv-PE40 in SCID mice was assessed in groups of three mice injected IV through the tail vein with concentrations of immunotoxin ranging from 0.16 to 0.81 mg/kg. Animals were observed for 7 days for overt signs of toxicity.
For rat toxicity studies, groups of four male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) received single IV injections of G28-5 sFv-PE40 at 0.5, 0.1, 2, or 4 mg/kg and were observed for 8 days. Rats that died during the study were subjected to gross necropsy.
Monkey toxicity studies were performed in cynomolgus monkeys (Charles River Primate Corp, Houston, TX) receiving a single dose of 0.08, 0.42, 0.83, 1.67, or 3.34 mg/kg IV into the saphenous vein. Clinical signs, body weights, food intake, drug plasma levels, and clinical pathology parameters were monitored up to day 29. Necropsies were conducted on animals that were killed because of poor physical condition.
Comparative binding to human and monkey tissue samples.Five-micron sections were cut and mounted on glass slides from a panel of frozen monkey and human tissues. Individual slides were stained using either G28-5 MoAb or G28-5 sFv-PE40 as primary staining reagents at a concentration of 1 μg/mL. Negative controls consisting of isotype-matched murine IgG1 or PE40 were included for each sample tested. Binding of G28-5 sFv-PE40 was detected using anti-PE40 MoAb EXA2-1H8 (T. Siadak, Bristol-Myers Squibb). All staining was conducted using a Ventana ES automated slide stainer (Ventana Medical Systems, Tuscon, AZ).
RESULTS
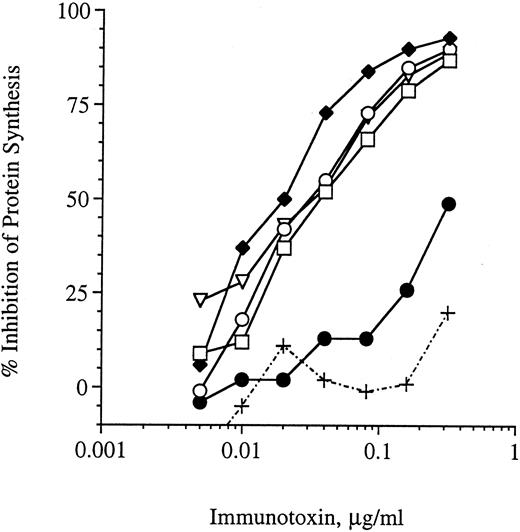
Stability of G28-5 sFv-PE40 in mouse serum and binding analysis.Previous studies have indicated that some single-chain immunotoxins are unstable in vitro and lose activity when incubated at 37°C for relatively short periods of time.30 Before initiating in vivo studies, we analyzed the stability of the anti-CD40 immunotoxin in vitro. G28-5 sFv-PE40 was incubated at 37°C in serum obtained from SCID mice and, at the indicated times, samples were removed and analyzed for protein synthesis inhibition activity using the human Burkitt's lymphoma cell line Ramos (Fig 1). Ramos cells were previously found to be sensitive to this anti-CD40 immuntoxin with an EC50 value of 20 ng/mL.11 G28-5 sFv-PE40 retained 100% of its cytotoxic activity after incubation in serum at 37°C for up to 6 hours. After 24 hours of incubation in serum, the immunotoxin retained less than 10% of its protein synthesis inhibition activity (Fig 1). Serum by itself was not cytotoxic to the cells. Thus, G28-5 sFv-PE40 was relatively stable in mouse serum at physiologic temperatures.
Stability of G28-5 sFv-PE40 in SCID mouse serum. G28-5 sFv-PE40 was incubated at 37°C in mouse serum at 20 μg/mL for 0 (□), 2 (♦), 4 (○), 6 (▿), or 24 hours (•) and then analyzed for protein synthesis inhibition activity on Ramos cells. Dilutions of serum (+) were used as a control. Data shown are the averages of three samples per time point.
Stability of G28-5 sFv-PE40 in SCID mouse serum. G28-5 sFv-PE40 was incubated at 37°C in mouse serum at 20 μg/mL for 0 (□), 2 (♦), 4 (○), 6 (▿), or 24 hours (•) and then analyzed for protein synthesis inhibition activity on Ramos cells. Dilutions of serum (+) were used as a control. Data shown are the averages of three samples per time point.
Binding analysis showed that the affinity of the monovalent immunotoxin was within 15-fold of the divalent parental MoAb. The apparent affinity, kd, of the immunotoxin was 3 nmol/L compared with 0.2 nmol/L for G28-5 MoAb. Thus, whereas there was some loss in affinity in generating the single-chain form of G28-5, the immunotoxin retained a high affinity for CD40.
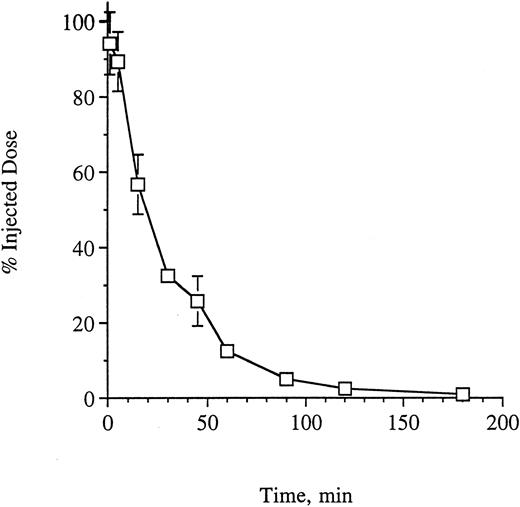
Pharmacokinetics and toxicity of G28-5 sFv-PE40 injected IV into SCID mice.To best determine a schedule and dose for treatment of xenografted mice with this anti-CD40 single-chain immunotoxin, we first investigated the pharmacokinetics of the agent in vivo. Clearance was assessed in groups of mice injected through the tail vein with 0.26 mg/kg of G28-5 sFv-PE40. At the indicated times, up to 3 hours, mice were bled and the plasma was isolated. The concentration of immunotoxin in each sample was analyzed by ELISA binding to immobilized CD40-Ig as described in the Materials and Methods. As shown in Fig 2, the data were consistent with a biphasic clearance, with a t1/2α of 16.7 minutes and a t1/2β of 45.3 minutes.
Clearance of G28-5 sFv-PE40 from SCID mice. Mice (3 per time point) were injected with G28-5 sFv-PE40. At the indicated times, the animals were bled, the plasma was isolated, and the amount of immunotoxin present in each sample was determined by ELISA analysis as described in the Materials and Methods.
Clearance of G28-5 sFv-PE40 from SCID mice. Mice (3 per time point) were injected with G28-5 sFv-PE40. At the indicated times, the animals were bled, the plasma was isolated, and the amount of immunotoxin present in each sample was determined by ELISA analysis as described in the Materials and Methods.
We next examined the single-dose toxicity of G28-5 sFv-PE40 in SCID mice. Mice (6/6) that received either 0.81 or 0.65 mg/kg immunotoxin died within 5 days of injection. All of the mice receiving the lower doses of immunotoxin (0.16, 0.32, or 0.48 mg/kg) survived for the entire 7-day study (Table 1). Thus, the maximun tolerated dose (MTD) of G28-5 sFv-PE40 in SCID mice was 0.48 mg/kg. For comparison, we also examined the toxicity of BR96 sFv-PE40, a similar single-chain immunotoxin that recognizes a human tumor antigen.27 The MTD of BR96 sFv-PE40 in SCID mice was found to be 0.32 mg/kg (Table 1). The MTD of G28-5 sFv-PE40 in athymic (nude) mice was 0.75 to 1.0 mg/kg, approximately twice that in SCID mice (data not shown).
Single-Dose MTD of G28-5 sFv-PE40 in SCID Mice
| . | No. of Survivors . | ||||
|---|---|---|---|---|---|
| . | 0.16 mg/kg . | 0.32 mg/kg . | 0.48 mg/kg . | 0.65 mg/kg . | 0.81 mg/kg . |
| G28-5 sFv-PE40 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| BR96 sFv-PE40 | 3/3 | 3/3 | 2/3 | 0/3 | 0/3 |
| . | No. of Survivors . | ||||
|---|---|---|---|---|---|
| . | 0.16 mg/kg . | 0.32 mg/kg . | 0.48 mg/kg . | 0.65 mg/kg . | 0.81 mg/kg . |
| G28-5 sFv-PE40 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| BR96 sFv-PE40 | 3/3 | 3/3 | 2/3 | 0/3 | 0/3 |
Groups of three 6- to 8-week-old female C.B.-17 SCID mice were injected with the indicated concentrations of immunotoxin. The mice were observed daily for 7 days.
Establishment of Ramos xenografted SCID mice.Previous studies have shown that SCID mice represent an appropriate host for human lymphoma xenografts, because mice injected IV develop disseminated disease in a manner analogous to the disease progression in humans; in contrast, human lymphoma cells injected into athymic mice grow as solid tumors.31,32 We therefore used SCID mice as the host for Ramos lymphoma xenografts. The mice were injected via the tail vein with 1 × 106 Ramos cells diluted in phosphate-buffered saline and were observed daily for the onset of hind-leg paralysis, which served as the experimental end-point. As seen previously,31 hind-leg paralysis preceded death by 1 to 3 days. Although it has been reported that γ-irradiation may be required for the successful engraftment of human hematologic malignancies into SCID mice,33 in the present study all mice, whether or not they had been exposed to irradiation, developed hind-leg paralysis and were killed before day 28 (data not shown). Thus, γ-irradiation was not required for successful establishment of Ramos xenografted SCID mice. At the onset of hind-leg paralysis, histopathologic examination showed that the mice carried well-differentiated lymphomas. Neoplastic lymphoma nodules were observed in the salivary gland, the medullary tissue of the kidney, in the renal pelvis and at the meninges near the rostral portion of the brain. The liver, adrenal gland, spleen, lung, heart, pancreas, and gastrointestinal tract were all within normal parameters.
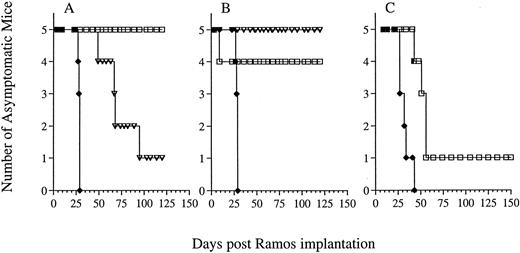
Therapy of Ramos xenografted SCID mice with G28-5 sFv-PE40.Previous studies have shown that the effectiveness of treatment with immunotoxins or immunoconjugates is greatly dependent on the schedule of administration.27 34 The efficacy of G28-5 sFv-PE40 in Ramos lymphoma bearing SCID mice was therefore investigated using schedules of every second (q2d), third (q3d), or fourth day (q4d). In each case, 5 injections of immunotoxin, at either 0.13 or 0.26 mg/kg/injection, were administered. Mice were implanted with 1 × 106 Ramos cells IV and, 24 hours later, therapy was initiated. Untreated mice rapidly succumbed to the effects of disease (Fig 3), as did mice receiving control immunotoxin BR96 sFv-PE40 (data not shown). Administration of the immunotoxin every second or every third day had a profound effect on the survival of Ramos-bearing SCID mice. At 0.26 mg/kg, five of five mice that received G28-5 sFv-PE40 q2dx5 (Fig 3A) and four of four evaluable mice that received the immunotoxin q3dx5 (Fig 3B) survived for greater than 135 days, the length of this study. One of five mice in the q3dx5 group died on day 9 of toxicities that did not appear to be either immunotoxin or disease related (Fig 3B). The immunotoxin was less effective when administered q4dx5 at 0.26 mg/kg. In this case, only one of five mice remained disease free, with the other four mice developing hind-leg paralysis by day 55 (Fig 3C).
Efficacy of G28-5 sFv-PE40 on Ramos xenografted SCID mice and the effect of administration schedule. Ramos xenografted SCID mice treated with G28-5 sFv-PE40 with a schedule of q2dx5 (A), q3dx5 (B), or q4dx5 (C) with a dose of either 0.13 mg/kg (▿) or 0.26 mg/kg (□). Control mice (♦) received no immunotoxin. Animals in (C) received only the 0.26 mg/kg dose.
Efficacy of G28-5 sFv-PE40 on Ramos xenografted SCID mice and the effect of administration schedule. Ramos xenografted SCID mice treated with G28-5 sFv-PE40 with a schedule of q2dx5 (A), q3dx5 (B), or q4dx5 (C) with a dose of either 0.13 mg/kg (▿) or 0.26 mg/kg (□). Control mice (♦) received no immunotoxin. Animals in (C) received only the 0.26 mg/kg dose.
The lower dose of immunotoxin (0.13 mg/kg) was also effective when administered q3dx5, with five of five animals exhibiting complete responses (Fig 3B). However, at this dose, the q2dx5 schedule was less efficacious, because three of five mice receiving 0.13 mg/kg immunotoxin q2dx5 became paralyzed by day 67 and only one mouse remained disease free (Fig 3A). Thus, G28-5 sFv-PE40 was capable of inducing complete responses in human lymphoma xenografted SCID mice. The efficacy of the treatment was dependent on the administration schedule, with q3dx5 being the best tested.
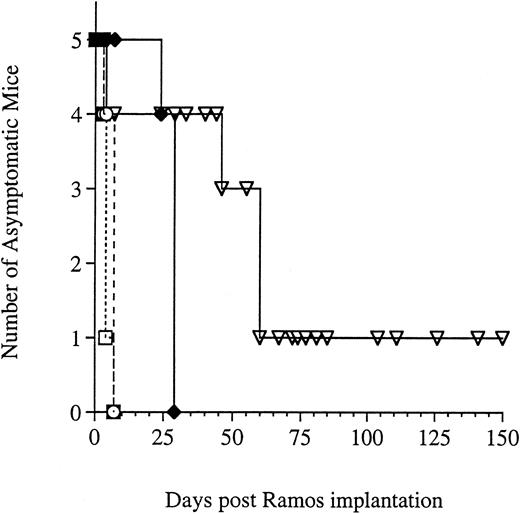
The efficacy of G28-5 sFv-PE40 was also investigated using daily injections of 0.16 or 0.32 mg/kg. As in the previous studies, mice receiving no treatment became paralyzed and were killed within 30 days (Fig 4). Treatment with 0.32 mg/kg of either G28-5 sFv-PE40 or the control immunotoxin, BR96 sFv-PE40, on a daily basis was toxic to mice after two doses. At 0.16 mg/kg, one mouse died after the second injection of G28-5 sFv-PE40 and therapy was halted. Of the remaining four mice, one survived disease free for the length of the experiment (>150 days). In the other three mice, the progression of the disease was altered from what was seen previously. These mice did not develop hind-leg paralysis but rather developed large solid tumor masses in the upper spinal and abdominal regions and were killed by day 60. The manifestation of solid tumor growth was not seen in any of the previous studies using schedules of q2d, q3d, or q4d.
Effect of G28-5 sFv-PE40 administered q1dx2 on Ramos xenografted SCID mice. Mice were injected IV with Ramos cells and were left untreated (♦) or were treated q1dx2 with 0.16 mg/kg G28-5 sFv-PE40 (▿), 0.32 mg/kg G28-5 sFv-PE40 (□), or 0.32 mg/kg BR96 sFv-PE40 (○). Treatment was initiated on day 1 after Ramos implantation.
Effect of G28-5 sFv-PE40 administered q1dx2 on Ramos xenografted SCID mice. Mice were injected IV with Ramos cells and were left untreated (♦) or were treated q1dx2 with 0.16 mg/kg G28-5 sFv-PE40 (▿), 0.32 mg/kg G28-5 sFv-PE40 (□), or 0.32 mg/kg BR96 sFv-PE40 (○). Treatment was initiated on day 1 after Ramos implantation.
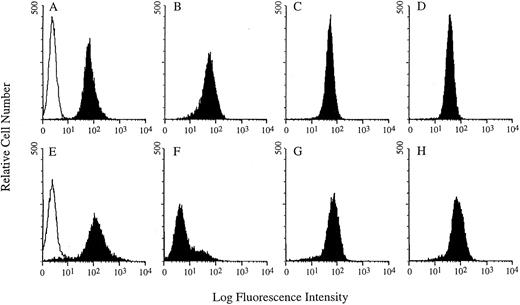
Microscopic analysis of the solid tumors showed that these masses were composed of solid, dense sheets of lymphoma cells separated by broad areas of necrotic cells sometimes containing mineralized debris or individual necrotic cells. FACS analysis of the solid lymphoma masses showed that the cells expressed CD40 and thus were not a selected subpopulation that was CD40−. Consistent with the expression of CD40, these cells subsequently grown in culture were sensitive to the cytotoxicity of G28-5 sFv-PE40 (data not shown). Although the expression of the B-cell antigens CD40, CD19, and CD22 on the cells removed from the mice was similar to that of cultured Ramos cells, the expression of CD20 was greatly reduced compared with Ramos cells (Fig 5). Whether the cells that grew as solid tumors were a subset of Ramos cells that initially did not express CD20 or downregulation of CD20 occurred after the establishment of solid tumor growth is unknown.
Comparative FACS analysis of Ramos cells cultured in vitro (A through D) and solid tumor samples excised from SCID mice (E through H). The solid histograms show CD19 (A and E), CD20 (B and F ), CD22 (C and G), and CD40 (D and H) expression. The open histograms in (A) and (E) represent the background fluorescence due to the binding of a nonspecific antibody.
Comparative FACS analysis of Ramos cells cultured in vitro (A through D) and solid tumor samples excised from SCID mice (E through H). The solid histograms show CD19 (A and E), CD20 (B and F ), CD22 (C and G), and CD40 (D and H) expression. The open histograms in (A) and (E) represent the background fluorescence due to the binding of a nonspecific antibody.
Toxicity of G28-5 sFv-PE40 in rats and cynomolgus monkeys.To establish a therapeutic window for G28-5 sFv-PE40, the toxicity of the immunotoxin in immunocompetent rats and in cynomolgus monkeys was evaluated. It has been shown that rats represent an appropriate model to evaluate vascular leak syndrome (VLS) induced by Pseudomonas exotoxin-based immunotoxins.35 VLS has been the dose-limiting toxicity in a number of immunotoxin clinical trials8 but is not seen in mice. In rats, no significant toxicity was noted in animals treated with either 0.5 or 1 mg/kg G28-5 sFv-PE40. One of four rats treated with 2 mg/kg and all rats (4/4) treated with 4 mg/kg died by day 3. Gross necropsy showed hydrothorax (>2 mL of fluid in the pleural cavity) indicative of VLS. Thus, the dose-limiting toxicity in rats was VLS and the MTD was 1 mg/kg.
Because antihuman CD40 antibodies cross-react with macaque CD40,36 these primates represent an appropriate model to study the toxicity of anti-CD40 immunotoxins to normal tissues. Before examining the toxicity of G28-5 sFv-PE40 in cynomolgus monkeys, we compared the binding of G28-5 sFv-PE40 and its parental MoAb, G28-5, in monkey and human tissue samples. G28-5 and G28-5 sFv-PE40 stained similar tissues in both monkey and human samples, with qualitatively similar staining intensities using both reagents (Table 2). Strong binding was detected in B-cell–dependent areas of primary lymphoid organs (germinal centers of spleen and lymph nodes) as well as on thymic epithelium. Some diffuse binding was detected in the liver, although no binding was found in the heart, brain, or other postmitotic tissues.
Immunohistochemical Staining of Cynomolgus Monkey and Human Tissues With G28-5 MoAb and G28-5 sFv-PE40
| Organ . | G28-5 . | G28-5 sFv-PE40 . | Staining Pattern . |
|---|---|---|---|
| Lymph node | +/− to +++ | +/− to +++ | B-cell–dependent areas |
| Spleen | +/− to +++ | +/− to +++ | B-cell–dependent areas |
| Thymus* | ++ to +++ | ++ | Medulla |
| Liver | + to ++ | +/− | Hepatocytes |
| Heart | − | − | |
| Brain | − | − | |
| Stomach | − | − | |
| Colon | − | − |
| Organ . | G28-5 . | G28-5 sFv-PE40 . | Staining Pattern . |
|---|---|---|---|
| Lymph node | +/− to +++ | +/− to +++ | B-cell–dependent areas |
| Spleen | +/− to +++ | +/− to +++ | B-cell–dependent areas |
| Thymus* | ++ to +++ | ++ | Medulla |
| Liver | + to ++ | +/− | Hepatocytes |
| Heart | − | − | |
| Brain | − | − | |
| Stomach | − | − | |
| Colon | − | − |
Abbreviations: −, no staining observed; +/−, mixed areas of no staining and low levels of staining; +, low levels of staining seen throughout; ++, intermediate level of staining; +++, high level of staining.
Data for monkey tissue only.
For toxicity studies, monkeys were injected with a single dose of G28-5 sFv-PE40, with one or two animals per group. The immunotoxin was well tolerated at doses up to 1.67 mg/kg. At doses ranging from 0.42 to 1.67 mg/kg, there was an elevation in liver enzymes, an increase in neutrophil count, and a decrease in peripheral blood lymphocytes, serum albumin, and total blood protein. All of these toxicities were transient. The one animal that received 3.34 mg/kg was killed on day 2 after displaying moderately decreased activity and decreased body temperature, although the cause of its moribund condition was not discernible from clinicopathologic, gross necropsy, or histopathologic examinations. Based on these results, the MTD in cynomolgus monkeys was estimated to be 1.67 mg/kg.
DISCUSSION
Despite advances in chemotherapeutic treatment, B-lineage NHL remains essentially incurable for the majority of patients.1-3 We have previously shown that G28-5 sFv-PE40, a single-chain immunotoxin targeted to CD40, is potently and specifically cytotoxic in vitro against B-cell malignancies that express the CD40 antigen.11 In this report, we further characterized the in vitro properties of G28-5 sFv-PE40 and have shown that this immunotoxin is efficacious in treating SCID mice carrying disseminated human lymphoma xenografts.
SCID mice have been shown to be a useful model for studying the engraftment and treatment of human hematologic malignancies.31,32 Treatment with G28-5 sFv-PE40 had a profound effect on the survival of Ramos xenografted SCID mice with the therapeutic efficacy dependent on both the dose and schedule of administration. With a schedule of q3dx5, complete antitumor responses were obtained at doses (0.13 to 0.26 mg/kg) that were well tolerated in mice, rats, and monkeys. Administration of the immunotoxin at suboptimal schedules, q2dx5 or q4dx5, also produced some cures, although to a lesser degree than q3dx5. The dependence of efficacy on administration schedule has been seen with other targeted antitumor agents.27 34 Nevertheless, this indicates that the schedule of administration of G28-5 sFv-PE40 must be carefully evaluated before advancing to clinical trials.
Although tumor-bearing mice are useful for studying the efficacy of immunotoxins, they have not been predictive of toxicities in humans. For example, the major toxicity associated with a number of immunotoxin clinical trials has been VLS, which has not been seen in mice. In contrast, rats have previously been shown to represent a model for immunotoxin-induced VLS.35 Rats that received G28-5 sFv-PE40 up to 1.0 mg/kg showed no toxicity. The dose-limiting toxicity was found to be VLS with hydrothorax present in one of four animals receiving 2 mg/kg and in four of four receiving 4 mg/kg.
Because CD40 has been found on a variety of normal human tissues, including endothelial cells, it was important to evaluate the toxicity of G28-5 sFv-PE40 in a cross-reactive species. Whereas antibodies to human CD40 do not cross-react with rat or mouse CD40, they have been shown to bind to monkey CD40 (Clark and Draves36 and this study). Therefore, we also examined the toxicity of G28-5 sFv-PE40 in cynomolgus monkeys. In monkeys, the immunotoxin was well tolerated at doses up to 1.67 mg/kg but was toxic at 3.34 mg/kg, although the dose-limiting toxicity was not determined.
The major issues confronting the use of immunotoxins as antitumor agents are their toxicity and immunogenicity. Despite the best preclinical data, toxicologic analysis can only assist in predicting doses that will be tolerated in the clinic. Nonetheless, the use of multiple animal species to analyze the toxicology of specific agents such as G28-5 sFv-PE40 are helpful in preparing appropriate clinical protocols for the goal of drug registration. Immunogenicity has limited most immunotoxins in clinical trials to a single course of therapy over a 1-week period. However, patients with hematologic malignancies are often more immunosuppressed than patients with solid tumors, thereby limiting this liability. Additionally, preliminary data indicate that G28-5 sFv-PE40 inhibits immune activation by directly targeting activated antigen-presenting cells (unpublished observations), suggesting that G28-5 sFv-PE40 may have reduced immunogenicity when compared with other immunotoxins.
CD40 is highly expressed on a wide range of hematologic malignancies and some carcinomas but is also expressed on some normal tissues. However, the fact that G28-5 sFv-PE40 was well tolerated in monkeys at doses that provided complete antitumor responses in xenografted mice indicates that the expression of CD40 on normal tissues may not limit the potential clinical utility of this anti-CD40 immunotoxin. We recently showed that both endothelial cells and monocytes, when grown under normal culture conditions, were insensitive to G28-5 sFv-PE40.24 This lack of primary cell sensitivity to G28-5 sFv-PE40 despite the presence of CD40 may explain its limited toxicity in monkeys. The high level of CD40 expression on a large number of human malignancies, the sensitivity of malignant B cells to G28-5 sFv-PE40, and the therapeutic efficacy of this immunotoxin at doses that are well tolerated in mice, rats, and monkeys indicate that this immunotoxin should be considered for clinical studies.
ACKNOWLEDGMENT
The authors thank J. Emswiler for performing BIACore binding analysis and Drs M. Steinitz, P. Fell, and K. E. Hellström for helpful comments.
Address reprint requests to Clay B. Siegall, PhD, Bristol-Myers Squibb Pharmaceutical Research Institute, 3005 First Ave, Seattle, WA 98121.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal