Abstract
Tissue factor pathway inhibitor (TFPI) is a Kunitz-type plasma protease inhibitor that inhibits factor Xa and the factor VIIa/tissue factor catalytic complex. It plays an important role in feedback inhibition of the coagulation cascade (Broze, Annu Rev Med 46:103, 1995). TFPI has also been used successfully to prevent lethality and attenuate coagulopathic responses in a baboon model of septic shock (Creasey et al, J Clin Invest 91:2850, 1993; and Carr et al, Circ Shock 44:126, 1995). However, the mechanism of reduced mortality in these animals could not be explained merely by the anticoagulant effect of TFPI, because TFPI-treated animals also had a significantly depressed interleukin-6 response. Moreover, inhibition of coagulopathic responses by other anticoagulants has failed to block the organ damage or lethal effect of endotoxic shock (Coalson et al, Circ Shock 5:423, 1978; Warr et al, Blood 75:1481, 1990; and Taylor et al, Blood 78:364, 1991). We show here that recombinant TFPI can bind to endotoxin in vitro. This binding prevents interaction of endotoxin with both lipopolysaccharide binding protein and CD14, thereby blocking cellular responses.
ENDOTOXIN (lipopolysaccharide [LPS]) is a bacterial cell wall component responsible for remarkable stimulation of host cells and initiation of the event leading to septic shock. Systemic exposure to endotoxin activates a cascade of inflammatory responses leading to increased secretion of cytokines such as tumor necrosis factor (TNF ), interleukin-1 (IL-1), and IL-6 from inflammatory cells. These cytokines are believed to be responsible for most clinical manifestations of septic shock. Endotoxin can also induce coagulation and lead to disseminated intravascular coagulation (DIC). DIC is thought to arise from a shift in the vascular endothelium from an anticoagulant to a procoagulant state caused by induction of tissue factor (TF ) by inflammatory cytokines or endotoxin. A role for TF in DIC was demonstrated earlier by showing that infusion of TF-induced DIC in rabbit and antibody to TF reduced the incidence of endotoxin-induced DIC.1
Tissue factor pathway inhibitor (TFPI) is a multivalent, Kunitz-type plasma protease inhibitor known to inhibit factor Xa directly and factor VIIa/TF complexes in a factor Xa-dependent manner.2 TFPI exists in three different pools (1) associated with lipoproteins, (2) stored in platelets, and (3) associated with endothelium such that it can be released into circulation by heparin.3 The inhibitory role of TFPI in DIC was shown in animal studies in which depletion of endogenous TFPI sensitized rabbits to DIC induced by TF or endotoxin.4,5 On the other hand, infusion of recombinant TFPI clearly reduced the mortality of baboons injected with lethal doses of Escherichia coli.6 7 In these studies, TFPI-treated animals showed attenuation of the coagulopathic responses compared with placebo-treated animals. However, TFPI-treated animals also showed a strongly decreased cytokine response (IL-6), suggesting that TFPI had an inhibitory effect on inflammatory responses initiated by gram-negative bacteria. A result from a methodologically similar study suggests that the anticoagulant effect of TFPI is not sufficient to explain reduced mortality. When an active-site blocked factor Xa (DEGR-factor Xa) was used as an anticoagulant, shock, organ damage, or death was not prevented, despite effective blockage of DIC.8
CD14 and LBP play a crucial role in mediating cellular responses to endotoxin. CD14 binds LPS9 and complexes of LPS and CD14 play a key role in initiating responses.9-11 CD14 is found on the surface of monocytes and polymorphonuclear leukocyte (PMN) as a glycosylphosphatidylinositol-linked protein. It also exists as a soluble form in plasma and this form is necessary for responses of cell types such as endothelium that do not express membrane CD14.12 LBP, a lipid transfer protein, is present in plasma associated with a lipoprotein particle.13,14 LBP transfers LPS molecules from LPS micelles to CD14 in a catalytic manner. Spontaneous binding of LPS to CD14 occurs only slowly but is dramatically enhanced by LBP.9 15 Consequently, PMN or monocytes respond poorly to LPS alone, but show a very strong and rapid response in the presence of LBP.
To explain the anti-inflammatory effect of TFPI observed in the above-mentioned studies, we hypothesized that TFPI may have an effect on early inflammatory responses involving transfer of LPS to CD14 by LBP. In this report, we present evidence showing that, indeed, TFPI interferes with the LBP-mediated transfer of LPS to CD14 and that TFPI has this effect by directly binding to LPS.
MATERIALS AND METHODS
Materials.LPS, Re595 from Salmonella minnesota or Rb from E coli K12 was purchased from List Biological Laboratories (Campbell, CA). Recombinant human LBP (rhLBP) and rh soluble CD14 (sCD14) were generous gifts from Dr Henri Lichenstein (Amgen, Thousand Oaks, CA). rhTNF-α was purchased from Genzyme (Cambridge, MA). Recombinant human TFPI was prepared as described.16 Briefly, nonglycosylated TFPI with an additional alanine attached to the amino terminus of the wild-type molecule (ala-TFPI) was expressed in E coli and purified using chromatographic methods. The final product used in the study was formulated in arginine citrate buffer (200 mmol/L arginine, 20 nmol/L sodium citrate, pH 6.0, containing 150 mmol/L sodium chloride and 0.01% polysorbate 80). rTFPI13-161 was also expressed in E coli and purified using chromatographic methods used for full-length ala-TFPI. Reconstituted high-density lipoprotein (rHDL) was prepared as described previously17 with the ratio of phosphatidylcholine:cholesterol:apolipoprotein AI equaling 80:8:1.
PMN adhesion assay.Details of the PMN adhesion assay were described previously.18 Briefly, freshly isolated PMN from healthy volunteers were labeled with carboxyfluorescein diacetate, succinimidyl ester (Molecular Probe, Eugene, OR), incubated with stimuli for 10 minutes at 37°C, washed, and then transferred to terasaki plates coated with fibrinogen. Adhesion of the PMN was determined by reading the fluorescence before and after washing the plate, and the amount of fluorescence remaining after wash was calculated and expressed as the percentage of adhesion.
Transfer of LPS to sCD14 or rHDL.Fluorescently labeled LPS (BODIPY-LPS) was prepared from Re595 of S minnesota as described previously.15 In aqueous buffer, this preparation shows fluorescence quenching, but quenching is relieved and fluorescence increases upon movement of LPS monomers into sCD1415 or rHDL.19 For LBP-mediated LPS transfer to sCD14, rLBP and sCD14 were quickly mixed with the BODIPY-LPS in phosphate-buffered saline (PBS) containing 2 mg/mL of bovine serum albumin (BSA) and the fluorescence increase upon binding of BODIPY-LPS to sCD14 was recorded in real time (every 0.2 seconds). From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated. (The curve was linear in this period [R2 > .95].) For other BODIPY-LPS transfer assays, BODIPY-LPS was incubated with various reagents in PBS containing 2 to 3 mg/mL of BSA for the indicated amount of time either at room temperature or 37°C. At the end of the incubation, the fluorescence of each sample was measured before and after adding sodium dodecyl sulfate (SDS). SDS disaggregates LPS and yields maximal fluorescence efficiency. When transfer was expressed as RF (%), it represents the ratio of fluorescence measured to the total amount of fluorescence in the sample (fluorescence measured in 4% SDS).
Binding of LBP or TFPI to LPS.To coat the plastic surfaces, 0.01 μg of RbLPS from E coli K12 diluted in methanol was added to each well of a terasaki plate and incubated at 37°C until dry. The plates were then blocked with 10 mg/mL of BSA in PBS for 1 hour at room temperature. rLBP or rTFPI was added to wells and incubated for 1 hour at room temperature or overnight at 4°C. rLBP or rTFPI bound on the surface was detected with rabbit polyclonal anti-LBP or anti-TFPI antibodies and alkaline phosphatase-conjugated secondary antibody followed by a fluorogenic substrate (Attophos; JBL Scientific, San Luis Obispo, CA). Fluorescence was measured by a fluorescence plate reader.
RESULTS
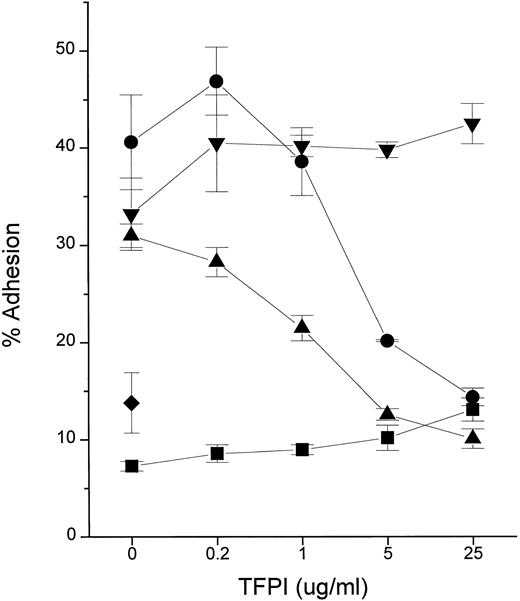
We tested the hypothesis that TFPI blocks cellular responses to endotoxin by using the PMN adhesion assay. The adhesive response of PMN to various inflammatory stimuli is rapid, requiring only 10 to 15 minutes of incubation time.18 Recombinant TFPI (rTFPI) effectively inhibited the PMN response to LPS (endotoxin) in the presence of plasma or purified recombinant LBP (Fig 1). In contrast, TFPI had no effect on the response to TNF-α, suggesting that inhibition is specific to endotoxin.
TFPI inhibits PMN adhesion in response to endotoxin. Fluorescently labeled PMN were incubated for 10 minutes 37°C in the presence of stimuli and the indicated concentrations of rTFPI. After washing, adhesion to fibrinogen-coated surface was measured. Stimuli were LPS (10 ng/mL, Rb from E coli K12) with NHP (2%; •), LPS with rLBP (1 μg/mL; ▴), rTNF-α (0.5 ng/mL; ▾), and buffer alone (▪) in the presence of increasing doses of rTFPI. The average of triplicates and standard deviation are shown as a symbol and error bars. (♦) A PMN sample incubated with LPS only. Data are plotted on logarithmic X scale.
TFPI inhibits PMN adhesion in response to endotoxin. Fluorescently labeled PMN were incubated for 10 minutes 37°C in the presence of stimuli and the indicated concentrations of rTFPI. After washing, adhesion to fibrinogen-coated surface was measured. Stimuli were LPS (10 ng/mL, Rb from E coli K12) with NHP (2%; •), LPS with rLBP (1 μg/mL; ▴), rTNF-α (0.5 ng/mL; ▾), and buffer alone (▪) in the presence of increasing doses of rTFPI. The average of triplicates and standard deviation are shown as a symbol and error bars. (♦) A PMN sample incubated with LPS only. Data are plotted on logarithmic X scale.
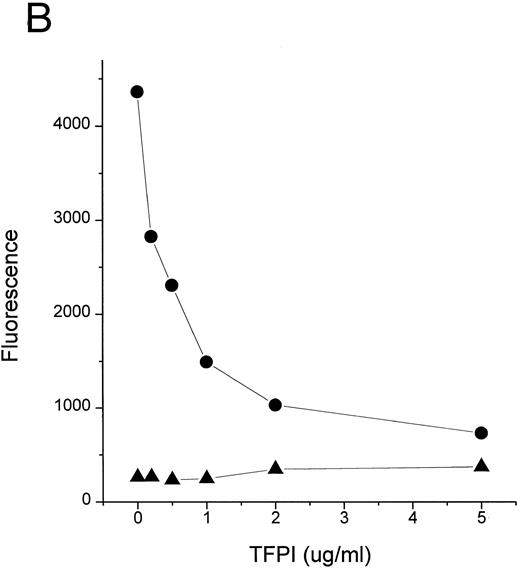
Complexes of LPS with CD14 are key intermediates in cell stimulation by LPS.9 11 Formation of LPS-CD14 complexes is catalyzed by LBP, a plasma lipid transfer protein. We thus tested whether TFPI could block the transfer of LPS to CD14 in a cell free system. Figure 2 shows that TFPI effectively blocked the movement of LPS to sCD14 facilitated by LBP (Fig 2A and C) or normal human plasma (Fig 2B). Blockade of LPS binding to CD14 would be predicted to block cellular responses to LPS and could contribute to the efficacy of TFPI in septic shock.
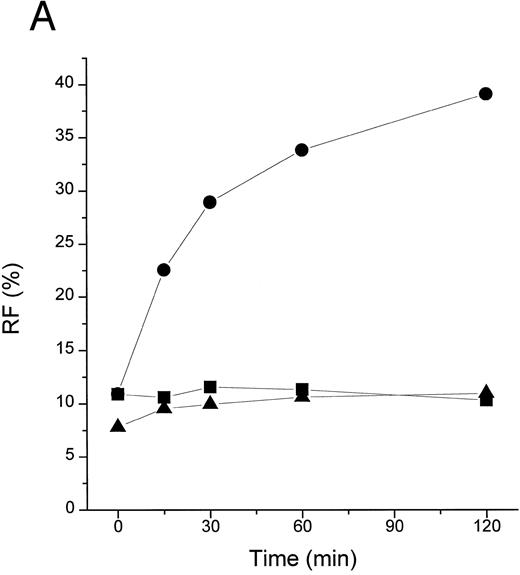
TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.
TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.
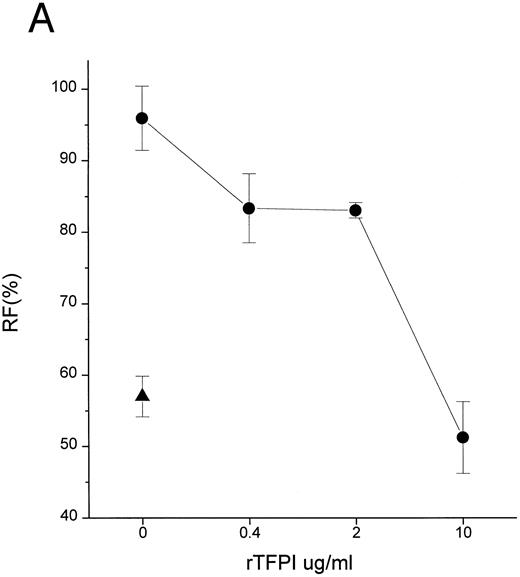
TFPI could block interaction of LPS with CD14 by interfering with LPS, LBP, or CD14. Additional studies were therefore performed to establish the target of TFPI. LBP is known to transfer LPS molecules not only to CD14 but also to rHDL.17 To determine if TFPI blocks LPS transfer by interacting with CD14, we tested whether TFPI could inhibit the movement of LPS to rHDL by LBP in the absence of CD14. Figure 3A shows that TFPI effectively inhibited the LBP-catalyzed movement of LPS to rHDL in the absence of CD14, thus suggesting that CD14 is not necessary for the effect of TFPI. Additional experiments showed that rTFPI blocks interaction between LBP and LPS. LBP binds to LPS-coated surfaces and this binding was effectively blocked by rTFPI (Fig 3B). Inhibition by TFPI was observed not only when LBP was added to the wells together with TFPI, but also when LBP was added after TFPI was incubated with LPS-coated surface and washed away (data not shown). These results strongly suggest that TFPI blocks interaction of LBP and LPS by binding to LPS. Direct evidence of TFPI binding to LPS was shown by measuring association of TFPI with LPS-coated surfaces (Fig 3C). TFPI thus binds LPS and thereby prevents LPS-LBP interaction. The apparent Kd of TFPI-LPS binding was approximately 0.23 nmol/L, a figure comparable to that reported for LBP.20 However, our competition assay (Fig 3B) showed that a sixfold molar excess of TFPI was needed for half-maximal blockade of LBP binding. This suggests that LBP binds to LPS more avidly than TFPI.
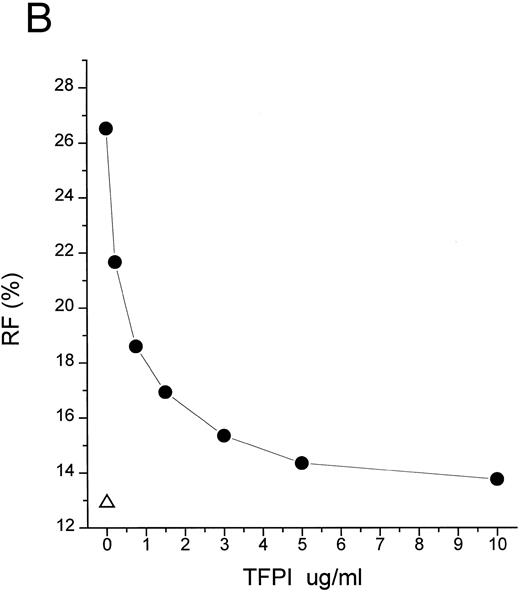
TFPI interferes with LBP-LPS interaction by binding to LPS. (A) TFPI blocks LBP-mediated transfer of LPS to rHDL. LBP-mediated BODIPY-LPS transfer to rHDL was measured as described in the Materials and Methods. BODIPY-LPS (0.5 μg/mL), rLBP (5 μg/mL), rHDL (20 μg/mL), and the indicated amounts of rTFPI were mixed and incubated for 2.5 hours in room temperature. At this time point, the transfer of BODIPY-LPS to rHDL was completed. The transfer was expressed as RF(%), which represents the ratio of fluorescence measured to the total amount of fluorescence in the sample. Samples were in triplicate, and average standard deviation are shown. The triangle represents a control sample in which BODIPY-LPS was incubated with LBP alone without rHDL. Experiments were repeated twice with essentially identical results. Data are plotted on logarithmic X scale. (B) TFPI interferes with binding of LBP to LPS-coated surface. Binding of rLBP to LPS-coated terasaki plates in the presence of rTFPI was measured by enzyme-linked immunosorbent assay (ELISA) methodology as described in the Materials and Methods. Plates were either coated with LPS (•) or buffer (▴) and rLBP (0.1 μg/mL) was added in the presence of the indicated amounts of rTFPI. (C) TFPI binds to LPS-coated surfaces. Binding of rTFPI to LPS-coated plates was measured by the ELISA methodology as described in the Materials and Methods. (○) Total binding; (▵) nonspecific binding on buffer-coated surface; (▪) specific binding to LPS (total binding subtracted with nonspecific binding).
TFPI interferes with LBP-LPS interaction by binding to LPS. (A) TFPI blocks LBP-mediated transfer of LPS to rHDL. LBP-mediated BODIPY-LPS transfer to rHDL was measured as described in the Materials and Methods. BODIPY-LPS (0.5 μg/mL), rLBP (5 μg/mL), rHDL (20 μg/mL), and the indicated amounts of rTFPI were mixed and incubated for 2.5 hours in room temperature. At this time point, the transfer of BODIPY-LPS to rHDL was completed. The transfer was expressed as RF(%), which represents the ratio of fluorescence measured to the total amount of fluorescence in the sample. Samples were in triplicate, and average standard deviation are shown. The triangle represents a control sample in which BODIPY-LPS was incubated with LBP alone without rHDL. Experiments were repeated twice with essentially identical results. Data are plotted on logarithmic X scale. (B) TFPI interferes with binding of LBP to LPS-coated surface. Binding of rLBP to LPS-coated terasaki plates in the presence of rTFPI was measured by enzyme-linked immunosorbent assay (ELISA) methodology as described in the Materials and Methods. Plates were either coated with LPS (•) or buffer (▴) and rLBP (0.1 μg/mL) was added in the presence of the indicated amounts of rTFPI. (C) TFPI binds to LPS-coated surfaces. Binding of rTFPI to LPS-coated plates was measured by the ELISA methodology as described in the Materials and Methods. (○) Total binding; (▵) nonspecific binding on buffer-coated surface; (▪) specific binding to LPS (total binding subtracted with nonspecific binding).
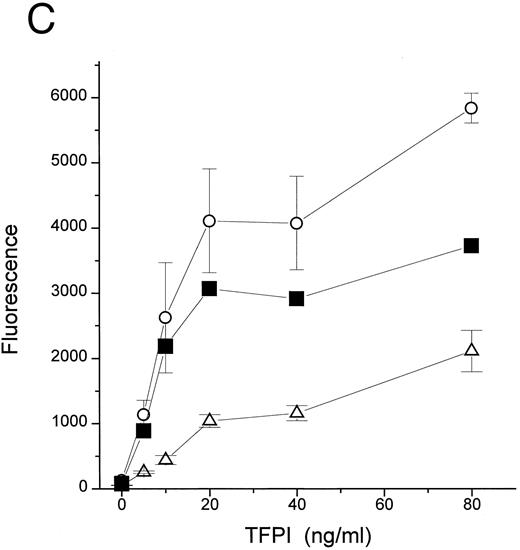
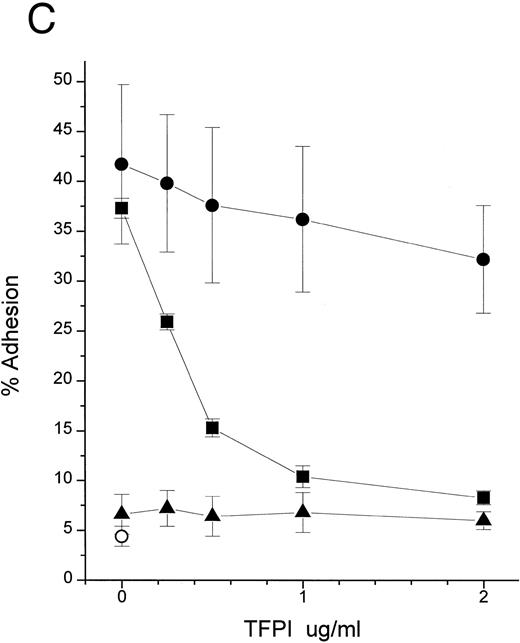
An additional observation confirmed the hypothesis that the primary target of TFPI is LPS, not LBP or CD14. LPS binds to CD14 at a very slow rate in the absence of LBP (Fig 4A and Hailman et al9 ). However, once LPS-CD14 complexes are formed, they can stimulate PMN in the absence of LBP.11 We observed that the movement of LPS to CD14 in the absence of LBP was efficiently blocked by rTFPI (Fig 4A) in a dose-dependent fashion (Fig 4B). As expected, blockade of the formation of LPS-CD14 complexes by TFPI prevented PMN stimulation (Fig 4C). However, once sCD14-LPS complexes were formed, TFPI could not block the cellular response (Fig 4C). These observations lead us to conclude that TFPI binds to LPS aggregates and thereby inhibits binding of LBP and transfer of a LPS monomer to sCD14. However, after the formation of stoichiometric complexes of LPS with CD14, TFPI is not effective.
TFPI blocks movement of LPS to sCD14 in the absence of LBP. (A) Time course. BODIPY-LPS (0.2 μg/mL) was incubated with sCD14 (5 μg/mL) alone (•), sCD14 plus rTFPI (20 μg/mL; ▪), or PBS alone (▴) at 37°C, and fluorescence was measured at different time points before and after adding SDS. RF(%) was calculated as described above. (B) Dose dependence. BODIPY-LPS transfer to sCD14 in the absence of LBP was performed in the presence of different doses of rTFPI. The concentration of BODIPY-LPS was 0.2 μg/mL and the concentration of sCD14 was 2.5 μg/mL. The samples were incubated at 37°C for 2 hours. (▵) A control sample with BODIPY-LPS alone without sCD14. (C) sCD14-LPS complexes formed in the presence of TFPI fail to activate PMN. The PMN adhesion assay was performed with sCD14-LPS complex prepared as follows. sCD14 and LPS (100 μg/mL and 5 μg/mL in PBS, respectively) were incubated at 37°C for 2 hours. Different amounts of rTFPI were added to the samples during (▪) or after (•) the 2 hours of incubation and the resulting mixture was added to the PMN adhesion assay. For stimulation of PMN, samples were diluted to yield a final concentration of 2 μg/mL sCD14, 0.1 μg/mL LPS, and the indicated amount of rTFPI. (▴) Control samples in which LPS alone was incubated with rTFPI without sCD14. (○) Buffer alone was added to PMN for the control of PMN adhesion assay.
TFPI blocks movement of LPS to sCD14 in the absence of LBP. (A) Time course. BODIPY-LPS (0.2 μg/mL) was incubated with sCD14 (5 μg/mL) alone (•), sCD14 plus rTFPI (20 μg/mL; ▪), or PBS alone (▴) at 37°C, and fluorescence was measured at different time points before and after adding SDS. RF(%) was calculated as described above. (B) Dose dependence. BODIPY-LPS transfer to sCD14 in the absence of LBP was performed in the presence of different doses of rTFPI. The concentration of BODIPY-LPS was 0.2 μg/mL and the concentration of sCD14 was 2.5 μg/mL. The samples were incubated at 37°C for 2 hours. (▵) A control sample with BODIPY-LPS alone without sCD14. (C) sCD14-LPS complexes formed in the presence of TFPI fail to activate PMN. The PMN adhesion assay was performed with sCD14-LPS complex prepared as follows. sCD14 and LPS (100 μg/mL and 5 μg/mL in PBS, respectively) were incubated at 37°C for 2 hours. Different amounts of rTFPI were added to the samples during (▪) or after (•) the 2 hours of incubation and the resulting mixture was added to the PMN adhesion assay. For stimulation of PMN, samples were diluted to yield a final concentration of 2 μg/mL sCD14, 0.1 μg/mL LPS, and the indicated amount of rTFPI. (▴) Control samples in which LPS alone was incubated with rTFPI without sCD14. (○) Buffer alone was added to PMN for the control of PMN adhesion assay.
TFPI is composed of 3 Kunitz type domains, a short acidic amino-terminus, and quite basic carboxy-terminus. Anticoagulant activity has been assigned to first 2 Kunitz domains.21 The physiologic function of the third Kunitz domain is not known, but heparin binding sites are known to reside in the third Kunitz domain and in the carboxy-terminus.22,23 We used a truncated recombinant protein to roughly map the domains needed for interaction of TFPI with LPS. rTFPI13-161 contains only the first 2 Kunitz domains and is missing the short amino-terminus, third Kunitz domain, and carboxy terminus. This protein did not show any inhibition of LBP-mediated LPS transfer to CD14 at concentration 50 to 100 times higher (25- to 50-fold higher molar ratio) than those sufficient for full-length rTFPI (Fig 2D). We also tested rTFPI13-161 for binding to LPS coated on plastic surface and did not observe binding at the concentration up to 100 times higher than those sufficient for full-length rTFPI (data not shown). This suggests that LPS binding and neutralizing activity is located on the third Kunitz domain, C-terminus, or N-terminal 12 amino acids. To further localize the endotoxin binding site, we tested whether heparin could inhibit the binding of TFPI to LPS. In the presence of heparin, the binding of rTFPI to LPS coated on plastic surfaces was substantially decreased (data not shown). These results strongly suggest that heparin binding sites, mapped on the third Kunitz domain and carboxy terminus,22 23 are involved in binding to endotoxin.
DISCUSSION
We propose here a new function for TFPI as an anti-inflammatory molecule. By binding to endotoxin, TFPI can halt transfer of LPS to CD14 (Fig 2), depress cellular responses to endotoxin (Fig 1), and may thereby attenuate the process of septic shock.6 7
Despite the relatively great affinity of pure TFPI for LPS in vitro (Fig 3C), strong inhibition of LPS action may require higher levels of TFPI than normally appear in plasma. TFPI must compete with LBP for binding to LPS (Fig 3B), and we observed that 6.9 nmol/L TFPI is needed to inhibit 50% of the effect of 16.7 nmol/L LBP (Fig 2A). Because LBP is abundant in plasma (∼85 nmol/L), the physiologic plasma concentration of TFPI (∼2.5 nmol/L) is not likely to be sufficient to neutralize endotoxin in endotoxemic patients. Despite these considerations, effective concentrations of TFPI may be obtained by infusion of the recombinant protein. Studies with baboons and recombinant TFPI6 7 achieved a high plasma level of TFPI (∼50 nmol/L) exceeding the predicted ID50 obtained from LBP-mediated LPS transfer study (Fig 2A), and these doses attenuated septic shock. Moreover, the plasma concentration of infused rTFPI probably reflects only a fraction of the rTFPI that binds to endothelium. We also would like to point out that the concentration of LPS we used in our in vitro LPS transfer studies (0.2 to 0.5 μg/mL) is supraphysiologic as compared with that of endotoxemic patients or animals. As shown in Fig 2C, more effective inhibition of LPS action could be achieved with lower LPS concentration.
Most (although not all, see Novotny et al3 and Bajaj et al24 ) recent studies show that plasma TFPI is increased in septic patients.25-30 These observations suggest that TFPI behaves as an acute-phase reactant. Although obviously not sufficient to prevent the systemic inflammatory response or DIC in all patients, this response may nevertheless serve to attenuate responses to endotoxin. Because cells including monocytes and endothelial cells may produce increased amounts of TFPI in response to inflammatory signals,29 31-33 TFPI may also accumulate and act in local tissue environment or confined body compartments. TFPI may thus represent an endogenous feedback inhibition mechanism for both the inflammation and coagulation responses, and these responses may be subject to modulation by intervention with TFPI.
Supported by National Institutes of Health Grant No. K08 AI 01333-02 (to C.T.P.).
Address reprint requests to C. Thomas Park, MD, Laboratory of Cellular Physiology and Immunology, The Rockefeller University, Box 303, 1230 York Ave, New York, NY 10021.

![Fig. 2. TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4268/3/m_bl_0049f2a.jpeg?Expires=1769254510&Signature=ZQpVbaHH9yCiSbEH~bts8TGfNnaJd69XcM8dRKN8pbnW7ABd9uFkDsh-DWa1qN~i-1ybpNXBdVId6dmxSGbod6pI0xWSZpTdaSt-eF6TI8-UfLovy1UL44TxqQNzrINLRLIESru7IGpl0Y3vYduFG0UZVAgp-aNSe5-1Oxz31ECS0Qn0DMKxxbTMcAqQ25lPoUVJoi1hzFBB21asUprtoI3hXlyZItV5A82kV82RFrm4Lzrcnv~Afvdwt7dd8pdRAePR4Oa5x6BdJQqeE~q6qeatdGHjnNeJSrm~97dvIEeWytQXDZ9e5tCK3RZ1JETGT439i4hdnRywA~3IKqxqpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4268/3/m_bl_0049f2b.jpeg?Expires=1769254510&Signature=ngg1n3xYs3BZI4kgjI7uW6tuMgA1Lxlq01cSazfSCF9CsVnj5eZU39xuhUpVZN-9xUo5khemyXc0lG-YldBuGtiZ4bpsBygOy275-b2BD5RiC6zys9yqa8tIT8oz~7GwavzFZXW2BGUvNnYfuGkSK5JSMCh49dXGluO-nLZYY~038sgMEYPXjlvmu1EtOqzh1zIRPpq6KsiDqdbkqzwxqL7lTJdmarEp3nT7RbcNVb1JM5nKB8lfJnveFCipmOw~K8eAfWc7CM6yhd8g7i~CYsrstKVdy7szQhsNNZsPeHfuB-G0KpFG2Rq8F19M8Gs-qTZp1JKE8uZ~QHthL3JIpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4268/3/m_bl_0049f2c.jpeg?Expires=1769254510&Signature=2akBilpwiJL1UpjbtZrbwi2NlReD0eMO1QzyxFcB3SxbFqsQFErXBNh6nsx-KNja5ZOuLpR0SHKmMqZSfgaG2dE85OnxgmIvtvAcq3T8NXs2Kz7-dY4wBfeBahmgXxjuLTsduq~Vx9LpI8J09PWAMznXHFl1-JPzAy85Nuw82xO9ZxD8p-TM7lQ9N~~gNc-6AinlAJf2dGxt8SJXBs7qcY5UVrGTnF8tsQVsUzdQBOBUkUIc22FlYrnVbvPhQKJFJowX7qpsCQIO3i3SG6sr1AXTmbsohT4E9T5YK9S4w8nF9jzSiI08yCfb9-Lap2e01LYhqwmrqu9Abt7kWeRQLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. TFPI blocks LBP- or plasma-mediated transfer of BODIPY-LPS to CD14. (A) rLBP (1 μg/mL), sCD14 (2.5 μg/mL), and the indicated amounts of rTFPI were mixed quickly with the BODIPY-LPS (0.2 μg/mL) and the fluorescence increase was recorded. From the recorded curve, the initial velocity (Vi) during the first 20 seconds was calculated and is shown on the Y axis. Error bars represent standard errors of linear regression of each data point. (▴) A control sample in which BODIPY-LPS was incubated with LBP alone in the absence of sCD14. TFPI alone did not show any effect on fluorescence compared with buffer controls (not shown). Experiments were repeated four times with similar results and shown is a representative experiment. Data are plotted on logarithmic X scale. (B) Normal human plasma (10%) was used instead of rLBP for the BODIPY-LPS transfer assay to see whether rTFPI could block the plasma-mediated LPS transfer to sCD14. BODIPY-LPS at 0.5 μg/mL and sCD14 at 5 μg/mL were mixed with the indicated amounts of rTFPI. The rest of the conditions were the same as above. Experiments were repeated twice with similar results. Data are plotted on logarithmic X scale. (C) Inhibition of LBP-mediated BODIPY-LPS transfer to sCD14 is overcome by high LPS concentrations. The experiment was performed as described above with fixed doses of TFPI (5 μg/mL), LBP (0.25 μg/mL), and sCD14 (5 μg/mL). A reverse linear relationship is shown between LPS doses and the percentage of inhibition. The percentage of inhibition was calculated as follows: (1 − [V − Vc]/[Vm − Vc]) × 100, where Vm is Vi of the sample without TFPI, V is Vi of the sample with TFPI, and Vc is Vi of the control sample with LBP alone in a given LPS dose. (D) The BODIPY-LPS transfer assay was performed as above with full-length rTFPI or rTFPI13-161 . BODIPY-LPS at 0.1 μg/mL, rLBP at 0.2 μg/mL, and sCD14 at 2 μg/mL were used. Control represents a sample with BODIPY-LPS plus 0.2 μg/mL of rLBP but without sCD14. Experiments were repeated twice with essentially the same results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4268/3/m_bl_0049f2d.jpeg?Expires=1769254510&Signature=UzGWXL437H22CDstPgOE2dw45c2N7tcT6QUasawU6dMr1CDFXpwQxHQRNyA~sX~bEqqUlMcC9MdXi7g9XL4Tg30To5FIADw6cwIuyAYRBfRuBmMC2ZMfI4tTYP03w-FEsYruZeOx~rl2ENw6JzZVrzxVK1YGp-pPmDwf1WU4CFSwKhuWslvtL2dO~KsQ3OhjPN5mzQCAk9v~LMd3q5aeZCceRZIORoU3cp8PfDdZtHKmjKQUEEK~IJlQibQeNAgLkQArUHC~zGEDKg-04GUF73ByEoghXsdTaFVvw2hosvtuROoe0bzDREeqRk5iiKbVc1731oTdwIOST0iSjBusOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal