Abstract
Using thiol deprivation, we have previously shown that the response of natural killer (NK) cells to interleukin-2 (IL-2) is subject to redox regulation downstream of IL-2 binding and internalization. We have now used the IL-2–dependent cell line, NK3.3 to study redox regulation of NK cells further, and found that NK3.3 cells neither incorporated [3H]-thymidine nor completed the G1-S phase transition in medium lacking the thiol-related compounds, L-cystine, and glutathione, despite the presence of sufficient IL-2. Thiol deprivation did not alter the induction of DNA interferon-γ activated sequence (GAS)-binding activity in response to IL-2. However, the retinoblastoma gene product (RB), a cyclin-dependent kinase (CDK) substrate, was phosphorylated within 24 hours after IL-2 stimulation in standard medium, but its expression and phosphorylation were reduced in thiol-depleted medium in both NK3.3 cells and freshly isolated NK cells. These reductions were not associated with an increased level of p27Kip1, an inhibitor of CDKs CDK6/2 in association with G1 cyclins. Reducing agents, N-acetylcysteine, reduced glutathione or 2-ME restored both RB phosphorylation and DNA synthesis in thiol-deprived NK3.3 cells. The in vitro kinase activities of CDK6 and CDK2 were prematurely increased by thiol deprivation. This enhancement was associated with CDK hyperphosphorylation and prolonged phosphorylation, and could be observed before and beyond IL-2 stimulation. The data suggest the possibility that the premature and prolonged enhancement of CDK activity in thiol-deprived NK cells is associated with, and therefore may contribute to, the reduced expression and phosphorylation of RB, and the associated cell cycle arrest.
WE PREVIOUSLY found, using thiol depletion, that interleukin-2 (IL-2)–induced activities of human natural killer (NK) cells require a reducing environment involving de novo synthesis of reduced intracellular glutathione (GSH). The most redox-sensitive event(s) following IL-2 stimulation were downstream of IL-2 internalization.1 Diminished GSH has been observed in sera2 or T cells3-5 from acquired immune deficiency syndrome (AIDS) patients, and decreased serum GSH6 or cystine7 has been reported in patients with other immunodeficiencies, suggesting that thiol sufficiency may be important for clinically normal immune function. Alteration of redox status has been suggested as a therapy for AIDS.3
Cell cycle progression requires a highly regulated series of signaling and transcriptional events. The retinoblastoma gene product (RB) undergoes phosphorylation during specific cell cycle stages8 resulting in the release of the E2F DNA-binding transcription factor(s).9 IL-2 induces expression and phosphorylation of RB in T cells10 by activating cyclin-dependent kinases (CDK), CDK2 and CDK4,11 and CDK6, a cyclin D-associated CDK4 T-cell homologue.12 Activities of CDKs are regulated by Tyr and Thr phosphorylation status; for example, dephosphorylation of Tyr15 and Thr14, and phosphorylation of Thr160 are required for CDK2 function.13 Several of the enzymes regulating phosphorylation in cell cycle progression contain cysteine residues and require a reducing microenvironment for optimal function,14-16 suggesting that certain events in cell cycle progression are thiol-dependent.
The current studies were designed to define the intracellular events induced by the absence of a reducing environment that result in decreased proliferation of NK cells. We report that the IL-2–induced expression and phosphorylation of RB was reduced in NK3.3 cells cultured in thiol-deficient medium, although CDK6 and CDK2 exhibited earlier and stronger kinase activity and prolonged phosphorylation under thiol-deprived conditions. Our findings suggest that inappropriate kinase activities, caused by excessive premature enhancement of phosphorylation, may account for the cell cycle arrest of NK cells in thiol-deficient medium.
MATERIALS AND METHODS
Antibodies.Antisera to RB and cyclin E were purchased from UBI (Lake Placid, NJ), to CDK2 from Transduction Laboratories (Lexington, KT), to CDK6 from Santa Cruz Biotechnology Inc (Santa Cruz, CA), and to cyclin D3 and p27kip1 from Pharmingen (San Diego, CA). Antibody to bcl-2 was from DAKO (Carpenteria, CA). Monoclonal antibody (MoAb) to CD5 (OKT1) was produced from the hybridoma (ATCC, Rockville, MD), and purified as described.1 Purified MoAb to CD36 (OKM5) was purchased from Ortho Diagnostic Systems (Raritan, NJ), and MoAb to CD22 was from Becton Dickinson (Mountain View, CA).
Reagents.p-Nitrophenylguanidinobenzoate (PNPGB), RNase A, NaF, EGTA, EDTA, tetrasodium pyrophosphate, 5-sulfosalicylic acid, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), glutathione (GSH) reductase, and NADPH were purchased from Sigma (St Louis, MO). Sodium vanadate was from Fisher Scientific (Fair Lawn, NJ) and propidium iodide (PI), Nonidet P-40 (NP-40), leupeptin and aprotinin were from Boehringer Mannheim (Indianapolis, IN). Methyl-[3H]-thymidine-deoxyribose (TdR), [32P]-γ-adenosine triphosphate (ATP), and [32P]-orthophosphate were from Dupont NEN (Boston, MA). H1 histone was purchased from Boehringer Mannheim, and 56-kD truncated RB from QED (San Diego, CA).
Cells.NK3.3 cells, kindly provided by Dr J. Kornbluth, University of Arkansas (Little Rock, AR) were maintained in RPMI 1640 medium containing 10% Lymphocult T (Biotest Diagnostic Corp, Denville, NJ), 10% fetal calf serum (FCS; Hyclone, Logan, UT), 25 mmol/L HEPES (BioWhittaker, Walkersville, MD) and 50 μg/mL gentamicin (Biofluids, Rockville, MD). They were deprived of Lymphocult T for 12 hours in standard RPMI 1640 medium and for an additional 12 hours in RPMI 1640 without L-cystine and GSH (CYS[-] medium). The second 12-hour period both depleted intracellular thiols and synchronized cell division (see Results). Unless specifically mentioned, thiol depletion was the same for all experiments. Viability after thiol depletion and synchronization was always >96%. Cells were then stimulated with 200 U/mL of IL-2 for 0 to 24 hours in CYS(−) medium or in the same medium to which sufficient L-cystine (200 μmol/L) was added back (CYS[+] medium). All media used for stimulation contained 25 mmol/L HEPES and 10% FCS, which had been dialyzed against phosphate-buffered saline (PBS) to remove small molecular weight compounds including thiols.
Fresh human NK cells were isolated as described previously1,17 from buffy coats obtained from healthy donors through the National Institutes of Health (NIH) Blood Bank, by centrifugation over Percoll (Pharmacia, Piscataway, NJ) and adherence to plastic followed by depletion of specific cells with MoAb. The negatively selected cells were always 85% to 90% CD16+ and/or CD56+ NK cells, as verified by flow cytometric analyses.1 Fresh NK cells were stimulated with IL-2 for 24 hours in CYS(+) or CYS(−) media without preculture.
Analyses of proliferation and cell cycle.Cytokine-starved NK3.3 cells were cultured at 2 × 104/well in 96-well plates in CYS(−) or CYS(+) media with or without 200 U/mL of rIL-2 and pulsed with 1 μCi of methyl-[3H]-TdR for 4 hours before harvest and determination of thymidine incorporation. Simultaneously, cell cycle progression was analyzed as described.18 19 Briefly, 106 cells were incubated 16 to 20 hours at 4°C in a 1:1 mixture of 95% ethanol/5% acetic acid: RPMI 1640 plus 10% FCS for fixation. After washing, cells were incubated for 30 minutes at 37°C in PBS containing 0.5 mg/mL of RNase A, and resuspended in 0.5 mL PBS containing 50 μg/mL of PI. Proportions of cells in G1 or S phase of cell cycle were analyzed by flow cytometry.
Measurement of intracellular reduced GSH.Intracellular GSH was measured using the DTNB-GSH reductase recycling assay20 with modifications as described.1
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting, and electrophoretic mobility shift assay (EMSA).Cell solubilization (lysis buffer: 0.5% NP-40, 150 mmol/L NaCl, 50 mmol/L Tris, 50 μg/mL aprotinin, 50 μg/mL leupeptin, 1 mmol/L Na3VO4 , 50 mmol/L NaF, 1 mmol/L EGTA, and 5 mmol/L EDTA), protein assays (BioRad Protein Assay Kit, Richmond, CA), SDS-PAGE (samples normalized for protein content), and Western blot were performed as described.21 After blocking, membranes were incubated with specific antibodies and then with horseradish perioxidase-conjugated second antibodies. Bands were visualized with the enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL).
EMSA was performed as reported22-24 using [32P]-labeled oligonucleotides containing interferon-γ (IFN-γ) activated sequences (GAS motifs) from the FcγRI promoter23,25 and the c-fos promoter sis-inducible element m67 (SIE).26 Nuclear extracts were prepared, specific oligonucleotide probes synthesized and labeled, and DNA-binding proteins assessed, exactly as described.24
Immunoprecipitation, in vitro kinase assay, phosphorylation, and dephosphorylation of CDKs.To measure in vitro kinase activity, NK3.3 cells that had been cultured in CYS(+) or CYS(−) media for the indicated times, were immunoprecipitated with antisera to CDK6 or CDK2 adsorbed to protein G-coupled Sepharose beads (Pharmacia). Immunoprecipitates were incubated with 0.2 mmol/L [32P]-γATP (50 μCi/μL) and 5 μg/μL 56-kD truncated RB (for CDK6) or H1 histone (for CDK2) in reaction buffer containing 50 mmol/L tris (pH 7.4), 10 mmol/L MgCl2 , and 1 mmol/L dithiothreitol at 37°C for 30 minutes. Supernatants were subject to SDS-PAGE (9% gels) and signals were visualized by autoradiography.
To measure the effect of thiol compounds on CDK phosphorylation, synchronized NK3.3 cells were first precultured in phosphate-free standard medium for 30 minutes and then cultured with 0.5 mCi/mL [32P]-orthophosphate in the same medium for an additional 3 hours at 1 × 108 cells/mL. Cells were stimulated with 200 U/mL of rIL-2 in CYS(−) or CYS(+) medium for time indicated, and solubilized as for Western analyses. Assessment of the influence of thiols on dephosphorylation was similar, except that labeled cells were stimulated with IL-2 only in CYS(−) medium for 2 hours, and then cultured in CYS(−) or CYS(+) medium with IL-2 for indicated times. Protein concentrations were normalized and samples immunoprecipitated with antisera to CDK6 or CDK2. Immunoprecipitates were subjected to SDS-PAGE (12% gels)27 and transferred to polyvinylidene fluoride (PVDF) membranes. Phosphorylation signals were detected by autoradiography, and membranes were incubated with antibodies to CDK6 or CDK2 to verify specific protein content.
Diminished (A) DNA synthesis and (B) cell cycle progression induced by IL-2 in NK3.3 cells cultured in CYS(+) (closed symbols) or CYS(-) medium (open symbols). (A) [3H]-TdR incorporation. (B) Percentage of NK3.3 cells in G1 (squares) or S (circles) phase. NK3.3 cells were depleted of intracellular thiols before initiation of experiment as described in Materials and Methods. Data are from one experiment, representative of two to three independent experiments.
Diminished (A) DNA synthesis and (B) cell cycle progression induced by IL-2 in NK3.3 cells cultured in CYS(+) (closed symbols) or CYS(-) medium (open symbols). (A) [3H]-TdR incorporation. (B) Percentage of NK3.3 cells in G1 (squares) or S (circles) phase. NK3.3 cells were depleted of intracellular thiols before initiation of experiment as described in Materials and Methods. Data are from one experiment, representative of two to three independent experiments.
RESULTS
NK3.3 cells were arrested in the G1 phase of the cell cycle in thiol-deficient (CYS[-]) medium.NK3.3 cells cultured in CYS(-) medium failed to incorporate [3H]-TdR at any time point (Fig 1A), despite the presence of sufficient IL-2 (200 U/mL), suggesting that cells were unable to synthesize DNA and were arrested in G1. Cells were also cultured in CYS(+) or CYS(−) medium for various time intervals and stained with PI to assess cell cycle stage (Fig 1B). The number of cells in S phase increased after IL-2 stimulation in CYS(+) medium to 41% in 24 hours, whereas cells cultured in CYS(−) remained in G1 phase. These data suggest that L-cystine is required for cell cycle progression to DNA synthesis in NK3.3 cells stimulated with IL-2.
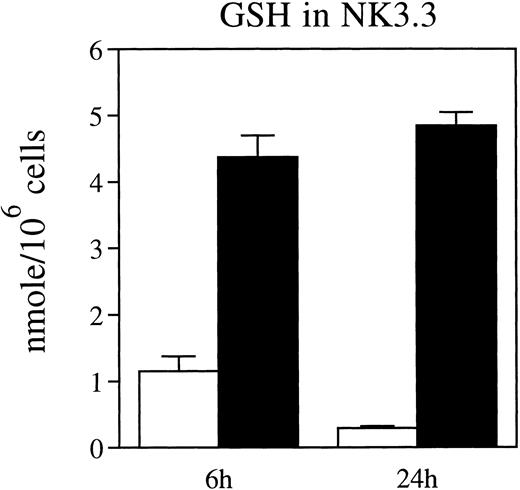
Intracellular GSH levels are depressed in NK cells cultured in thiol-depleted medium.We previously found that intracellular GSH was critical for maintaining the reducing environment in IL-2–stimulated fresh NK cells.1 Therefore, we measured the effect of exogenous L-cystine on intracellular GSH in IL-2–stimulated NK3.3 cells. Intracellular GSH in NK3.3 cells cultured with IL-2 for 6 or 24 hours in CYS(−) medium was significantly lower than that in cells cultured in CYS(+) medium, to which L-cystine had been restored (Fig 2), confirming that exogenous L-cystine maintains intracellular GSH levels in NK3.3 cells.
Thiol depletion reduces GSH levels in NK3.3 cells in a time-dependent manner. NK3.3 cells that were thiol-depleted and then cultured with rIL-2 (200 U/mL) in CYS(−) medium (□) or CYS(+) medium (▪) for 6 and 24 hours. Data are representative of two independent experiments.
Thiol depletion reduces GSH levels in NK3.3 cells in a time-dependent manner. NK3.3 cells that were thiol-depleted and then cultured with rIL-2 (200 U/mL) in CYS(−) medium (□) or CYS(+) medium (▪) for 6 and 24 hours. Data are representative of two independent experiments.
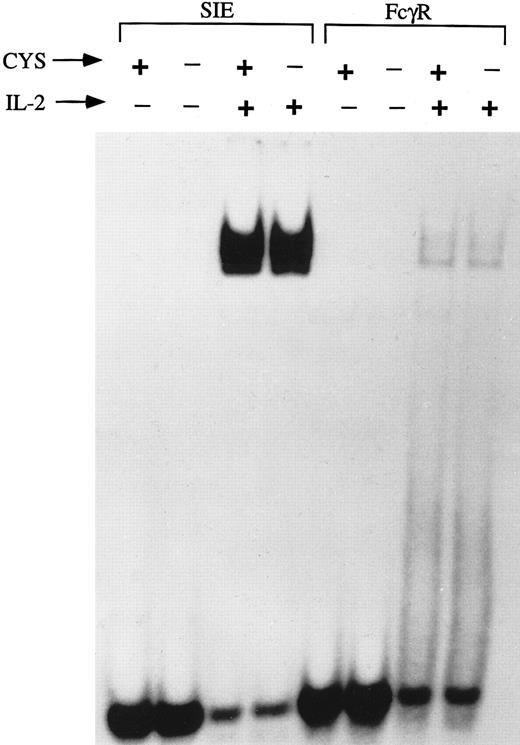
Activation of GAS-binding proteins is unaltered by thiol depletion.We previously observed that thiol deprivation reduced the IL-2 response in NK cells without altering IL-2–binding and internalization,1 and therefore examined the effect of reduced thiols on downstream events in IL-2 activation of NK3.3 cells. Because IL-2 stimulation involves activation of Janus family kinases (JAKs) that activate several proteins termed signal transducers and activators of transcription (STAT)22,24 28 that bind to GAS motifs, EMSA were performed using two probes containing GAS motifs. However, no differences were observed between cells stimulated with IL-2 (200 U/mL, 15 minutes) after IL-2 deprivation followed by 12-hour preculture and stimulation in thiol-depleted compared to thiol-sufficient media (Fig 3). Thus, thiol deprivation inhibits IL-2–induced cell cycle progression without affecting activation of the JAK-STAT pathway.
GAS-binding proteins are activated normally by IL-2 stimulation in thiol-deficient medium. EMSA were performed, using GAS motif-containing probes, SIE and FcγRI, on nuclear extracts of NK3.3 cells (3 × 106/condition) that had been IL-2–deprived and precultured for 12 hours in medium in the presence or absence of L-cystine and GSH followed by stimulation with IL-2 (200 U/mL, 15 minutes). No difference was seen in GAS-binding activities between cells cultured in the thiol-deficient compared to thiol-sufficient medium.
GAS-binding proteins are activated normally by IL-2 stimulation in thiol-deficient medium. EMSA were performed, using GAS motif-containing probes, SIE and FcγRI, on nuclear extracts of NK3.3 cells (3 × 106/condition) that had been IL-2–deprived and precultured for 12 hours in medium in the presence or absence of L-cystine and GSH followed by stimulation with IL-2 (200 U/mL, 15 minutes). No difference was seen in GAS-binding activities between cells cultured in the thiol-deficient compared to thiol-sufficient medium.
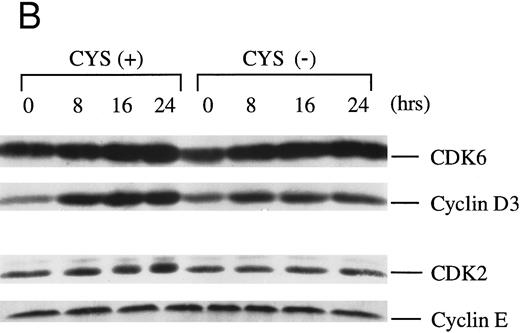
Degradation of the CDK inhibitor p27Kip1, and expression of CDK/cyclin and are not altered by thiol deprivation. Activity of CDKs is essential for cell cycle progression and CDK inhibitors must be proteolysed for CDKs to become phosphorylated and express kinase activity.29 Because the CDK inhibitor p27Kip1 is degraded in IL-2–stimulated T cells,30 we considered that its degradation may be compromised in thiol-deprived cells, resulting in reduced CDK activity. Therefore, we examined p27Kip1 levels in IL-2–stimulated NK3.3 cells (0 to 24 hours with 200 U/mL) in CYS(+) and CYS(−) medium, but found equivalently decreased p27Kip1 in both media (Fig 4A).
Levels of CDKs and CDK inhibitors are not affected under conditions of thiol deprivation. Western blot analyses of (A) p27Kip1 and (B) CDK6/cyclin D3 and CDK2 and cyclin E in NK3.3 cells revealed little dependency on medium thiols. NK3.3 cells were depleted of residual thiols and then stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−), and lysed at the indicated time points. Each figure is representative of at least two independent experiments. Experiments performed with different quantities of cell extracts yielded consistent results.
Levels of CDKs and CDK inhibitors are not affected under conditions of thiol deprivation. Western blot analyses of (A) p27Kip1 and (B) CDK6/cyclin D3 and CDK2 and cyclin E in NK3.3 cells revealed little dependency on medium thiols. NK3.3 cells were depleted of residual thiols and then stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−), and lysed at the indicated time points. Each figure is representative of at least two independent experiments. Experiments performed with different quantities of cell extracts yielded consistent results.
We next examined levels of G1 cyclins and CDKs in NK3.3 cells, and preliminary Western blot analysis revealed that cyclins D3 and E, and cyclin dependent kinases CDK6 and CDK2 were dominant in NK3.3 cells, while cyclins D1 and D2, and CDK4 were minor to undetectable components (data not shown). However, protein levels of cyclin D3/CDK6 and cyclin E/CDK2 were quite detectable but were unaffected by cystine deprivation (Fig 4B).
Cystine depletion decreased RB protein expression and phosphorylation.The RB gene product is a substrate for CDKs, and the expression31 and phosphorylation10 of RB protein on ser/thr residues regulates cell cycle progression. The level of phosphorylated RB protein was therefore examined in NK3.3 cells cultured in CYS(+) or CYS(−) medium using Western blot analysis in which phosphorylated RB migrates more slowly than nonphosphorylated RB.32 33 RB protein exhibited reduced expression and was essentially unphosphorylated in synchronized NK3.3 cells stimulated with IL-2 (200 U/mL) for 0 to 24 hours in CYS(−) medium compared to identically treated cells in CYS(+) medium (Fig 5A).
Thiol depletion of NK3.3 cells reduced RB expression and greatly reduced or abrogated phosphorylation of RB in response to IL-2. (A) Western blot analysis of RB protein in NK3.3 cells cultured with rIL-2 (200 U/mL) for 0 to 24 hours in CYS(+) medium or CYS(−) medium. Phosphorylated RB migrates slower than nonphosphorylated RB.32 33 (B) Addition of thiol-related reducing reagents, GSH, 2-ME, or NAC to CYS(−) medium restored RB phosphorylation and expression in response to IL-2 (Western blot) and [3H]-TdR incorporation (bar graph) in a dose-dependent manner. Controls included cells without IL-2 in CYS(−) medium (IL-2[−]), and NK3.3 cells cultured in CYS(−) medium with IL-2 (200 U/mL) (Cystine [0]). (C) RB phosphorylation is induced by IL-2 in freshly isolated human NK cells. Immediately after isolation (time 0), cells were cultured with IL-2 (200 U/mL) in CYS(−) or CYS(+) medium. Each data set is representative of two independent experiments.
Thiol depletion of NK3.3 cells reduced RB expression and greatly reduced or abrogated phosphorylation of RB in response to IL-2. (A) Western blot analysis of RB protein in NK3.3 cells cultured with rIL-2 (200 U/mL) for 0 to 24 hours in CYS(+) medium or CYS(−) medium. Phosphorylated RB migrates slower than nonphosphorylated RB.32 33 (B) Addition of thiol-related reducing reagents, GSH, 2-ME, or NAC to CYS(−) medium restored RB phosphorylation and expression in response to IL-2 (Western blot) and [3H]-TdR incorporation (bar graph) in a dose-dependent manner. Controls included cells without IL-2 in CYS(−) medium (IL-2[−]), and NK3.3 cells cultured in CYS(−) medium with IL-2 (200 U/mL) (Cystine [0]). (C) RB phosphorylation is induced by IL-2 in freshly isolated human NK cells. Immediately after isolation (time 0), cells were cultured with IL-2 (200 U/mL) in CYS(−) or CYS(+) medium. Each data set is representative of two independent experiments.
We previously reported that certain chemical antioxidants could restore proliferation of fresh NK cells in response to IL-2, a reducing environment and intracellular GSH when added to CYS(−) medium. Therefore, NK3.3 cells were cultured with 200 U/mL IL-2 for 24 hours in CYS(−) medium to which antioxidants, 2-ME, GSH, or N-acetylcysteine (NAC) were added. Chemical antioxidants restored both IL-2–induced-phosphorylation of RB and [3H]-TdR incorporation by NK3.3 cells (Fig 5B). Reduced RB protein and no RB phosphorylation was also seen in fresh NK cells after stimulation with 200 U/mL IL-2 in CYS(−) medium for 24 hours compared to those cultured in CYS(+) medium with IL-2 (Fig 5C). These results suggest that a reducing environment, produced by cystine or other reducing agents, is necessary for the RB production and phosphorylation required for DNA synthesis in NK3.3 and fresh NK cells.
Kinase activity and phosphorylation of CDK6 and CDK2.Because RB is a CDK substrate, we examined the in vitro kinase activity of CDK6 and CDK2. The data revealed that kinase activities of CDK6 and CDK2 appeared early, ie, even before IL-2 stimulation, and exhibited prolonged enhancement in thiol-deprived cells, compared to cells maintained and stimulated in thiol-sufficient medium (Fig 6A). This surprising but reproducible finding, that the lack of RB phosphorylation in vivo under conditions of thiol deprivation is associated with early and strong enhancement of CDK activities, suggests that reduced RB phosphorylation is associated with inappropriately early and strong induction of CDK activity rather than to low CDK activity.
Thiol deprivation induced premature enhancement of both enzymatic activity and phosphorylation of CDK6 and CDK2. (A) NK3.3 cells were stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−) medium for the indicated time, and equivalent protein amounts in each lysate were immunoprecipitated with antibodies to CDK6 or CDK2. In vitro kinase assays were performed on the immunoprecipitates using truncated RB (56 kD) or H1 histone as substrates for CDK6 or CDK2, respectively. (B) NK3.3 cells were labeled with [32P]-orthophosphate and stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(-) media for the indicated times, and lysates were immunoprecipitated with antibodies to CDK6 or CDK2. Equivalent protein content in each lane and identity of each band were verified by Western blot analysis with antibody to CDK6 or CDK2, respectively. (C) Dephosphorylation of CDK6 was assessed in cells that had been labeled with [32P]-orthophosphate in CYS(−) medium in the presence of IL-2 (200 U/mL), and split into two cultures, one continued in CYS(−) medium, and one placed in CYS(+), each with IL-2, for the indicated times. CDK6 protein amount in each lane was verified by Western blot analysis in one of two independent experiments (not shown). Each set of data is representative of two independent experiments.
Thiol deprivation induced premature enhancement of both enzymatic activity and phosphorylation of CDK6 and CDK2. (A) NK3.3 cells were stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−) medium for the indicated time, and equivalent protein amounts in each lysate were immunoprecipitated with antibodies to CDK6 or CDK2. In vitro kinase assays were performed on the immunoprecipitates using truncated RB (56 kD) or H1 histone as substrates for CDK6 or CDK2, respectively. (B) NK3.3 cells were labeled with [32P]-orthophosphate and stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(-) media for the indicated times, and lysates were immunoprecipitated with antibodies to CDK6 or CDK2. Equivalent protein content in each lane and identity of each band were verified by Western blot analysis with antibody to CDK6 or CDK2, respectively. (C) Dephosphorylation of CDK6 was assessed in cells that had been labeled with [32P]-orthophosphate in CYS(−) medium in the presence of IL-2 (200 U/mL), and split into two cultures, one continued in CYS(−) medium, and one placed in CYS(+), each with IL-2, for the indicated times. CDK6 protein amount in each lane was verified by Western blot analysis in one of two independent experiments (not shown). Each set of data is representative of two independent experiments.
Because CDK enzymatic activity requires phosphorylation, we compared the phosphorylation levels of CDKs in cells labeled with [32P]-orthophosphate and stimulated in CYS(−) or CYS(+) medium. Labeled cells were immunoprecipitated with anti-CDK2 or anti-CDK6. Phosphorylation of CDK6 and CDK2 in NK3.3 cells cultured in CYS(−) was much earlier and stronger than in cells cultured in CYS(+) medium (Fig 6B). Next, cells were labeled with [32P]-orthophosphate and IL-2–stimulated for 2 hours in CYS(−) medium to test whether thiol depletion inhibited dephosphorylation of CDK6. Cells were then split into two groups and cultured in CYS(+) or CYS(−) medium in the presence of IL-2 for varying time periods. CDK6 remained strongly phosphorylated after 16 hours in cells cultured in CYS(−) medium (Fig 6C), while in cells cultured in CYS(+) medium, phosphorylation was much less enhanced by IL-2 at that time point. In aggregate, these findings are consistent with the notion that achieving the correct phosphorylation status of CDK6 and CDK2 requires adequate thiols, such as supplied as extracellular L-cystine, and resulting in adequate intracellular GSH.
DISCUSSION
We previously reported that NK cells are subject to redox regulation and do not proliferate in response to IL-2 in culture medium depleted of L-cystine and GSH.1 We now show that the proliferation of NK3.3 cells is also inhibited such that cells do not progress into S phase when stimulated with IL-2 in thiol-depleted medium. Cell cycle arrest was associated with decreased production and phosphorylation of the RB gene product in NK3.3 cells deprived of thiols (L-cystine and GSH), whereas RB protein was strongly phosphorylated within 24 hours in NK3.3 cells cultured in thiol-sufficient medium. The thiol-providing chemical antioxidants, 2-ME, GSH or NAC, restored IL-2–induced phosphorylation and production of RB, as well as DNA synthesis in NK3.3 cells. DNA binding of STAT proteins in NK3.3 cells was also unaffected by thiol deprivation. Moreover, thiol deprivation did not alter serine (by Western analysis34 ) or total (by [32P]-orthophosphate labeling and immunoprecipitation) phosphorylation of src family kinase, p56Lck, nor total phosphorylation of p59Fyn, suggesting that thiol depletion did not induce a generalizable change in phosphorylation of kinases (unpublished data). In addition, although IL-2 enhanced expression of the bcl-2 protooncogene in NK3.3 cells, this increase was not altered by cystine deprivation (unpublished data), suggesting that cystine depletion did not induce a generalized decrease in protein synthesis. The current findings support the conclusion that thiol-related reducing reagent(s) are required for RB phosphorylation.
RB phosphorylation appears to be essential for IL-2–induced lymphocyte proliferation,10 because phosphorylation of RB results in release of the E2F transcription factor, which recognizes binding sequences in genes including α DNA polymerase, c-myc, c-myb, and cdc2, as well as the RB gene, and E2F-1 gene, all of which are important for cell cycle progression.9 35 Our findings are therefore consistent with the possibility that RB dysfunction induced by thiol depletion results in reduced release of E2F-binding factor with concomitantly reduced expression of RB and other genes essential for cell cycle progression.
RB is phosphorylated by CDKs, the activities of which are regulated by CDK inhibitor(s) and phosphorylation. In mammalian cells induced into cell cycle by growth factor, the CDK inhibitor, p27Kip1, is degraded through the ubiquitin-proteasome pathway,29 and degradation of p27Kip1 in IL-2–stimulated T cells is inhibited by rapamycin.30 In NK3.3 cells, p27Kip1 was degraded in cells cultured in CYS(−) medium similarly to cells in CYS(+) medium, suggesting that the degradation of p27Kip1 neither requires thiols, nor is subject to redox regulation.
Paradoxically, the in vitro kinase activities of CDK6 and CDK2 from NK3.3 cells cultured in CYS(−) medium was enhanced to a greater degree than from cells cultured in CYS(+) medium. Moreover, the greater level of kinase activity was observable abnormally early, even before IL-2–stimulation. This observation prompts the speculation that early CDK activity inducing premature phosphorylation of RB may induce release of the E2F transcription factor inappropriately early during G1, ultimately resulting in the observed reduced production of RB, and perhaps other products of E2F target genes as well, culminating in cell cycle arrest.
Because phosphorylation/dephosphorylation events regulate CDK enzymatic activities,13 we compared CDK phosphorylation levels. Our data show that phosphorylation of CDK2, as well as of the CDK4 homologue, CDK6, was enhanced within 2 hours and decreased by 4 hours in NK3.3 cells cultured with IL-2 in thiol-sufficient CYS(+) medium. In contrast, CDK2 and CDK6 in cells stimulated with IL-2 in CYS(−) medium were phosphorylated significantly earlier, higher, and longer. By analogy, prolonged phosphorylation of a CDK4 tyrosine residue has been shown to be critical for irradiation-induced cell-cycle arrest.15 Our data further show that dephosphorylation of CDK6 was significantly delayed in cells cultured in CYS(−) medium compared to cells in CYS(+) medium, suggesting that inadequate dephosphorylation could cause premature and prolonged enhancement of CDK6 or CDK2 enzymatic activities in thiol-deprived cells, ultimately resulting in decreased phosphorylation of RB and cell cycle arrest. These findings suggest the hypothesis that thiol deprivation may reduce the activity of phosphatase(s), which may result in the premature and prolonged phosphorylation of CDKs. Recently Geng et al36 reported that cyclin E expression is negatively regulated by RB, although the molecular basis for this regulation was unclear. Their findings support our experimental evidence in suggesting a reciprocal relationship between enhancement of RB expression and CDK activity in the presence or absence of thiol compounds.
Evidence for the clinical importance of thiol-related compounds in immune function includes diminished levels of GSH in serum2 or T cells3-5 from AIDS patients, and decreased serum GSH6 or cystine7 in patients with other immunodeficiencies, which include reduced NK-cell function. Alteration of redox status has been suggested as a therapy for AIDS.3 We speculate that reduction of NK function in immunodeficiency may be due, in part, to disrupted cell cycling, and that thiols function, in part, to maintain normal cell division by regulating the function of CDKs.
ACKNOWLEDGMENT
NK3.3 was kindly provided by Dr Jackie Kornbluth and rIL-2 by Amgen Corp (Thousand Oaks, CA). We thank Dr Jian-Xin Lin, Laboratory of Molecular Immunology, National Heart Lung and Blood Institute, NIH (Bethesda, MD) for supplying labeled probes for gel shift analyses. We thank Howard Mostowski for flow cytometric analyses, and Drs Mark Hayes and Parris Burd for critical review of the manuscript.
Address reprint requests to Eda T. Bloom, PhD, Chief, Laboratory of Cellular Immunology (HFM-518), Division of Cellular and Gene Therapies, Center for Biologics Evaluation Research, Food and Drug Administration, 8800 Rockville Pike, Bethesda, MD 20892.

![Fig. 1. Diminished (A) DNA synthesis and (B) cell cycle progression induced by IL-2 in NK3.3 cells cultured in CYS(+) (closed symbols) or CYS(-) medium (open symbols). (A) [3H]-TdR incorporation. (B) Percentage of NK3.3 cells in G1 (squares) or S (circles) phase. NK3.3 cells were depleted of intracellular thiols before initiation of experiment as described in Materials and Methods. Data are from one experiment, representative of two to three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f1a.jpeg?Expires=1769093198&Signature=Z-zIDlLH7yJmdSNyNBMMyn-JS2i89Jj3SF1vlYQFEYGcQ~yMqwTGOLHavGNTQlfGeOg5EQWEWzxQ~jMgpFVxFOhWL5KtG-zMoAULAa7-NVanbjmrWSS3d5BQJECU6lcNinAzEk395DICxD1CSaby-BPqef6lvt1axqrO0Y1G80T6iaMZwBW5gGvAtKkCDYz2gzW17vHlZfpqLvAlA5NvrDf8qXKcbNCj6lPjBGqv5NmCuBK~Tsx4-5aYdSrwLTZfHHL1lLYoJ1XgacR2PiL9gnn7jIU3lKquKbr8aPy-TaxGHom39Z-oCKKvAxGHIPy86MzUn0I-zoSbsiPaZBGHyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Diminished (A) DNA synthesis and (B) cell cycle progression induced by IL-2 in NK3.3 cells cultured in CYS(+) (closed symbols) or CYS(-) medium (open symbols). (A) [3H]-TdR incorporation. (B) Percentage of NK3.3 cells in G1 (squares) or S (circles) phase. NK3.3 cells were depleted of intracellular thiols before initiation of experiment as described in Materials and Methods. Data are from one experiment, representative of two to three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f1b.jpeg?Expires=1769093198&Signature=qi2L~qJxHDG1cEUVcIcl0nXrNfOdtBN93-LFZCmiuOXg0vUS~dIyE5k2UNC2caua3GlQmHT59nMQRzzrN9YcLU3tho~xoE~vb6EF5iywPOInjqdcUnsGyd2hxJLKqteP48Gzy3i-xQFE-34E-~qiUeqx3X7S9TwosJ4-bA9sLPm2Zg5Vg0ro9rs4k3x7w0Du~kLjtA2tYPgxJzpanmYqdDA34G1H6E3I0VgYwMNh9eGuAE8boBv3~U5RrzfVjQcBeYWKpoxg-pY0HA5Cz8O~aGcbgbYgQj-iD-AJffJOKbpqPYPU7nYjys79AC4rj2jayjMLma8KS1339n-nlZQyPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Thiol depletion of NK3.3 cells reduced RB expression and greatly reduced or abrogated phosphorylation of RB in response to IL-2. (A) Western blot analysis of RB protein in NK3.3 cells cultured with rIL-2 (200 U/mL) for 0 to 24 hours in CYS(+) medium or CYS(−) medium. Phosphorylated RB migrates slower than nonphosphorylated RB.3233 (B) Addition of thiol-related reducing reagents, GSH, 2-ME, or NAC to CYS(−) medium restored RB phosphorylation and expression in response to IL-2 (Western blot) and [3H]-TdR incorporation (bar graph) in a dose-dependent manner. Controls included cells without IL-2 in CYS(−) medium (IL-2[−]), and NK3.3 cells cultured in CYS(−) medium with IL-2 (200 U/mL) (Cystine [0]). (C) RB phosphorylation is induced by IL-2 in freshly isolated human NK cells. Immediately after isolation (time 0), cells were cultured with IL-2 (200 U/mL) in CYS(−) or CYS(+) medium. Each data set is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f5a.jpeg?Expires=1769093198&Signature=Au28AkNJYtq5dLyEqlxPZxQYGQn7fLvbHsxkwlyyPTiqsT8j5Rr13Pe~~DC9mUPrd3lcV4bdFLQ2Y1zR0jWye2~TSlc2ZBGl3rsjrNBxJCPZ4CRrCkKYyu0O9jLYBCf~iKCYlUxQT3-qnKguA8xC7ROf6Dn8-u932XOfhnWd5U1m7Y7pBIOiukzv7ukBq-NadMvVjhVlX0XWbL0PtXngWuCHYjB9V~lSLY7VR8jwYSWPZ5kbidKdm0~57xbHDssTRsJ7TwHG3Bvc8GsAzlo~boNLreA9EuSCjct~4~I1U42OBO9zcpNwo84mWLZ4xweOJuKz8FESUG~VID2dDRH8bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Thiol depletion of NK3.3 cells reduced RB expression and greatly reduced or abrogated phosphorylation of RB in response to IL-2. (A) Western blot analysis of RB protein in NK3.3 cells cultured with rIL-2 (200 U/mL) for 0 to 24 hours in CYS(+) medium or CYS(−) medium. Phosphorylated RB migrates slower than nonphosphorylated RB.3233 (B) Addition of thiol-related reducing reagents, GSH, 2-ME, or NAC to CYS(−) medium restored RB phosphorylation and expression in response to IL-2 (Western blot) and [3H]-TdR incorporation (bar graph) in a dose-dependent manner. Controls included cells without IL-2 in CYS(−) medium (IL-2[−]), and NK3.3 cells cultured in CYS(−) medium with IL-2 (200 U/mL) (Cystine [0]). (C) RB phosphorylation is induced by IL-2 in freshly isolated human NK cells. Immediately after isolation (time 0), cells were cultured with IL-2 (200 U/mL) in CYS(−) or CYS(+) medium. Each data set is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f5b.jpeg?Expires=1769093198&Signature=jPZXj6vWGVLKV1ur2R37XtwbuLcd6Jtg~da71seXH8r69TszTFzV6z6tl9hgA3LZDuKclyeQbow5b6MlzEHhEKBf8NWXfBLg4xNjpV9bV9T8fToCGegEI4h4rU3hoqRkmgq3MOnlAzaGsdwlV99-Xt9GSBYGhV-2pd7sRHj~117NR02DuA0EU5IpaMJfeA0pEhk0WPGP0h1KGwEjrKDgFFu9flQy6LjfVtZQCfGndkpeAOwa-DvmARsrXJpMn6KPW5eSxQDZAlFnQeb3QqE5saupvbj-DKyFYhnexVJw5yK84hVcrnkqXx-6Dhu0HDJm5JXXLpTgjaLrfHdXfVEKgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Thiol depletion of NK3.3 cells reduced RB expression and greatly reduced or abrogated phosphorylation of RB in response to IL-2. (A) Western blot analysis of RB protein in NK3.3 cells cultured with rIL-2 (200 U/mL) for 0 to 24 hours in CYS(+) medium or CYS(−) medium. Phosphorylated RB migrates slower than nonphosphorylated RB.3233 (B) Addition of thiol-related reducing reagents, GSH, 2-ME, or NAC to CYS(−) medium restored RB phosphorylation and expression in response to IL-2 (Western blot) and [3H]-TdR incorporation (bar graph) in a dose-dependent manner. Controls included cells without IL-2 in CYS(−) medium (IL-2[−]), and NK3.3 cells cultured in CYS(−) medium with IL-2 (200 U/mL) (Cystine [0]). (C) RB phosphorylation is induced by IL-2 in freshly isolated human NK cells. Immediately after isolation (time 0), cells were cultured with IL-2 (200 U/mL) in CYS(−) or CYS(+) medium. Each data set is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f5c.jpeg?Expires=1769093198&Signature=hpXo~sWa45KYpeDFi3k5e-bVZmQjIb9Lfrcw-2BydRbK2P917voNOJZiWm6C38sfWy0Tn1sUNXQ~SWwmlpHo9q2NwnOmeDDUEp4FnVSq~6JNXx~USBe3RhjNz1a5ZVqvObx3~Q-IkVr7KVgn0GS8HwX~xC1tH0G7HZlMjOGebB1RidzM3j2VoyLNJXqes2Hhdlufpslwb3GgzxOTFDV5ddU4d1WQhM-weS07DszuyDOEyYpxUx2KZatqZZSkuwC0EGS4lbdTfCBwG3reBHwDqhBgJk59qxbImlRy7DmvMBYdlgm4s~8vT-3gjXTkcKUdNugVzpx-2Uw11L~E4T6mbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Thiol deprivation induced premature enhancement of both enzymatic activity and phosphorylation of CDK6 and CDK2. (A) NK3.3 cells were stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−) medium for the indicated time, and equivalent protein amounts in each lysate were immunoprecipitated with antibodies to CDK6 or CDK2. In vitro kinase assays were performed on the immunoprecipitates using truncated RB (56 kD) or H1 histone as substrates for CDK6 or CDK2, respectively. (B) NK3.3 cells were labeled with [32P]-orthophosphate and stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(-) media for the indicated times, and lysates were immunoprecipitated with antibodies to CDK6 or CDK2. Equivalent protein content in each lane and identity of each band were verified by Western blot analysis with antibody to CDK6 or CDK2, respectively. (C) Dephosphorylation of CDK6 was assessed in cells that had been labeled with [32P]-orthophosphate in CYS(−) medium in the presence of IL-2 (200 U/mL), and split into two cultures, one continued in CYS(−) medium, and one placed in CYS(+), each with IL-2, for the indicated times. CDK6 protein amount in each lane was verified by Western blot analysis in one of two independent experiments (not shown). Each set of data is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f6a.jpeg?Expires=1769093198&Signature=Vc0-VjzBncbNxizTLpDjPtcOh75IZEXjI2q8q72fZhhhJw-O2eMf89v3g0r4iNbziHh2whWtxcpIwrqzw2-rq6wCif3~7CYb90TvkSL~weHuA9aTFVf5jwB904tsYjC~E2hQdPN5beoORdPxOxj~h43UKhevBV3Onk6cLS-OQR9A5ntY9STfJI9yH-8M~rX1pu3XggoBWUbyz5CyRXIQT59k7Bz2oA6j4xtA2qSH5hK8Z~y5OHQeDxR3JIoMDrti~Np7p9e5L8tESxfty1stEXjrg8RRSHdxFqsQ-dYUiHVGj31UNpsvzQMVjd2SJRhvE-qsuFQlzJPJNeNASu9ztQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Thiol deprivation induced premature enhancement of both enzymatic activity and phosphorylation of CDK6 and CDK2. (A) NK3.3 cells were stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−) medium for the indicated time, and equivalent protein amounts in each lysate were immunoprecipitated with antibodies to CDK6 or CDK2. In vitro kinase assays were performed on the immunoprecipitates using truncated RB (56 kD) or H1 histone as substrates for CDK6 or CDK2, respectively. (B) NK3.3 cells were labeled with [32P]-orthophosphate and stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(-) media for the indicated times, and lysates were immunoprecipitated with antibodies to CDK6 or CDK2. Equivalent protein content in each lane and identity of each band were verified by Western blot analysis with antibody to CDK6 or CDK2, respectively. (C) Dephosphorylation of CDK6 was assessed in cells that had been labeled with [32P]-orthophosphate in CYS(−) medium in the presence of IL-2 (200 U/mL), and split into two cultures, one continued in CYS(−) medium, and one placed in CYS(+), each with IL-2, for the indicated times. CDK6 protein amount in each lane was verified by Western blot analysis in one of two independent experiments (not shown). Each set of data is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f6b.jpeg?Expires=1769093198&Signature=o~zQ9jy1gpMYFoNzI13D2XSJzPR8oWUbqBC0u~MxYQxyOrJpFLob7c6s7AeCz-cmgebh3dKrawuzuGnW0Qos2I52jOHT4Aboitf3oqGyVpgGLB-xa7K9s~UHasK7Kl3YWgzOz4TGIg6-qgCGJGsnpLn5bFTLgnR0AdYu5hdArX2K7MUQpX927JBKNbd3X9d5o~MB1Q03Tg7BQFuSiqyv8NyToJHDfVoh86Vm8uB8huI1wOPP66rTavUD44PBY-Qt~ql3BJM3MEL7mZLdockpNzcyQbhDzfW0DQpvKusB~c~1Q2i8fUPuXh4SRGHEN5L0JlJujsKroRyMMvYuq2sGhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Thiol deprivation induced premature enhancement of both enzymatic activity and phosphorylation of CDK6 and CDK2. (A) NK3.3 cells were stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(−) medium for the indicated time, and equivalent protein amounts in each lysate were immunoprecipitated with antibodies to CDK6 or CDK2. In vitro kinase assays were performed on the immunoprecipitates using truncated RB (56 kD) or H1 histone as substrates for CDK6 or CDK2, respectively. (B) NK3.3 cells were labeled with [32P]-orthophosphate and stimulated with IL-2 (200 U/mL) in CYS(+) or CYS(-) media for the indicated times, and lysates were immunoprecipitated with antibodies to CDK6 or CDK2. Equivalent protein content in each lane and identity of each band were verified by Western blot analysis with antibody to CDK6 or CDK2, respectively. (C) Dephosphorylation of CDK6 was assessed in cells that had been labeled with [32P]-orthophosphate in CYS(−) medium in the presence of IL-2 (200 U/mL), and split into two cultures, one continued in CYS(−) medium, and one placed in CYS(+), each with IL-2, for the indicated times. CDK6 protein amount in each lane was verified by Western blot analysis in one of two independent experiments (not shown). Each set of data is representative of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4092/4/m_bl_0046f6c.jpeg?Expires=1769093198&Signature=vnyQlXegq0H8~NshHEjKj1iJAFqvjxO44ifJXOAKFi6NvHz~u3jcaAGOOT0tsKpQ4mgarNpGcu7jUDHHGsDBhNfGRSLTRdgyctN4stmmD~AoGaoKQ7NZN7othcagzpO-usX88sgkoBR9taS1JuYaT-Ewtn6J54YUC3oVR1WKOCoXZrBiWZI4Xr5m2yN8fJHkuK51oMchPa4472APB~dPxC1zOCNHrH7xHFFd3ENj1s9WVoZ-nRDD2-VH0w8rhCQ~yCE~2SkT0Otfoz0nk~BCjhCScXKJHFw3FMtoYwhNPbljvrYKeK6jhzdLusMGF0MrwBnFVlQMwXYADLYQQBPpNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal