Abstract
Platelets contain two main types of secretory organelles, the dense granules and the α-granules. P-selectin, a specific receptor for leukocytes that is present in the α-granule membrane, has also been demonstrated to be associated with the dense granule limiting membrane, showing that a relationship exists between these two types of secretory granules. We have previously shown that the plasma membrane receptors glycoproteins (Gp) IIb-IIIa and Ib are also present in the α-granule membrane. To document further the composition of the dense granule membrane, we have used immunoelectron microscopy in the present work to determine if the dense granule membrane also contains these glycoproteins. First, the cytochemical method of Richards and Da Prada (J Histochem Cytochem 25:1322, 1977), which specifically enhances dense body electron density, was combined with immunogold-labeled anti–Gp IIb-IIIa or anti–Gp Ib antibody. A consistent and reproducible labeling for Gp IIb-IIIa, but less for Gp Ib, was found in the membrane of platelet dense granules. Subsequently, double immunogold labeling was performed on frozen thin sections of resting platelets using antibodies directed against the dense body components granulophysin or P-selectin, followed by anti–Gp IIb-IIIa or anti–Gp Ib. Consistent labeling for Gp IIb-IIIa and weaker labeling for Gp Ib were detected in dense bodies. The possibility that the granulophysin-positive structures could be lysosomes was excluded by the presence of P-selectin. Immunogold labeling of isolated dense granule fractions confirmed these results. Identical findings were made on human cultured megakaryocytes using double immunolabeling. In conclusion, this study demonstrates the presence of Gp IIb-IIIa and Gp Ib on the dense granule membrane. This observation provides additionnal evidence of similarities between the α-granule and dense granule membranes and raises the possibility of a dual mechanism responsible for the formation of dense granules similar to that of α-granules, ie, endogenous synthesis as well as endocytosis from the plasma membrane.
BLOOD PLATELETS contain a variety of storage organelles that contribute to their hemostatic function by releasing their contents to the extracellular surroundings. There are two main types of secretory granules: the α-granules, which are the most prominent population in size and number, and the dense granules. The dense granules contain the vasoconstrictive agent 5-hydroxytryptamine (5-HT), the nonmetabolic pool of adenosine triphosphate and adenosine diphosphate, Ca2+ and Mg2+ have a fundamental role in hemostasis. Dense granules and α-granules are morphologically distinct,1 but recent studies showing the presence of P-selectin, a component of the α-granule membrane, in the dense granule membrane2 suggest a relationship between these organelles. It has been shown that α-granule biogenesis is a complex phenomenon involving both endogenous synthesis and endocytosis. The soluble proteins stored in the α-granule matrix are derived via exclusive synthesis in the megakaryocytes (MK), such as von Willebrand factor and thrombospondin,3,4 or through endocytosis of plasma proteins such as fibrinogen and albumin.3,5,6 Moreover, the proteins of the α-granule membrane can also be categorized in two groups. Some receptors are restricted to the α-granule limiting membrane and are absent from the plasma membrane such as P-selectin.7 Alternatively, several components of the platelet plasma membrane are also present in the α-granule membrane, including glycoprotein (Gp) IIb-IIIa8 and the Gp Ib-IX-V complex.9 In the present study, we have used cytochemistry and immunoelectron microscopy to investigate the different components of the dense granule membrane, focusing on two plasma membrane markers, Gp IIb-IIIa and Gp Ib.
MATERIALS AND METHODS
Cells
Blood samples were drawn from normal healthy volunteers by venipuncture into plastic tubes containing ACD-C buffer (13 mmol/L citric acid, 6.8 mmol/L sodium citrate, 11.2 mmol/L glucose, pH 4.2). The platelet-rich plasma was obtained by centrifugation of the anticoagulated blood for 10 minutes at 100g at 22°C. The isolated platelets were obtained by centrifugation of the platelet-rich plasma for 10 minutes at 1,100g at 22°C and washed three times with Tyrode's buffer (360 mmol/L citric acid, 5 mmol/L KCl, 2 mmol/L CaCl2 , 1 mmol/L MgCl2 , 103 mmol/L glucose, pH 7.4) containing 3.5 mg/mL bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) and 100 nmol/L prostaglandin E1 (Sigma). When platelets were treated for the cytochemical method of Richards and Da Prada,10 blood samples were directly harvested into the adequate fixative.
Antibodies
Three antihuman polyclonal rabbit antibodies (anti–Gp IIb-IIIa,8 antiglycocalicin, and anti–P-selectin9 ) were kindly provided by Dr Michael Berndt (Victoria, Australia) and were used at 10 μg/mL. Antihuman granulophysin, a monoclonal mouse antibody kindly provided by Dr J.M. Gerrard (Winnipeg, Manitoba, Canada) was also used at 10 μg/mL.11 Gold-conjugated (10 and 15 nm) protein A purchased from the Department of Cell Biology (University of Utrecht, Utrecht, The Netherlands) was used at an 1/80 and 1/35 dilution, respectively. Goat antimouse and goat antirabbit IgG fractions coupled to 10 or 15 nm gold particles were purchased from British Biocell International (Cardiff, UK).
Electron Microscopy
Normal platelets and MK were prepared for immunoelectron microscopy by fixation in 1% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.4, for 1 hour at 22°C; washed three times with the same buffer; embedded in sucrose; and frozen in liquid N2 . Alternatively, the cytochemical reaction of Richards and Da Prada10 was performed after fixation of blood in 3% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.2) for 90 minutes, and the isolated platelets were obtained as described above and washed three times with 0.9% NaCl for 15 minutes. The cytochemical reaction was performed in 4% aqueous uranyl acetate (pH 3.9) overnight (18 hours) and the platelets were then washed once more in 0.9% NaCl. Platelets were embedded in glycolmethacrylate (GMA) followed by the performance of the immunochemical reactions on thin sections collected on grids according to the method of Bendayan.12 Sections were labeled by first incubating with the polyclonal rabbit antibodies diluted in Tris-buffered saline (TBS) containing 1% BSA and 4% normal goat serum for 2 hours at 22°C, washed three times with TBS containing 0.1% BSA, and then incubated with goat antirabbit gold (10 nm) for 1 hour at room temperature. The sections were counterstained with uranyl acetate and lead citrate. To identify the nature of labeled granules, double immunolabeling was performed on frozen thin sections using different-sized gold protein A conjugates or alternatively goat antirabbit antibody (GAR) coupled to 10-nm gold particles and goat antimouse antibody (GAM) coupled to 10-nm gold particles. To avoid cross-reactions between antibodies of the same species, a short fixation with 1% glutaraldehyde and saturation with 0.1% BSA was performed before the second round of labeling, according to the method of Slot et al.13 Samples were examined with a Philips CM10 electron microscope (Philips, Eindhoven, The Netherlands).
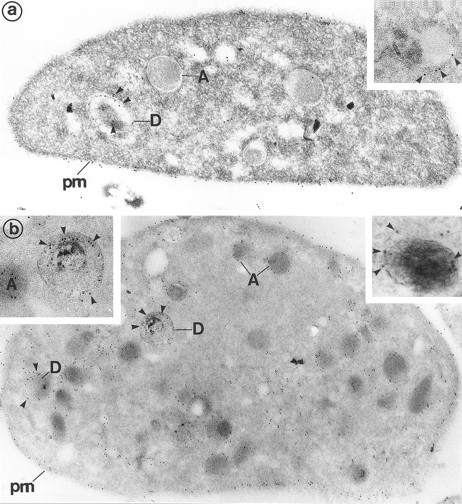
(a) Platelets treated for the cytochemical detection of dense granules by the uranaffin technique of Richards and Da Prada10 and then immunolabeled for Gp Ib (polyclonal anti-Gp Ib/GAR 10 nm). The gold label lines the plasma membrane (pm) and is occasionally found on α-granules (A). Moreover, a granule with a dense content (D) is also labeled for Gp Ib. (Original magnification × 40,000.) (Inset) High magnification of a dense granule displaying immunolabeling for Gp Ib. (Original magnification × 59,000.) (b) Platelets pretreated by the uranaffin technique of Richards and Da Prada,10 embedded in GMA, and then immunolabeled for Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/GAR 10 nm). Gold label is evident along the plasma membrane (pm) as well as along the α-granules (A). Note that the limiting membrane of the two dense granules (D) is also strongly labeled. (Original magnification × 32,000.) (Insets) High magnification of dense granules labeled for Gp IIb-IIIa. (Original magnification × 59,000 and × 92,000.)
(a) Platelets treated for the cytochemical detection of dense granules by the uranaffin technique of Richards and Da Prada10 and then immunolabeled for Gp Ib (polyclonal anti-Gp Ib/GAR 10 nm). The gold label lines the plasma membrane (pm) and is occasionally found on α-granules (A). Moreover, a granule with a dense content (D) is also labeled for Gp Ib. (Original magnification × 40,000.) (Inset) High magnification of a dense granule displaying immunolabeling for Gp Ib. (Original magnification × 59,000.) (b) Platelets pretreated by the uranaffin technique of Richards and Da Prada,10 embedded in GMA, and then immunolabeled for Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/GAR 10 nm). Gold label is evident along the plasma membrane (pm) as well as along the α-granules (A). Note that the limiting membrane of the two dense granules (D) is also strongly labeled. (Original magnification × 32,000.) (Insets) High magnification of dense granules labeled for Gp IIb-IIIa. (Original magnification × 59,000 and × 92,000.)
(a and b) Frozen sections of resting platelets labeled with the dense granule markers granulophysin and P-selectin to identify the morphology of these organelles on cryosections. (a) Labeling of P-selectin (polyclonal anti–P-selectin/protein A 10 nm). P-selectin is present along the α-granules (A) and labels the dense granules (D) that have been partially extracted. The absence of P-selectin permits distinction of the vacuolar structures of the OCS from the dense bodies. (Original magnification × 48,000.) (b) Labeling of granulophysin (monoclonal anti-granulophysin/GAM 10 nm). The gold marker labels vacuolar structures, such as the dense granule limiting membrane (D). Using this technique, the dense granule core appears to have been partially lost, which gives the dense granule a vacuolar appearance. α-Granules (A) and plasma membrane (pm) are negative. (Original magnification × 48,000.)
(a and b) Frozen sections of resting platelets labeled with the dense granule markers granulophysin and P-selectin to identify the morphology of these organelles on cryosections. (a) Labeling of P-selectin (polyclonal anti–P-selectin/protein A 10 nm). P-selectin is present along the α-granules (A) and labels the dense granules (D) that have been partially extracted. The absence of P-selectin permits distinction of the vacuolar structures of the OCS from the dense bodies. (Original magnification × 48,000.) (b) Labeling of granulophysin (monoclonal anti-granulophysin/GAM 10 nm). The gold marker labels vacuolar structures, such as the dense granule limiting membrane (D). Using this technique, the dense granule core appears to have been partially lost, which gives the dense granule a vacuolar appearance. α-Granules (A) and plasma membrane (pm) are negative. (Original magnification × 48,000.)
Immunogold Analysis of Isolated Dense Granules
Dense granules were isolated as described by Rendu et al.14 Dense granules were pelleted at the bottom of the metrizamide gradient in an almost pure fraction. The enhancement of the intrinsic opacity of dense granules was obtained either according to the method of White1 and further embedded in Epon for morphologic examination or according to the method of Richards and Da Prada10 and embedded in GMA for immunolabeling.
Controls
To avoid the detection of IgG contained in the platelets, the following controls were performed: replacement of the primary antibody by a nonimmune serum or omission of the primary antibody from the reaction.
RESULTS
Immunoelectron Microscopic Detection of Gp Ib and Gp IIb-IIIa in the Dense Granule Membrane
Immunolabeling of Platelets Using a Dense Body Cytochemical Reaction
When platelets were treated with the cytochemical method of Richards and Da Prada,10 dense granules were identified because of their density due to the cytochemical reaction of 5′- phosphonucleotides of dense granules and the uranyl ions. Incubation of thin sections in the various sequential immunolabeling media tended to fade the electron density of dense granules, presumably because of the water solubility of the reaction products. Immunolabeling for Gp Ib was evident along the plasma membrane. The membrane of some of the α-granules stained weakly for Gp Ib, as described previously.9 Some labeling for Gp Ib was also found occasionally in the dense granule limiting membrane (Fig 1a). Immunogold staining for Gp IIb-IIIa gave the following pattern: conspicuous labeling of the plasma membrane as well as the α-granule membrane. In addition, the dense granule membrane was also consistently labeled (Fig 1b). Thus, Gp Ib and Gp IIb-IIIa appear to be present in the dense granule membrane.
Detection of Gp Ib in dense granules by double immunolabeling on frozen thin sections of platelets. (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). P-selectin is present in the α-granules (A) and dense granules (D). Gp Ib is evident along the plasma membrane (pm), occasional OCS, and α-granules. The dense granule membrane (D) that is marked with P-selectin also contains gold marker for Gp Ib. (Original magnification × 46,500; inset, × 101,000.) (b) Granulophysin (monoclonal anti-granulophysin/GAM 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). Granulophysin labels the dense granule membrane (D) but not the α-granules (A). Gp Ib is found essentially along the plasma membrane (pm). The OCS and some α-granules (A) are occasionaly labeled. It also double-labels the dense granule membrane (D) identified by the presence of granulophysin. (Original magnification × 46,500; inset, × 84,000.)
Detection of Gp Ib in dense granules by double immunolabeling on frozen thin sections of platelets. (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). P-selectin is present in the α-granules (A) and dense granules (D). Gp Ib is evident along the plasma membrane (pm), occasional OCS, and α-granules. The dense granule membrane (D) that is marked with P-selectin also contains gold marker for Gp Ib. (Original magnification × 46,500; inset, × 101,000.) (b) Granulophysin (monoclonal anti-granulophysin/GAM 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). Granulophysin labels the dense granule membrane (D) but not the α-granules (A). Gp Ib is found essentially along the plasma membrane (pm). The OCS and some α-granules (A) are occasionaly labeled. It also double-labels the dense granule membrane (D) identified by the presence of granulophysin. (Original magnification × 46,500; inset, × 84,000.)
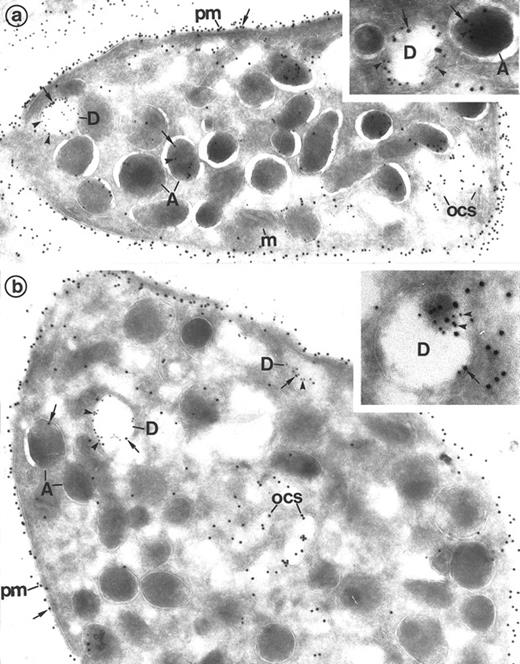
Detection of Gp IIb-IIIa on dense granules by double immunolabeling performed on frozen thin sections of platelets. (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). The presence of P-selectin identifies α-granules (A) and the dense granule membrane (D). Gp IIb-IIIa lines the plasma membrane (pm) and the α-granule membrane (A). Note that it is present in the dense granule membrane (D), where it colocalizes with P-selectin. (Original magnification × 36, 000; inset, × 84,000.) (b) Granulophysin (monoclonal anti-granulophysin/GAM 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). The anti-granulophysin labels the dense granule membrane, thus allowing identification of these structures (D). Gp IIb-IIIa is also found at their level. Gp IIb-IIIa labels the α-granule membrane (A) and the plasma membrane (pm) as well. (Original magnification × 44,000; inset, × 84,000.)
Detection of Gp IIb-IIIa on dense granules by double immunolabeling performed on frozen thin sections of platelets. (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). The presence of P-selectin identifies α-granules (A) and the dense granule membrane (D). Gp IIb-IIIa lines the plasma membrane (pm) and the α-granule membrane (A). Note that it is present in the dense granule membrane (D), where it colocalizes with P-selectin. (Original magnification × 36, 000; inset, × 84,000.) (b) Granulophysin (monoclonal anti-granulophysin/GAM 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). The anti-granulophysin labels the dense granule membrane, thus allowing identification of these structures (D). Gp IIb-IIIa is also found at their level. Gp IIb-IIIa labels the α-granule membrane (A) and the plasma membrane (pm) as well. (Original magnification × 44,000; inset, × 84,000.)
Ultrastructural Identification of Dense Granules on Frozen Thin Sections
During this type of cell preparation, the dense content of the dense granules is extracted and dense granules appear as vacuolar organelles.2 To distinguish these organelles from other vacuolar structures such as the open canalicular system (OCS), immunolabeling was performed using antibodies against the known dense granule marker granulophysin and against P-selectin, because it had been shown that the dense granule membrane contains this receptor.2 Immunolabeling for P-selectin was observed in the α-granules and strongly labeled some vacuolar structures that are dense granules that have been partially extracted (Fig 2a). Moreover, the OCS cisternae that appear as vacuoles on thin sections did not display immunolabeling for P-selectin and could thus be distinguished from the dense granules. Immunolabeling for granulophysin was also present on the electron luscent granules and vacuole-like structures that had been positively labeled for P-selectin and mostly restricted to these structures (Fig 2b). Because of this labeling pattern, we could thus identify dense granules on frozen thin sections. Occasional small granules with a dense matrix were labeled and were identified as platelet primary lysosomes.
Human MK double-labeled for the α-granule and dense granule marker P-selectin, combined with Gp Ib or Gp Iib-IIIa. (Inset) Human cultured MK are large in size and have an indented nucleus, numerous secretory granules, and a prominent demarcation membrane system. (Original magnification × 16,000.) (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). P-selectin lines the α-granules (A) and the dense granule membrane (D) and is absent from the plasma membrane (pm) and demarcation membranes (dm). In contrast, Gp Ib lines the plasma membrane and demarcation membranes (dm). Gp Ib is also found within the dense granule membrane (D) colocalized with P-selectin. Mitochondria (m), as control structures, do not label. (Original magnification × 72,000.) (b) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). Gp IIb-IIIa is classically located on the plasma membrane (pm) and α-granules (A). It also colocalizes with P-selectin in the α-granules and, noteworthily, within the dense granules (D). (Original magnification × 33,500.)
Human MK double-labeled for the α-granule and dense granule marker P-selectin, combined with Gp Ib or Gp Iib-IIIa. (Inset) Human cultured MK are large in size and have an indented nucleus, numerous secretory granules, and a prominent demarcation membrane system. (Original magnification × 16,000.) (a) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp Ib (polyclonal anti–Gp Ib/protein A 15 nm; arrows). P-selectin lines the α-granules (A) and the dense granule membrane (D) and is absent from the plasma membrane (pm) and demarcation membranes (dm). In contrast, Gp Ib lines the plasma membrane and demarcation membranes (dm). Gp Ib is also found within the dense granule membrane (D) colocalized with P-selectin. Mitochondria (m), as control structures, do not label. (Original magnification × 72,000.) (b) P-selectin (polyclonal anti–P-selectin/protein A 10 nm; arrowheads)/Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/protein A 15 nm; arrows). Gp IIb-IIIa is classically located on the plasma membrane (pm) and α-granules (A). It also colocalizes with P-selectin in the α-granules and, noteworthily, within the dense granules (D). (Original magnification × 33,500.)
Double Immunolabeling
To confirm that both Gp Ib and Gp IIb-IIIa were associated with the dense granule membrane, double immunolabeling experiments were performed using antibodies against the dense granule markers granulophysin and P-selectin on frozen thin sections of resting platelets and MKs.
Platelets.When platelets had undergone double immunolabeling for the granule protein, P-selectin, together with Gp Ib, P-selectin was located in the α-granule membrane and the dense granules as described above. The same organelles also displayed labeling for Gp Ib. Plasma membrane was regularly and conspicuously labeled with Gp Ib, whereas the OCS was only occasionaly labeled, confirming that Gp Ib is essentially a plasma membrane component on nonstimulated platelets (Fig 3a). A similar result was obtained using double immunolabeling for granulophysin and Gp Ib, ie, the presence of Gp Ib in dense bodies that were also lined by granulophysin, with the exception that granulophysin only labeled the dense granule limiting membrane and not the α-granules (Fig 3b).
Using a similar approach, labeling for Gp IIb-IIIa was present in the membrane of the same vacuolar structures in which P-selectin and granulophysin were detected, permitting identification of these structures as dense granules. Gp IIb-IIIa was also present on the α-granule and plasma membranes (Fig 4a and b).
The mitochondria were negative for the four markers.
MK.Double immunolabeling of the frozen thin sections of the mature MK with P-selectin and Gp Ib showed colocalization of the two glycoproteins in the dense granule membrane (Fig 5a). Similarly, Gp IIb-IIIa was colocalized with P-selectin in the dense granule membrane (Fig 5b). Gp Ib and Gp IIb-IIIa were also observed on the plasma membrane and the demarcation membrane system of the MK. Prominent labeling of Gp IIb-IIIa and occasional labeling of Gp Ib was also present on the α-granule membrane as previously described. α-Granules displayed P-selectin labeling but were easily distinguished from the dense granules because of their characteristic morphology, size, dense nucleoid, and general electron density. It is noteworthy that, in mature MK, dense granule morphology was more identifiable than in platelets, with better preservation of their dense contents for unclear reasons.
Controls
The various control experiments led to the disappearance of immunogold labeling (not shown).
Dense Granule Fraction Analysis
Ultrastructural Aspects of the Dense Granule Fraction
An isolated dense granule subcellular fraction appeared to be highly pure when examined by electron microscopy (Fig 6a). Indeed, the most abundant recognizable organelles were well-preserved, intact granules of high electron density that were identified as dense bodies. Only scant broken granules and membranes were present.
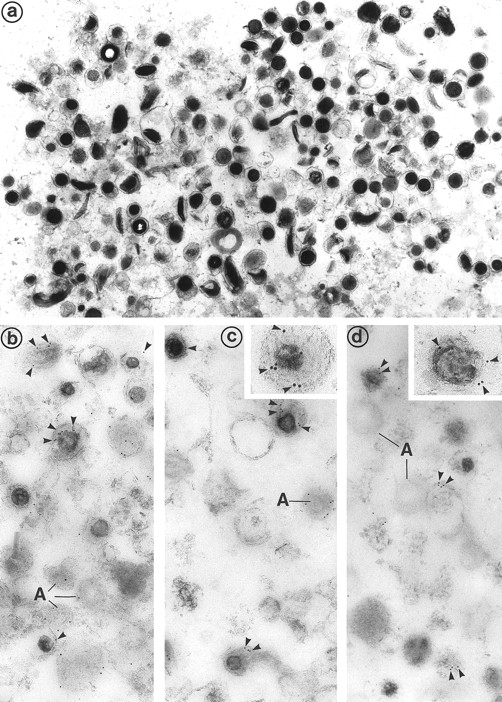
(a) Electron microscopic examination of the dense granule fraction whose electron density has been enhanced by White's cytochemical technique. This shows the high purity of the fraction that is almost exclusively composed of dense bodies. Only few membranes and platelet granules of other types can be identified. (Original magnification × 18,500.) (b, c, and d) Immunolabeling of the dense granule fraction pretreated by the uranaffin technique of Richards and Da Prada10 and embedded in GMA. (b) Labeling of P-selectin (polyclonal anti–P-selectin/GAR 10 nm). P-selectin is present in the membrane of α-granules (A) and in some of the dense granules (D) that are still identifiable, but only discrete labeling is obtained. This might be caused by the interaction of the chemical solutions with the antigenic sites, which could have decreased their reactivity. (Original magnification × 38,500.) (c) Immunolabeling for Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/GAR 10 nm) is similar to immunolabeling for P-selectin and is present in the membrane of some dense granules (D) as well as α-granules (A). (Original magnification × 38,500; inset, × 100,500.) (d) Immunolabeling for Gp Ib (polyclonal anti–Gp Ib/GAR 10 nm) is occasionaly found in the membrane of dense granules (D). α-Granules (A) present in this field are not labeled. (Original magnification × 38,500; inset, × 74,500.)
(a) Electron microscopic examination of the dense granule fraction whose electron density has been enhanced by White's cytochemical technique. This shows the high purity of the fraction that is almost exclusively composed of dense bodies. Only few membranes and platelet granules of other types can be identified. (Original magnification × 18,500.) (b, c, and d) Immunolabeling of the dense granule fraction pretreated by the uranaffin technique of Richards and Da Prada10 and embedded in GMA. (b) Labeling of P-selectin (polyclonal anti–P-selectin/GAR 10 nm). P-selectin is present in the membrane of α-granules (A) and in some of the dense granules (D) that are still identifiable, but only discrete labeling is obtained. This might be caused by the interaction of the chemical solutions with the antigenic sites, which could have decreased their reactivity. (Original magnification × 38,500.) (c) Immunolabeling for Gp IIb-IIIa (polyclonal anti–Gp IIb-IIIa/GAR 10 nm) is similar to immunolabeling for P-selectin and is present in the membrane of some dense granules (D) as well as α-granules (A). (Original magnification × 38,500; inset, × 100,500.) (d) Immunolabeling for Gp Ib (polyclonal anti–Gp Ib/GAR 10 nm) is occasionaly found in the membrane of dense granules (D). α-Granules (A) present in this field are not labeled. (Original magnification × 38,500; inset, × 74,500.)
Immunolabeling of the Dense Granule Fraction
Immunolabeling for P-selectin, as a marker of dense granules, was observed in the membrane of some dense granules but not in all of them (Fig 6b). This might be caused by the interaction of the different chemical treatments (pH 3.9, presence of uranyl acetate complexes in the dense granules and GMA embedding), which decreased reactivity of the antigenic sites. Immunolabeling for Gp IIb-IIIa resembled immunolabeling for P-selectin (Fig 6c). Fewer dense granules were labeled for GPIb, with the granules displaying less gold particles than for Gp IIb-IIIa and P-selectin; small amounts of Gp Ib was detected in the α-granules, as previously described.9
DISCUSSION
Platelet dense granules have engendred considerable interest because, like nervous tissue, they actively take up 5-HT and histamine and release 5-HT after reserpine treatment of platelets. They also greatly contribute to the hemostatic function of platelets. In this study, we have used cytochemical and immunogold labeling techniques at the ultrastructural level to specifically detect the dense granules of platelets and MK. Using combined immunolabeling, we investigated whether certain receptors found in the platelet plasma membrane as well as in the α-granule membrane would also be found in the dense granule limiting membrane. Dense granules are bound by a typical unit membrane. The proteins anchored in their membrane include granulophysin,11,15 a protein originally described as present in platelet lysosomes,16 as well as P-selectin, a leukocyte receptor that was first described in the α-granule membrane and that can redistribute to the plasma membrane after secretion.2 The occurence of P-selectin both in the α-granule and dense granule membrane is an argument in favor of searching for other markers of similarity. Moreover, the existence of a congenital platelet disease, α-δ storage pool disease, involving the association of α-granule abnormalities with dense granule deficiencies, is additional evidence for a close relationship.17,18 Finally, disorders of secretion of one kind of granule is often linked to abnormal secretion of the other. This was described in the Grey platelet syndrome, a congenital α-granule deficiency19 in which dense granule release is impaired, and in storage pool disease, defined as a decrease in the number and content of dense granules that is also classically characterized by abnormal α-granule release.20
α-Granules are formed by a dual mechanism: endogenous MK synthesis of proteins such as von Willebrand factor and thrombospondin3,4 and a mechanism of endocytosis of circulating proteins, eg, fibrinogen and Igs.3,5,6 The composition of the α-granule limiting membrane also reflects these two pathways. Some components are specifically restricted to their membrane, such as P-selectin,7 osteonectin,21 and GMP 33,22 whereas other receptors are located both in the plasma membrane and within the α-granules. It has been hypothesized that they enter the α-granule membrane by means of endocytosis and recycling from the plasma membrane. This appears to be the case for Gp IIb-IIIa, the Gp Ib-IX-V complex, Gp IV, PECAM1, and CD9, which are all found in the α-granule membrane, but at different levels.8,9,23 24 To investigate the components and biogenesis of dense granules, we used the same technical approach as has been used for α-granule studies. We show that the two main platelet membrane receptors, Gp IIb-IIIa and Gp Ib, are also components of the dense granule limiting membrane. Indeed, when platelets were pretreated by the uranaffin reaction to enhance the electron density of dense granules, a consistent immunolabeling for Gp IIb-IIIa and weaker labeling for Gp Ib was observed in about 50% of identifiable dense bodies.
However, because the solubility of the cytochemical reaction product in the immunolabeling incubation medium sometimes rendered the identification of dense granules questionable, we confirmed these results by double immunolabeling performed on frozen sections. Because sucrose embedding does not preserve the electron density of the dense bodies (presumably because their content is lost during the procedure), we had to use specific markers to recognize their membrane from other vacuolar structures. This problem was formerly encountered by Israels et al,2 who described the ultrastuctural appearance of dense bodies as visualized on platelet cryosections and whose data are similar to our observations. To distinguish these empty granules from the vacuole-like channels of the OCS, we used P-selectin, which is absent from the former and present in the latter. The other dense granule marker used was granulophysin. Because it is similar, if not identical, with the lysosomal membrane protein CD63,25 it was also found in platelet small granules that have been identified as primary lysosomes. However, their small size and electron dense matrix made them easily distinguishable from the dense granules, which are often located close to the plasma membrane.26 The observation that Gp Ib and IIb-IIIa are present in the dense granule membrane, just as they are present in the α-granule membrane (Gp IIb-IIIa immunolabeling being prominent in the dense granules like in α-granules and Gp Ib immunolabeling being scanter in both structures),9 shows a close relationship between the limiting membrane of the two types of granules. Although the dense granule-associated pool of glycoproteins only represents a small amount of the total platelet receptor pool (which is mainly expressed by the platelet membrane), the specific localization described here may illuminate the mechanism of formation of these organelles. Indeed, little is known about the constituents of the 5-HT organelle membrane and their synthesis in MK. Our findings might signify that dense granules and α-granules share a similar mechanism of formation through an endocytic process originating from the plasma membrane. Indeed, platelet 5-HT originates in the enterochromaffin cells of the intestinal mucosa; it is taken up by circulating platelets and subsequently stored within the dense granules. When platelets are activated, they release 5-HT from storage granules. The precursor cells of platelets, the MK, also have the ability to take up serotonin, although these cells do not synthesize 5-HT.27 28 We show here that Gp Ib and IIb-IIIa expression in dense granules is also an early occuring phenomenon and already well established at the MK stage.
It would be of interest to investigate the content of the dense granule membrane in pathologic states such as storage pool deficiency in which the dense granule membrane is present but its contents are abnormal.29,30 These studies might indicate if there exists a relationship between the presence of receptors in the membrane and the uptake (or retention) of the granule contents. Indeed, as far as α-granule abnormalities are concerned, the relationship between the absence of a receptor (Gp IIb-IIIa) in their membrane and the lack of α-granule content (fibrinogen) has been well documented in Glanzmann's thrombasthenia.31 Finally, the functional relevance of the receptors Gp IIb-IIIa and Gp Ib in the dense granule membrane might be that, after slight platelet stimulation leading to dense granule secretion only, the exposure of these receptors may help to anchor platelets close to their target to deliver their active transmitters.
In conclusion, we provide ultrastructural and biochemical evidence for the presence of two plasma membrane receptors, Gp IIb-IIIa and Gp Ib, in the dense granules of normal platelets and MKs. We also confirm that P-selectin is a component of the dense granule membrane using a combination of cytochemical and immunogold techniques. These findings suggest that the dense granule membrane, like α-granules, may originate from a dual mechanism: direct targeting from the Golgi complex as well as endocytosis from the plasma membrane. The functional relevance of this dense granule-associated pool of glycoproteins remains to be understood.
Peritoneal mast cell from a Beige rat undergoing anaphylactic degranulation of giant secretory granules. Swollen, less electron-dense secretory granules in the process of extrusion are enlarged beyond the giant, electron-dense cytoplasmic secretory granules not so involved. Original magnification × 12,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
Peritoneal mast cell from a Beige rat undergoing anaphylactic degranulation of giant secretory granules. Swollen, less electron-dense secretory granules in the process of extrusion are enlarged beyond the giant, electron-dense cytoplasmic secretory granules not so involved. Original magnification × 12,000. (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
The authors acknowledge Drs Gaëtan Berger and Anne-Marie Cieutat for helpful comments, Dr Samuel Burstein for carefully editing the English of the manuscript, and Dr Paul-Henri Roméo for his constant support.
Supported in part by the Association pour la Recherche contre le Cancer (ARC) and the Fondation pour la Recherche Médicale (FRM).
Address reprint requests to Elisabeth M. Cramer, MD, PhD, INSERM U.91, Hôpital Henri Mondor, 94010 Créteil, France.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal