Abstract
An animal model of gram-positive septicemia was developed to evaluate the effects of antithrombin (AT) concentrates on morbidity, mortality, and laboratory consequences of disseminated intravascular coagulation (DIC). DIC was induced in guinea pigs by infusing Staphylococcus aureus (SA) isolated from blood cultures of patients with DIC (DIC-SA) or without DIC (non–DIC-SA). The non–DIC-SA animals and animals infused with sterile saline served as controls. Varying doses of AT were administered either 30 minutes or 24 hours after infusion of SA. DIC was confirmed within 4 hours by changes in prothrombin time, activated partial thromboplastin time, fibrinogen, fibrinogen-fibrin degradation products, and AT activity. Clinical bleeding was also evident. Mortality of untreated DIC-SA animals was 36% within 24 hours and up to 75% by 72 hours. Intervention with any dose of AT between 125 and 1,000 IU/kg 30 minutes after DIC-SA infusion was associated with 100% survival (P ≤ .05 in the 250 IU/kg group) and sustained increases in AT activity and fibrinogen concentrations (P ≤ .05). When AT was administered in combination with low molecular weight heparin (LMWH) or if LMWH was adminstered alone, mortality from DIC-SA was slightly, but not significantly reduced compared with untreated DIC-SA. Gross hemorrhage was observed premortem and at autopsy in all of the DIC-SA animals but in substantially fewer animals that received AT (P ≤ .001 in the 250, 500, and 1,000 IU/kg groups). In contrast, groups treated with LMWH, alone or with AT, experienced hemorrhage and appeared to develop pathologic DIC. Fibrin formation in end-organs was detected in all guinea pigs in the untreated DIC-SA group and in the groups treated with 125 IU/kg AT and LMWH alone. AT doses between 250 and 1,000 IU/kg administered 30 minutes after DIC-SA infusion prevented fibrin formation in end-organs (P ≤ .001 in the 250 and 1,000 IU/kg groups). AT administered 24 hours after DIC-SA could not reverse pre-existing histopathologic evidence of DIC but favorably affected survival, which reached statistical significance in the 1,000 IU/kg AT group (P ≤ .025). In summary, suprapharmacologic doses of AT concentrate significantly decreased morbidity and mortality and ameliorated adverse changes in laboratory measures induced by DIC-SA in this guinea pig model and were not associated with untoward hemorrhagic complications. These findings provide justification for studying the use of AT therapy in patients with DIC-SA.
DISSEMINATED intravascular coagulation (DIC) represents the composite contributions of the coagulation cascade and its modulators, platelet activation, endothelial cell perturbation and release, inflammatory cytokines, and circulating mononuclear cells. Although various factors and events may influence how these components interact and determine which will predominate, the ultimate pathophysiology of DIC is caused by the consequences of excessive thrombin generation in vivo with fibrin formation in end-organs. This process is normally impeded by circulating inhibitors of the activated coagulation factors, the most abundant of which is the serine protease inhibitor antithrombin (AT).1-3
Early in the course of DIC, AT activity significantly decreases by virtue of its consumption during complex formation with activated clotting factors and after proteolytic degradation by elastase secreted from polymorphonuclear leukocytes.4,5 This initial decrease in AT may have particular prognostic significance in the clinical management of DIC, with almost absolute lethality observed among septic patients when AT levels decreased to less than 50% to 60% of normal.6-8 Clinical observations and several animal models have generally shown an ameliorating effect of AT replacement on the laboratory alterations and severity or duration of DIC symptoms produced during gram-negative sepsis.9-13 Survival was improved in the experimental animal models only when AT concentrate was administered simultaneously with or before the DIC-inducing gram-negative bacteria insult11-13 and in high doses.11,13,14 The mortality of clinical DIC in a small, uncontrolled study of polytrauma patients15 was decreased with high doses of AT concentrate. Preliminary results in sepsis patients also suggest that the administration of AT concentrate may reduce mortality.16
The present investigation monitored the mortality, morbidity, and laboratory consequences of DIC in an animal model of gram-positive septicemia, which has become an increasingly common clinical phenomenon and is a prevalent life-threatening complication of long-term indwelling venous access catheters in cancer patients receiving chemotherapy and in hemophiliacs participating in prophylaxis and immune tolerance induction regimens. The effects of the administration of suprapharmacologic doses of AT concentrate on the consequences of DIC were also investigated.
MATERIALS AND METHODS
A single lot of human plasma-derived purified AT concentrate (6.8 IU/mg) was used in this study (kindly supplied by Dr James Brown, Bayer, Inc, Berkeley, CA) and was determined to contain less than 1% of normal plasma concentration of protein S antigen and less than 2% protein C antigen per vial of AT concentrate diluted with supplied diluent per manufacturer's specifications.
Suspensions of pure isolates of Staphylococcus aureus (SA) were prepared from the 18- to 24-hour cultures of organisms isolated from the blood of septic patients either with documented DIC (DIC-SA) or without laboratory or clinical evidence of DIC (non–DIC-SA). Spontaneous platelet aggregation was induced when a suspension of DIC-SA (50 μL) was added to 450 μL of stirred platelet-rich plasma from normal humans and guinea pigs (final ratio of bacteria to platelets, 1:1) maintained at 37°C in a dual-channel aggregometer apparatus (Peyton Associates, Buffalo, NY) attached to a chart recorder. No changes in light transmission and no detectable release of platelet factor 4 or β-thromboglobulin (measured only in human platelet-rich plasma) were observed after adding the non–DIC-SA strain. These in vitro results confirmed the pattern noted previously in our laboratory17 18 that predicted the DIC potential in vivo for various strains of SA in our guinea pig model. Both the DIC and control bacteria were processed simultaneously for in vitro and in vivo use and were quantitated spectrophotometrically from a previously calibrated standard curve using viable bacteria from quantitative culture plates.
Production of gram-positive septicemia.Experimental gram-positive septicemia with SA was produced in normal male Hartley guinea pigs (Bio Lab Corp, White Bear Lake, MN) weighing 350 to 500 g, adhering to a protocol approved by the Institutional Review Board for Animal Research and consistent with National Institutes of Health guidelines. Briefly, after at least 1 week of quarantine, each animal was anesthetized with Ketamine and a sterile 18-gauge polyethylene indwelling catheter was surgically inserted into the carotid artery. Catheters were maintained patent by introducing sterile 3.0% trisodium citrate into the catheter in a volume just adequate to fill the lumen and were used for all infusions and blood withdrawals. Immediately thereafter, guinea pigs were randomly assigned to receive intravenous boluses of a suspension of pure isolates of SA obtained either from DIC or non-DIC patients at a dose of 7 × 108 cfu/100 g body weight or sterile saline. At 30 minutes or 24 hours later, varying bolus doses of AT concentrate or sterile saline were administered and the animals were observed for an additional 48 hours with laboratory monitoring. Two additional groups of guinea pigs were treated with either low molecular weight heparin (LMWH) only (3 mg/kg Enoxaparin subcutaneously every 12 hours) to simulate the conventional approach to the treatment of clinical DIC or were treated with LMWH in combination with 125 IU/kg AT concentrate beginning 30 minutes after infusion of DIC-SA.
Postmortem pathologic assessment.Surviving guinea pigs were euthanized at the termination of the observation period and all guinea pigs were autopsied (animals treated with AT at 30 minutes were autopsied at 48 hours postinfusion of SA; animals treated at 24 hours were autopsied at 72 hours postinfusion of SA) to examine for gross and microscopic evidence of DIC, intraorgan abscess formation, and hemorrhage. Histopathologic tissue slides were prepared from tissue taken from the lung, myocardium, liver, stomach, spleen, and kidneys of all guinea pigs and were stained with hematoxylin-eosin and phosphotungstic acid-hematoxylin (PTAH) for analysis of histopathology and fibrin deposition.
Coagulation assays.A two-syringe technique was used for all blood draws from the indwelling carotid artery catheters with the second polypropylene syringe containing 1/10 vol of 3.8% trisodium citrate. Platelet-poor plasma was prepared by centrifugation of citrated whole blood at 3,500g for 10 minutes at 22°C and then used for coagulation assays. The prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured in an automated coagulometer (ST-4; Stago Diagnostica Labs, Asnieres/Seine, France) with celite as the activator for the aPTT. Plasma fibrinogen was determined chronometrically according to the method of Clauss19 and fibrin degradation products (FDPs) were detected by a latex agglutination procedure (Diagnostica Stago Labs). AT activity was measured in plasma via a modified chromogenic substrate assay (Stachrom AT III; American Bioproducts Co, Parsipanny, NJ) and platelets were counted in a Baker System 9000 Series, research mode (Biochem Immunosystems, Allentown, PA).
The laboratory diagnosis of DIC in guinea pigs20 required the presence of the following abnormalities: (1) PT 3 or more seconds greater than control and/or aPTT 5 or more seconds greater than the upper limit of normal; (2) an absolute decrease in plasma fibrinogen concentrations ≥25%; (3) the presence of elevated FDPs; (4) an absolute decrease in platelet counts; and (5) the presence of positive PTAH staining for intravascular fibrin formation on postmortem tissue sections. Clinically, DIC in guinea pigs was recognized by spontaneous epistaxis and bleeding at multiple sites and by evidence of gross internal hemorrhage at autopsy.
Statistical analysis.Results are expressed as the mean ± standard error of the mean. Comparisons of plasma markers for DIC in DIC-SA and non–DIC-SA groups were made using an analysis of variance (ANOVA) with repeated measures. Selected group comparisons at various time points were conducted using an unpaired Students's t-test. Bonferroni's correction for multiple comparisons was used to adjust the level of significance (ie, P ≤ .017 was necessary for statistical significance). Comparisons of plasma markers between the various treatments for DIC were made using an ANOVA with repeated measures. Tukey post-hoc tests were used to discriminate between groups when significant differences were found. Fisher's test was used to analyze the pathologic assessments of end-organ data. A value of P < .05 was considered statistically significant.
RESULTS
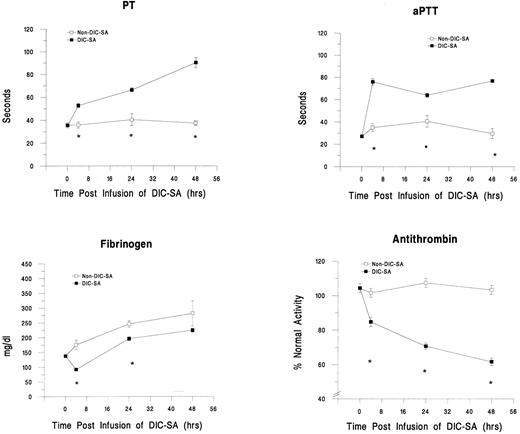
Development of DIC.This animal model successfully induced DIC, as evidenced by changes in plasma markers for DIC and clinical observations. Results for PT, aPTT, fibrinogen, and AT are shown in Fig 1. Significant decreases in mean platelet counts (P < .01, data not shown), AT activity levels (P ≤ .001), and fibrinogen concentrations (P ≤ .001) were noted in the animals receiving DIC-SA (n = 14) within 4 hours after infusion and, except for fibrinogen, persisted or progressed over time. Simultaneous significant prolongations of PT (P ≤ .001) and aPTT (P ≤ .001) and elevations of FDPs (data not shown) also occurred. These changes, in conjunction with evidence of clinical hemorrhage in the animals, ie, epistaxis, oozing around catheter sites, etc, were consistent with the development of DIC. In contrast, these parameters were not significantly affected (P > .05) in control animals receiving the non–DIC-SA strains (n = 5; Fig 1) or sterile saline (n = 5; data not shown). Although gradual increases in fibrinogen levels, which reached statistical significance 24 hours postinfusion (P = .002), were observed over time in the non–DIC-SA group and in the DIC-SA group at 24 and 48 hours (P ≤ .001), this most likely reflects a physiologic acute-phase response to the catheter placement surgery under anesthesia.
Changes in plasma markers of DIC over time in guinea pigs infused with DIC-SA and non–DIC-SA. *Significant difference between groups (P ≤ .05).
Changes in plasma markers of DIC over time in guinea pigs infused with DIC-SA and non–DIC-SA. *Significant difference between groups (P ≤ .05).
Treatment with AT concentrates 30 minutes after DIC-SA infusion.The progressive depression of AT levels and the clinical manifestations and morbidity of DIC in the DIC-SA group prompted the therapeutic intervention with AT concentrate infusions at various weight-based doses. In the initial set of experiments, guinea pigs received AT concentrate at 125 IU/kg (n = 3), 250 IU/kg (n = 11), 500 IU/kg (n = 4), or 1,000 IU/kg (n = 7) 30 minutes after infusion of DIC-SA, non–DIC-SA, or saline. Changes in plasma markers for DIC for the DIC-SA groups treated with AT and the untreated DIC-SA group are shown in Fig 2 (results for the non–DIC-SA and saline groups are not shown). The ANOVA results for each variable indicated significant main effects for group (P < .001), time (P < .001), and significant group × time interactions (P < .001). Compared with the untreated guinea pigs (DIC-SA alone), AT levels after 4 hours of DIC-SA infusion were significantly (P < .05) and proportionately increased over baseline according to the dosage in all groups treated with AT. These significant increases over baseline were sustained for 24 hours after DIC-SA infusion in all AT-treated groups, except for the group receiving the lowest dose (125 IU/kg). Although AT levels continued to decrease over time in the treated animals, AT remained elevated (P < .05) in the groups treated with the three highest doses (250, 500, and 1,000 IU/kg) compared with the DIC-SA alone group and the lowest AT dose group (125 IU/kg) at 48 hours after DIC-SA infusion. Peak AT levels at 4 hours postinfusion in the DIC-SA and non–DIC-SA groups treated with the 1,000 IU/kg AT were similar (non–DIC-SA, 739% ± 60.4%; DIC-SA, 725% ± 41%) and significantly greater (P < .001) than the untreated DIC-SA group (85% ± 3%).
Changes in plasma markers of DIC over time after the administration of varying doses of AT concentrate 30 minutes postinfusion of DIC-SA. *DIC-SA alone group different from all other groups (P ≤ .05). ρDIC-SA alone group different from 1,000 IU/kg, 500 IU/kg, and 250 IU/kg groups (P ≤ .05). †DIC-SA alone group different from 1,000 IU/kg and 500 IU/kg groups (P ≤ .05). ¶1,000 IU/kg and 500 IU/kg groups different from 125 IU/kg and 250 IU/kg groups (P ≤ .05).
Changes in plasma markers of DIC over time after the administration of varying doses of AT concentrate 30 minutes postinfusion of DIC-SA. *DIC-SA alone group different from all other groups (P ≤ .05). ρDIC-SA alone group different from 1,000 IU/kg, 500 IU/kg, and 250 IU/kg groups (P ≤ .05). †DIC-SA alone group different from 1,000 IU/kg and 500 IU/kg groups (P ≤ .05). ¶1,000 IU/kg and 500 IU/kg groups different from 125 IU/kg and 250 IU/kg groups (P ≤ .05).
The administration of AT concentrates also appeared to alter fibrinogen levels. The higher doses (500 and 1,000 IU/kg) ameliorated the fibrinogen consumption that was observed in the untreated DIC-SA animals and the low-dose AT animals (P < .05). Significantly higher fibrinogen levels were observed at 4, 24, and 48 hours in the higher dose groups, including the 250 IU/kg dose at 48 hours (P < .05).
The PTs and aPTTs also tended to respond in a dose-dependent manner. The significantly prolonged PTs and aPTTs at 4 and 24 hours in the 125 IU/kg group compared with the other AT groups (P < .05) suggests that this dose is virtually ineffective in impeding the consumption of coagulation proteins in the intrinsic and/or extrinsic pathways. At 48 hours postinfusion, the DIC-SA alone group continued to show significantly prolonged PT and aPTT compared with all other groups (P < .05).
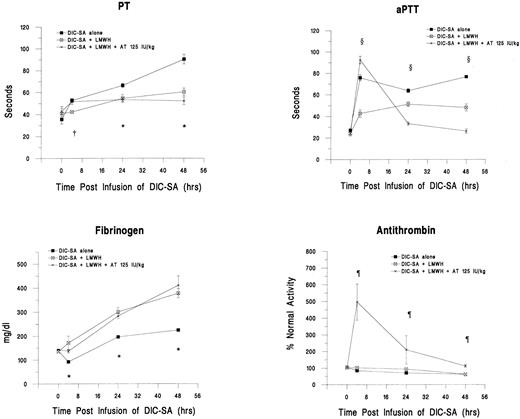
Treatment with LMWH.To simulate the conventional approach to treatment of clinical DIC, an additional group of DIC-SA animals was treated with LMWH (n = 4). Anti-factor Xa antibody (anti-Xa) levels were monitored and confirmed that a range of anticoagulation between 0.6 and 0.9 anti-Xa U/mL was maintained throughout the study period. In addition, another group of guinea pigs was treated with LMWH in combination with 125 IU/kg AT (n = 5). Changes in plasma markers for DIC in these groups are shown in Fig 3. LMWH effectively modulated fibrinogen consumption. Significantly higher fibrinogen levels were observed in both LMWH groups compared with the DIC-SA alone group (P < .05). Fibrinogen levels in the LMWH alone group and the LMWH in combination with AT group did not differ (P > .05). The LMWH also prevented the prolongation of PT and aPTT, particularly at 24 and 48 hours postinfusion (P < .05). AT levels were significantly higher at all time points in the LMWH plus 125 IU/kg AT group compared with the LMWH and DIC-SA alone groups (P < .05).
Changes in plasma markers of DIC over time after the administration of LMWH alone and in combination with 125 IU/kg AT 30 minutes postinfusion of DIC-SA. *DIC-SA group is different from other groups (P ≤ .05). †LMWH group different from other groups (P ≤ .05). ¶LMWH + 125 IU/kg AT group is different from other groups (P ≤ .05). ρAll groups are different (P ≤ .05).
Changes in plasma markers of DIC over time after the administration of LMWH alone and in combination with 125 IU/kg AT 30 minutes postinfusion of DIC-SA. *DIC-SA group is different from other groups (P ≤ .05). †LMWH group different from other groups (P ≤ .05). ¶LMWH + 125 IU/kg AT group is different from other groups (P ≤ .05). ρAll groups are different (P ≤ .05).
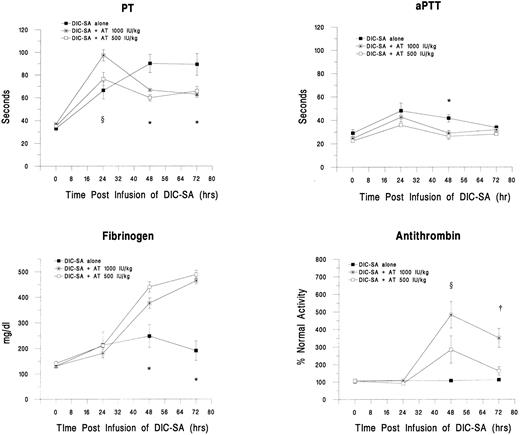
Treatment with AT concentrates 24 hours postinfusion.Subsequent groups of guinea pigs received AT concentrate at 500 IU/kg (n = 4) or 1,000 IU/kg (n = 8) at 24 hours after infusion of the DIC-SA. Changes in plasma markers for DIC in these groups are shown in Fig 4. The initially prolonged PTs associated with DIC significantly decreased (P < .05) and reverted toward the normal range within 24 hours after receiving AT concentrate, ie, 48 hours postinfusion of DIC-SA, and were maintained for 48 hours after AT infusions, ie, 72 hours after DIC-SA infusion (Fig 4). Similar, but less pronounced responses in aPTTs were seen. There was no statistically significant difference in responses between the two AT-treated cohorts. Similarly, fibrinogen levels were significantly increased 24 and 48 hours postadministration of AT in both the 500 IU/kg and 1,000 IU/kg groups compared with the untreated group (P < .05). Again, the responses to the two doses did not differ (P > .05). Alterations in AT concentrations were dose-related. At 24 hours post-AT administration (48 hours after DIC-SA infusion), AT levels in the 1,000 IU/kg group were significantly increased over the DIC-SA alone group (P < .05). Differences in AT levels between the 500 IU/kg dose group and the DIC-SA alone group at this time point approached but did not reach statistical significance (P > .05). By 48 hours after AT administration (72 hours after DIC-SA infusion), the elevation in AT persisted in the animals that received the 1,000 IU/kg dose (P < .05).
Changes in plasma markers of DIC over time and in response to the administration of AT 24 hours postinfusion of DIC-SA. *DIC-SA group is different from other groups (P ≤ .05). ρDIC-SA group and 1,000 IU/kg group are different (P ≤ .05). †1,000 IU/kg group is different from other groups (P ≤ .05).
Changes in plasma markers of DIC over time and in response to the administration of AT 24 hours postinfusion of DIC-SA. *DIC-SA group is different from other groups (P ≤ .05). ρDIC-SA group and 1,000 IU/kg group are different (P ≤ .05). †1,000 IU/kg group is different from other groups (P ≤ .05).
Postmortem pathologic assessment.All guinea pigs were autopsied either 48 hours after AT administration or upon death if before sacrifice. Pathologic assessment results comparing the DIC-SA group with the non-DIC and saline groups as well as the DIC-SA groups treated with AT 30 minutes after DIC-SA infusion are shown in Table 1. For statistical analysis, all groups were compared with the DIC-SA group (n = 14). In contrast to the DIC-SA animals, the saline controls (n = 5) and the non–DIC-SA controls (n = 5) manifested no evidence of gross or microscopic hemorrhage on examination of the lungs, myocardium, livers, stomachs, spleens, and kidneys (P ≤ .001). Hepatic abscess formation containing gram-positive cocci was noted in only one of these animals; no fibrin formation was detected on PTAH stain (P ≤ .001) and no early deaths occurred. All but one of these animals exhibited normal histology (P ≤ .001 and .01 for saline and non–DIC-SA groups, respectively). Thus, there was no indication of DIC. In contrast, all of the DIC-SA guinea pigs showed evidence of DIC with gross hemorrhage and fibrin formation in end-organs. Five of the animals (36%) died before the 48-hour study period was completed and five had liver abscesses. Administration of AT concentrate at all experimental doses 30 minutes after DIC-SA infusion appeared to eliminate mortality; however, statistical significance was reached in the 250 IU/kg group only (P ≤ .05). Fibrin formation was observed only in the AT 125 IU/kg group. In fact, none of the pathologic data in the animals who received this low dose differed from that of the DIC-SA group (P > .05). Significantly, gross or microscopic hemorrhage was absent in the animals which received the 3 larger doses of AT concentrate (P ≤ .001), despite achieving supraphysiologic plasma AT activity levels. Abscess formation occurred in some of these guinea pigs. Normal histology was observed in the groups receiving 1,000 IU/kg and 250 IU/kg (P ≤ .001). The treatment of the DIC-SA group with LMWH alone appeared to reduce the mortality rate (25% v 36%) when compared with the DIC-SA alone cohort; however, the reduction in mortality was not statistically significant (P > .05). Additionally, there was evidence of DIC with fibrin formation and hemorrhage in almost all of these animals and none exhibited normal histology. When AT 125 IU/kg was administered in conjunction with LMWH after DIC-SA infusion, the mortality rate (20%) appeared to be reduced but was not statistically different from the DIC-SA alone group, gross hemorrhage was ubiquitous, and abscess formation was common.
Pathologic Assessment of End-Organs After Guinea Pigs Received AT and/or LMWH 30 Minutes Postinfusion of DIC-SA
| . | Gross Hemorrhage . | Abscess Formation . | Mortality . | Histopathology (fibrin formation) . | Normal Histology . |
|---|---|---|---|---|---|
| Saline controls (n = 5) | 0* | 0 | 0 | 0* | 5* |
| Non–DIC-SA controls (n = 5) | 0* | 1 | 0 | 0* | 4† |
| DIC-SA alone (n = 14) | 14 | 5 | 5 | 14 | 0 |
| DIC-SA + AT concentrate | |||||
| 125 IU/kg (n = 3) | 3 | 1 | 0 | 3 | 0 |
| 250 IU/kg (n = 11) | 1* | 2 | 0‡ | 0* | 9* |
| 500 IU/kg (n = 4) | 0* | 3 | 0 | 0 | 1 |
| 1,000 IU/kg (n = 7) | 0* | 3 | 0 | 0* | 4* |
| DIC-SA + LMWH (n = 4) | 3 | 1 | 1 | 4 | 0 |
| DIC-SA + LMWH + 125 IU/kg AT (n = 5) | 5 | 3 | 1 | 0* | 0 |
| . | Gross Hemorrhage . | Abscess Formation . | Mortality . | Histopathology (fibrin formation) . | Normal Histology . |
|---|---|---|---|---|---|
| Saline controls (n = 5) | 0* | 0 | 0 | 0* | 5* |
| Non–DIC-SA controls (n = 5) | 0* | 1 | 0 | 0* | 4† |
| DIC-SA alone (n = 14) | 14 | 5 | 5 | 14 | 0 |
| DIC-SA + AT concentrate | |||||
| 125 IU/kg (n = 3) | 3 | 1 | 0 | 3 | 0 |
| 250 IU/kg (n = 11) | 1* | 2 | 0‡ | 0* | 9* |
| 500 IU/kg (n = 4) | 0* | 3 | 0 | 0 | 1 |
| 1,000 IU/kg (n = 7) | 0* | 3 | 0 | 0* | 4* |
| DIC-SA + LMWH (n = 4) | 3 | 1 | 1 | 4 | 0 |
| DIC-SA + LMWH + 125 IU/kg AT (n = 5) | 5 | 3 | 1 | 0* | 0 |
Significantly different from DIC-SA alone group (P ≤ .001).
Significantly different from DIC-SA alone group (P ≤ .01).
Significantly different from DIC-SA alone group (P ≤ .05).
All experimental animals receiving either 500 IU/kg or 1,000 IU/kg of AT concentrate 24 hours after DIC-SA infusion were observed for an additional 48 hours before being killed and autopsied. Pathologic data for these animals are shown in Table 2. Microscopic and gross evidence of hemorrhage, abscess, and intravascular fibrin formation was pervasive in all of the untreated DIC-SA animals. Animals in both AT-treated groups manifested evidence of fibrin formation. Abscess formation was variable but appeared to be less common in the AT 1,000 IU/kg DIC-SA cohort; however, the number of guinea pigs experiencing abscess formation did not significantly differ from the DIC-SA alone group (P > .05). Gross hemorrhage was found in 50% of the animals treated with 1,000 IU/kg AT and in all of the DIC-SA alone animals, but in none of the 500 IU/kg AT group (P ≤ .05). Despite these findings, administration of AT concentrate promoted 100% survival up to 72 hours after infusion of DIC-SA compared with the 75% mortality rate in DIC-SA alone animals observed for 72 hours. The lower mortality rate in the group receiving the 1,000 IU/kg dose reached statistical significance (P < .025).
Pathologic Assessment of End-Organs After Guinea Pigs Received AT 24 Hours Postinfusion of DIC-SA
| . | Gross Hemorrhage . | Abscess Formation . | Mortality . | Histopathology (fibrin formation) . | Normal Histology . |
|---|---|---|---|---|---|
| DIC-SA (n = 4) | 4 | 2 | 3 | 4 | 0 |
| DIC-SA + AT concentrate | |||||
| 500 IU/kg (n = 4) | 0* | 2 | 0 | 4 | 2 |
| 1,000 IU/kg (n = 8) | 4 | 1 | 0† | 6 | 4 |
| . | Gross Hemorrhage . | Abscess Formation . | Mortality . | Histopathology (fibrin formation) . | Normal Histology . |
|---|---|---|---|---|---|
| DIC-SA (n = 4) | 4 | 2 | 3 | 4 | 0 |
| DIC-SA + AT concentrate | |||||
| 500 IU/kg (n = 4) | 0* | 2 | 0 | 4 | 2 |
| 1,000 IU/kg (n = 8) | 4 | 1 | 0† | 6 | 4 |
Significantly different from DIC-SA alone group (P ≤ .05).
Significantly different from DIC-SA alone group (P ≤ .025).
DISCUSSION
The pathogenesis of DIC is complex, requiring the interaction of plasma procoagulant proteins, cytokines and mediators of inflammation, fibrinolytic enzymes, platelets, and, when triggered in the presence of sepsis, gram-positive or gram-negative microorganisms.21 The hierarchy of these components in mediating the progression of the process and the evolution of morbid and/or fatal events remains unclear; however, faulty inhibition of coagulation by the major naturally circulating inhibitors such as AT is critical. Clinical studies strongly suggest that AT consumption is an early and sensitive diagnostic indicator of DIC22 and may provide useful prognostic information regarding its potential lethality.6-8 Traditionally, DIC treatment is aimed toward the eradication or control of a specific underlying pathologic process, ie, infection, malignancy, etc; however, success is often delayed and limited, allowing the DIC process to accelerate and produce cumulative adverse effects. Intervention with burst replacement of a modulator(s) of coagulation would appear to be an attractive means of rapidly decelerating DIC and in polytrauma patients translated into improved survival15 when accomplished with concentrates of AT. Unfortunately, in the absence of well-controlled randomized clinical trials, this treatment is controversial and considered empiric. Therefore, we developed a guinea pig model for SA-induced DIC, an increasingly common clinical problem, to test this hypothesis.
The development of DIC in the experimental animals that received DIC-SA alone was confirmed in vitro with laboratory assays, ex vivo by autopsy evidence of fibrin formation in end-organs, and in vivo by the premortem and/or postmortem presence of hemorrhage. The mortality in this group was substantial. Treatment with AT concentrates 30 minutes after the DIC-SA infusion definitely ameliorated these findings and was associated with 100% survival. This improved survival reached statistical significance in the group receiving the 250 IU/kg dose. It is likely that statistical significance would have also been attained in the higher dose groups if the number of animals in these two groups was increased. Furthermore, the development of DIC was averted in a dose-response manner by increasing plasma AT levels well above the normal range.
The guinea pigs treated with 1,000 IU/kg AT concentrate alone or 30 minutes after DIC-SA injection achieved supranormal AT levels without sustaining any adverse hemorrhagic events, an important indicator of the safety profile of suprapharmacologic dosing if AT concentrate is to ultimately become a useful clinical adjunctive therapy for DIC. Similar findings were reported in a limited number of septic patients treated with high dose AT.16 The hemorrhage seen in the animals receiving AT 24 hours after DIC-SA was probably produced as a result of DIC and likely occurred before AT administration.
LMWH was administered to guinea pigs injected with DIC-SA either as a single agent or combined with a dose of AT concentrate (125 IU/kg) and appeared to reduce the mortality of DIC in this animal model, although statistical significance was not reached, but was associated with histopathologic alterations consistent with DIC. Interestingly, the addition of LMWH to the treatment regimen did not enhance any of the beneficial effects of AT, but rather permitted the development of histopathologic DIC and appeared to induce gross hemorrhage in almost all experimental animals.
Similarly, randomized studies in humans with shock and DIC observed that combined therapy with heparin and AT resulted in a higher frequency of bleeding complications and increased blood loss,23,24 and a patient with acquired heparan sulfate-like dysproteinemia was recently reported to have hemorrhaged to death after AT infusions.25 These findings may be related to heparin(an)-mediated enhancement of AT inactivation and proteolysis by neutrophil elastase released during fulminant bacteremia or stress.4,5,26 In addition, the naturally occurring circulating inhibitor, α1-proteinase inhibitor, which neutralizes elastase, is rapidly oxidized and inactivated by free oxygen radicals produced by stimulated neutrophils.27 This results in a net positive effect on promoting and propagating the DIC process.
Heparin, the traditional parenteral anticoagulant used for modulation of the DIC process, is variably successful as prophylaxis or treatment of DIC. In the current study, we chose to administer an LMWH preparation to prevent DIC for logistic reasons. Nevertheless, we did observe some favorable changes in the plasma markers for DIC with administration of this agent. Although the mortality data did not reach statistical significance, which was most likely due to the small number of animals examined, we do believe these preliminary results suggest that LMWH could be potentially useful as a single or combined agent in the treatment of DIC and deserves to be evaluated in a randomized, controlled clinical trial. Because LMWH possesses greater anti-factor Xa than antithrombin (IIa) effects and has less potential to activate platelets, it may have an improved safety profile over unfractionated heparin and may supplant the latter in DIC clinical scenarios, particularly if larger doses can be used to neutralize pathologically generated IIa.
The relevant antithrombotic effects of AT are based on the inhibition of factor Xa and IIa, which is accelerated 1,000-fold by heparin and presumably by the glycosoaminoglycans present on endothelial cell surfaces. An overwhelming excess of circulating AT, such as produced in these studies with suprapharmacologic dosing, may alter the kinetics of these interactions or the actions of other serine proteases, such as plasmin, kallikrein, or trypsin to attenuate DIC. Alternatively, infusions of large amounts of AT may exert hitherto unknown effects on coagulation. This may be particularly pertinent in SA-induced DIC because Staphylococci have been shown to induce tissue factor production by endothelial cells,28 which would result in local and systemic generation of thrombin and mediation of the DIC process.
This study involved the use of human AT concentrate in an animal model. Understandably, this is not an ideal situation. However, given that the administration of human AT did modulate both laboratory and clinical/pathologic evidence of thrombin formation and beneficially altered the clinical course of DIC in these animals, we believe that the use of homologous proteins (ie, human AT in humans or guinea pig AT in guinea pigs) may provide even more beneficial results.
The AT concentrate used in this study contained only trace contamination by protein C or protein S, which would not be expected to provide significant pharmacologic benefits for the modulation of hypercoagulability. Furthermore, high doses of AT concentrates do not induce any change in circulating protein C and protein S activity.14
In conclusion, the survival advantages and the microscopic and gross pathologic benefits conveyed by suprapharmacologic dosing of AT concentrate in this animal model of SA-induced DIC provide insight and justification to study a similar approach in clinical trials of DIC.
Address reprint requests to Craig M. Kessler, MD, Division of Hematology/Oncology, Georgetown University, 3800 Reservoir Rd NW, Washington, DC 20007.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal