Abstract
HLA-DR is one of the markers associated with hematopoietic cell differentiation, since expression of this molecule is modulated throughout hematopoiesis. We have previously described and cloned the gene encoding factor IK, which inhibits both interferon gamma (IFN-γ)-induced and constitutive HLA-DR expression. The current study demonstrates that IK gene transcripts are present in CD34+ cells purified from human umbilical cord blood. IK expression increased and was therefore inversely correlated with the gradual loss of HLA-DR during growth factor–induced CD34+ cell proliferation and differentiation. To study the possible role of IK in hematopoiesis, antisense probes were used. IK expression was specifically inhibited by an antisense oligodeoxynucleotide containing two phosphorothioate internucleotide linkages at each of the 3′ and 5′ ends and corresponding to the initiation site of IK mRNA. A control oligonucleotide was also tested in parallel. A specific decrease of IK transcripts was correlated with an increase of HLA-DR antigen expression level. In colony-forming assays, IK antisense oligonucleotide inhibited colony formation by multilineage early erythroid and granulomonocytic CD34+ progenitors. The mean colony size was decreased 70% by IK antisense oligonucleotide in comparison to controls. These results provide evidence that the IK molecule participates in the regulation of HLA-DR expression on hematopoietic cells and plays a role in growth factor–dependent CD34+ cell proliferation and differentiation by modulating HLA-DR expression.
HEMATOPOIESIS IS A multistep cell proliferation and differentiation process that underlies the production of highly specialized cells from stem and progenitor cells. This complex process is regulated by a cascade of both positive and negative signals mediated by cell-cell contacts and cytokine-receptor interactions.1,2 Due to recent progress in growth factor biology and the development of hematopoietic progenitor cell assays, several molecules have been identified that have a role in the regulation of hematopoiesis,3 4 and their genes have been cloned.
We have recently cloned the cDNA of factor IK,5 so named because it inhibits interferon gamma (IFN-γ)-induced expression of HLA class II antigen and was originally isolated and purified from the conditioned culture medium of the K562 erythroleukemic cell line.6 IK also inhibits HLA class II constitutive expression.7
It has been suggested that expression of the HLA-DR antigen, one of three distinct membrane glycoproteins of major histocompatibility complex (MHC) class II, is associated with hematopoietic differentiation.8-15 Although recent observations have documented the existence of differences in HLA-DR expression between fetal and adult primitive hematopoietic progenitor cells,10-12 there is some evidence suggesting that the HLA-DR antigen is associated with the proliferative potential of hematopoietic progenitors.13,14 Caux et al15 have shown that expression of both CD34 and HLA-DR antigens was gradually lost in response to interleukin-3 (IL-3) and that the decrease in HLA-DR expression was associated with a loss in the proliferative capacity of hematopoietic progenitor cells. Therefore, as an inhibitor of HLA-DR expression, we postulated that IK could act on hematopoietic progenitor proliferation and differentiation. We therefore examined IK expression in CD34+ progenitor cells purified from human umbilical cord blood and investigated its possible function in hematopoiesis using an antisense method. Our present results show that IK is expressed in CD34+ hematopoietic progenitors, and that its expression can be upregulated during growth factor–induced differentiation. Furthermore, specific inhibition of IK expression by antisense oligodeoxynucleotides (or oligonucleotides) resulted in increased HLA-DR expression and inhibition of growth factor–dependent colony formation, indicating that IK expression was required for in vitro CD34+ progenitor proliferation and differentiation.
MATERIALS AND METHODS
Cell line.The RJ2.2.5 cell line, a gift from R. Accolla (ABC, Genoa, Italy), was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Biological Industries, ATGC, Noisy-le-Grand, France). This human B-lymphocyte cell line, an HLA class IL–negative RAJI cell line mutant, was previously identified as IK-positive7 and used as a positive control for IK expression analysis.
Purification of CD34+ cells from human cord blood.Hematopoietic progenitors express CD34 antigen; this property allows their purification by anti-CD34 antibody panning. Briefly, low-density (LD) mononuclear cells from human cord blood were isolated by Ficoll-Hypaque (1.077 g/mL; Seromed, Berlin, Germany) centrifugation. After two washes, mononuclear cells were separated by Percoll (1.070 g/mL; Pharmacia, Uppsala, Sweden), centrifuged, and further depleted of monocytic cells by two to three adhesion periods of 1 hour in plastic culture flasks. Nonspecific fixation was blocked by incubating cells with 0.5% (wt/vol) heat-inactivated purified human Ig (Sandoz globulin; Sandoz, Basel, Switzerland) for 15 minutes at room temperature. CD34+ progenitors were then enriched by incubation on an anti-CD34 antibody (ICH3)-coated CELLector flask (Applied Immune Science, [AIS] Inc, Menlo Park, CA) for 1 hour at room temperature. Nonadherent cells (CD34− and CD34low) were eliminated by at least seven washes with phosphate-buffered saline (PBS). Adherent CD34high (CD34+) cells were recovered by incubation in Iscove's modified Dulbecco's medium (IMDM) containing 10% FCS for 1 hour at 37°C, gentle scraping, and centrifugation. In some experiments, CD34+ cells were further divided by anti–HLA-DR antibody panning on a surface-activated CELLector flask (AIS) in two subsets: CD34+DRlow and CD34+DRhigh.
Antigen expression analysis by flow cytometry.Multiparameter analysis of purified cells for cell-surface antigen expression was performed as follows: 2.5 × 104 cells were incubated with 2 μg (saturating concentration) fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) at 4°C for 30 minutes in 40 μL PBS/2% bovine serum albumin (BSA) and washed twice before flow cytometric analysis. The MoAbs were CD34 (8G12 PE; Becton Dickinson, Mountain View, CA) and HLA-DR (L243 FITC; Becton Dickinson). As a negative control, each MoAb was replaced by murine IgG of the same isotype. Flow cytometric analysis was performed on a Profile II Coulter counter (Coulter Immunology, Hialeah, FL). In double staining, adjustment of the crossover of fluorescence signals was obtained by compensation of the two single-stained samples to limit the superposition of fluorochrome emission spectra. Forward light-scattering and two fluorescence signals were determined for each cell and stored in list-mode data files. Each measurement contained 5,000 cells.
RNA isolation and cDNA synthesis.Total RNA was extracted from either freshly isolated cells or cells treated with antisense oligonucleotide or control oligonucleotide by the following technique. Cells (2 × 104) were homogenized with 200 μL TRIzol reagent (GIBCO BRL, Cergy-Pontoise, France), 40 μL chloroform was then added, and the mixture was centrifuged at 12,000g for 15 minutes at 4°C. The aqueous phase was transferred to a new tube, and an equal volume of cold isopropanol was added to precipitate the RNA for 2 hours at −20°C. After centrifugation, the RNA pellet was washed with 75% ethanol and then dissolved in RNase-free water. RNA solution was heated at 65°C for 10 minutes and quickly chilled on ice. Reverse transcription was performed for 1 hour at 39°C in 1× RT buffer (GIBCO BRL), 10 mmol/L dithiothreitol (GIBCO BRL), 2.5 μmol/L poly(d)T12-18 primer (Pharmacia, Saclay, France), 1 mmol/L dNTPs (Promega, Madison, WI), 200 U M-MLV reverse transcriptase (GIBCO BRL), and 8 U RNasin (Promega).
Polymerase chain reaction.Semiquantitative polymerase chain reaction (PCR) was performed in a final volume of 25 μL; 2 μL cDNA reaction mixture was added to 1× PCR buffer (Boehringer, Mannheim, Germany), 200 μmol/L each of the four dNTPs (Promega), 1 μmol/L of each primer, 1.25 U Tag DNA (Boehringer), and 0.1 μCi [α32P]dCTP (NEN Research Products, Boston, MA). Conditions for the PCR were as follows: 94°C for 1 minute, 68°C for 2 minutes, and 72°C for 3 minutes. For glyceraldehyde phosphodehydrogenase (GAPDH), which was used as a control, the PCR was set at 94°C for 1 minute, 58°C for 1.5 minutes, and 72°C for 2 minutes. Control reactions were performed to ensure that the conditions used were within the linear range of PCR amplification for all samples tested. PCR products were separated on 5% polyacrylamide gels and autoradiographed before quantification on a Molecular Dynamics Phosphorimager. For each experiment, the PCR was repeated in at least two different samples, and typical results are presented.
The primers were synthesized on an Oligo 1000 DNA synthesizer (Beckman). The following primers were used for IK amplification: upper, 5′CGGGGGAAGCTGGAAGAGAAGAAACCTCCTGACGCTGACA (position 1 to 40), and lower, 5′TTCCACTGGCGATCAAGCTCTGCTTTGTCATTGGTTTCCT (position 612 to 652).
The product derived from these primers contains the sequence corresponding to the target site of IK antisense oligonucleotide. They were therefore suitable for detection of IK mRNA degradation caused by RNase H when it was in the complementary complex with the oligonucleotide.16 Primer sequences for GAPDH amplification were as follows: upper, 5′GGTGAAGGTCGGAGTCAACGGA (position 76 to 97), and, lower, 5′GAGGGATCTCGCTCCTGGAAGA (position 273 to 294).
Antisense and control oligonucleotides.To test the validity of this antisense approach, some preliminary precautions were taken. Computer alignment of the relevant protein sequences that have homology with the IK sequence and were accessible from Genebank was performed. Specificity of the IK antisense oligonucleotide is therefore underlined by the lack of homology with other proteins and particularly since no homologies with the start codon region were detected.
Our choice of the antisense oligonucleotides was based on calculation of IK mRNA secondary structures with the computer software RNAFOLD (Infobiogen, Villejuif, France). These oligonucleotide sequences were also selected for their inability to produce low-energy internal complexes and self-complementarities. An evaluation of the Tm(s) of the proposed complex was taken into account. As a result, antisense oligonucleotide AS1 is complementary to the region of the translation initiation codon of IK mRNA (position −5, +19) and AS2 is complementary to the position 169 to 195 of IK mRNA.
As a control, we used oligonucleotides with the same length and base composition but with reverse chain direction.17 Only the ends of the dinucleotide sequences were changed to have the same compositions of nucleotide phosphorothioates for the antisense as for control oligonucleotides, allowing the proposed nuclease digestion.
The antisense and control oligonucleotides were synthesized and purified by Genset (Paris, France). Their sequences were as follows: antisense oligonucleotide AS1, TpsGpsTCTTCAAAAATATTCATGTCpsApsG; control NS1, CpsGpsTTGTACTTATAAAAACTTCTpsApsG; antisense oligonucleotide AS2, TpsCpsTCTTCTTTTCCTCTTCTCTCTCpsTpsC; and control NS2, TpsCpsTTCTTTCTTCTCCTCTTTCCCTpsTpsC.
Colony assays.CD34+ cells were cultured in semisolid medium to assess the effects of antisense oligonucleotide on the proliferating and differentiating capacity of CD34+ progenitors. Five hundred purified cells were plated in 35-mm dishes (Becton Dickinson) in 1 mL IMDM medium (ICN, Orsay, France) containing 1.1% methylcellulose (Fluka, Buchs, Switzerland), 20% preselected fetal calf serum (Flow Laboratories, Irvine, CA), 1% BSA (Sigma, St Louis, MO), 100 μmol/L 2-mercaptoethanol (Sigma), 1% L-glutamine (GIBCO BRL), 1% antibiotic (Genzyme, Boston, MA), 2 U/mL EPO (Dompe Biotechnology, Milan, Italy), 100 U/mL IL-3 (Sandoz), 100 U/mL IL-1β (Genzyme), 100 U/mL IL-6 (Genzyme), 100 U/mL granulocyte/macrophage colony-stimulating factor (GM-CSF; Sandoz), 100 U/mL G-CSF (Dompe Biotechnology), and 2 U/mL stem cell factor (SCF; Genzyme). We have previously established that this cytokine combination is optimal for maximal colony formation from either purified cord blood or bone marrow CD34+ cells.
Duplicate cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 . The colony-forming units (CFU; >50 cells) were scored using an inverted microscope after 10 to 18 days of culture. Immature burst-forming units-erythroid (iBFU-E) and mature BFU-E (mBFU-E) were scored using criteria already described.18 CFU-granulocyte/macrophage (CFU-GM) colonies with high cell density and greater than 0.5 mm in diameter were scored as large CFU-GM, and CFU-GM colonies with lower cell density and smaller than 0.5 mm in diameter were scored as small CFU-GM.
Cell treatment with antisense oligonucleotides.A dose-dependent assay was performed on CD34+ cells to verify that the oligonucleotides used did not cause toxicity. For this purpose, cell viability as assessed by trypan blue exclusion was determined after incubating the CD34+ cells for 6 days in liquid medium containing growth factors and oligonucleotides at the following concentrations: 2.5, 5, 10, 20, and 40 μmol/L.
To assess the effects of IK antisense oligonucleotides on CD34+ cell colony formation, antisense or control oligonucleotides were directly added to the semisolid medium on day 0 at a final concentration of 10 μmol/L. The effect of the oligonucleotides on the proliferative capacity of primitive hematopoietic progenitors has also been tested by counting the number of high–proliferative potential colony-forming cells (HPP-CFC) obtained when CD34+ cells were plated after a preincubation with IK antisense or control oligonucleotides for 2 weeks in liquid medium containing growth factors.
To investigate the effect of IK antisense oligonucleotide treatments on IK mRNA levels and HLA-DR antigen expression, purified CD34+ cells were cultured for 1 to 7 days at 37°C and 5% CO2 in IMDM medium supplemented with 10% FCS and growth factors (as indicated in the results) either with or without IK antisense or control oligonucleotides at 10 and 20 μmol/L.
RESULTS
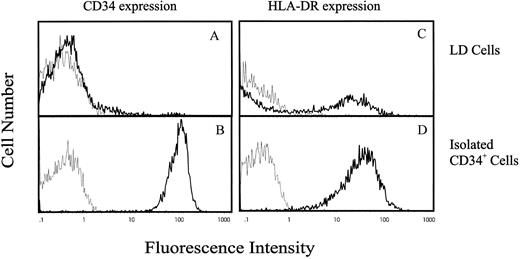
IK transcript is expressed in CD34+ hematopoietic progenitor cells, and expression is inversely correlated with HLA-DR antigen expression.To evaluate IK expression in human hematopoietic progenitor cells, CD34+ cells were isolated from either umbilical cord blood or bone marrow by density centrifugation followed by anti-CD34 antibody panning. Using this method, we can reproducibly obtain CD34+ cells expressing a high level of CD34 antigen (79.4 AU) with a purity ranging from 97% to 99% (Fig 1B). The majority of these purified CD34+ cells also expressed HLA-DR antigen (54.8 AU; Fig 1D) as compared with LD differentiated mononuclear cells, in which only 30% expressed HLA-DR antigen with a lower intensity (21.5 AU; Fig 1C). Since only a low proportion of CD34+ cells are found in either cord blood or bone marrow (≤0.1%), we used the sensitive RT-PCR technique to investigate IK mRNA expression in these cells. Total RNA was extracted from 2 × 104 cells and reverse-transcribed with poly(d)T12-18 oligonucleotides as primer. The resulting cDNAs were amplified by semiquantitative PCR using primers specific for a 692-base region of the IK mRNA, and using primers specific for a 240-base region of the GAPDH mRNA as an internal control for each sample. RJ2.2.5 cells were used as a positive control for analysis of IK expression. The negative control was the product of reverse transcriptase where RNA was omitted.
Flow cytometric analysis of CD34 and HLA-DR antigen expression on LD and CD34+ cells purified from human cord blood. The vertical axis indicates the relative number of cells on a linear scale, and the horizontal axis indicates fluorescence on a logarithmic scale. (A) and (B) CD34 antigen expression and (C) and (D) HLA-DR antigen expression on LD mononuclear cells or purified CD34+ cells, respectively. Cells were incubated either with antihuman FITC-conjugated anti-HLA-DR MoAb or PE-conjugated anti-CD34 MoAb (━) or with negative control antibodies (─).
Flow cytometric analysis of CD34 and HLA-DR antigen expression on LD and CD34+ cells purified from human cord blood. The vertical axis indicates the relative number of cells on a linear scale, and the horizontal axis indicates fluorescence on a logarithmic scale. (A) and (B) CD34 antigen expression and (C) and (D) HLA-DR antigen expression on LD mononuclear cells or purified CD34+ cells, respectively. Cells were incubated either with antihuman FITC-conjugated anti-HLA-DR MoAb or PE-conjugated anti-CD34 MoAb (━) or with negative control antibodies (─).
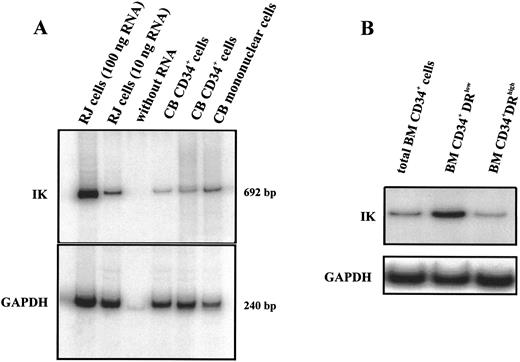
IK mRNA expression was detected in CD34+ cells purified from different cord blood samples (Fig 2A). Positive controls containing either 10 or 100 ng total RNA from RJ2.2.5 cells were included to ensure that for all tested samples amplification was in the linear region of the PCR curve. In comparison to LD mononuclear cells, CD34+ cells express lower IK transcripts (IK/GAPDH, 13.1% v 46.7%). Since the majority of mononuclear cells were composed of mature cells, it is reasonable that progenitors express less IK than differentiated hematopoietic cells. To verify this hypothesis, we picked cells from CFU-GM, CFU-G, CFU-M, and BFU-E colonies obtained from the proliferation and differentiation of CD34+ and analyzed IK expression. As expected, these cells expressed a higher level of IK transcripts than nondifferentiated CD34+ progenitor cells; this increase was not restricted to a specific lineage since cells from either myeloid or erythroid colonies expressed similar IK transcript levels (data not shown). We further examined IK expression in different subsets of CD34+ cells. CD34+DRlow cells purified from bone marrow cells expressed a higher level of IK transcripts than CD34+DRhigh cells (Fig 2B). Altogether, our results show that IK expression was inversely correlated with HLA-DR antigen expression in both CD34+ progenitors and differentiated hematopoietic cells.
IK mRNA expression in isolated CD34+ cells. RT-PCR products were analyzed by 5% polyacrylamide gel electrophoresis and autoradiographed. Positive controls (100 and 10 ng total RNA from IK-positive RJ cells) and a negative control (without RNA) were included to ensure that all tested samples were in the linear region of the PCR curve. GAPDH for each sample was amplified as an internal control. Signals were found at the expected sizes (692 bp for IK and 240 bp for GAPDH), indicating that the PCR was specific. (A) IK expression from LD mononuclear cells and 2 CD34+ cell samples purified from cord blood (CB). Scanning densitometry of the IK mRNA signal relative to the GAPDH mRNA signal shows the following values: lane 2 (RJ cells, 10 ng RNA), 24.6%; lanes 4 and 5 (CB CD34+ cells), 6.0% and 13.1%, respectively; lane 6 (CB mononuclear cells), 46.7%. (B) IK expression in total CD34+ cells purified from bone marrow and in 2 different subsets of medullar CD34+ cells: CD34+DRlow and CD34+DRhigh cells.
IK mRNA expression in isolated CD34+ cells. RT-PCR products were analyzed by 5% polyacrylamide gel electrophoresis and autoradiographed. Positive controls (100 and 10 ng total RNA from IK-positive RJ cells) and a negative control (without RNA) were included to ensure that all tested samples were in the linear region of the PCR curve. GAPDH for each sample was amplified as an internal control. Signals were found at the expected sizes (692 bp for IK and 240 bp for GAPDH), indicating that the PCR was specific. (A) IK expression from LD mononuclear cells and 2 CD34+ cell samples purified from cord blood (CB). Scanning densitometry of the IK mRNA signal relative to the GAPDH mRNA signal shows the following values: lane 2 (RJ cells, 10 ng RNA), 24.6%; lanes 4 and 5 (CB CD34+ cells), 6.0% and 13.1%, respectively; lane 6 (CB mononuclear cells), 46.7%. (B) IK expression in total CD34+ cells purified from bone marrow and in 2 different subsets of medullar CD34+ cells: CD34+DRlow and CD34+DRhigh cells.
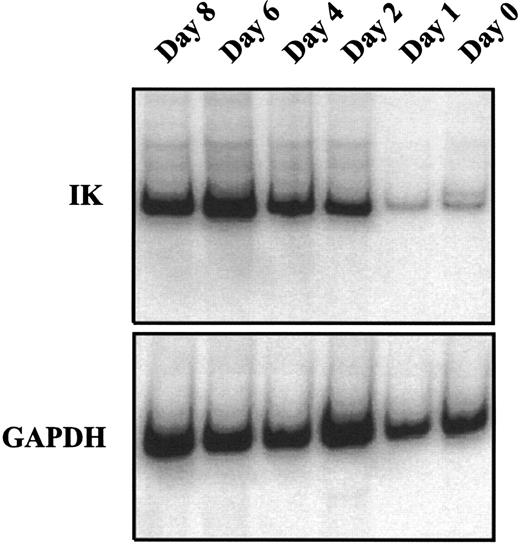
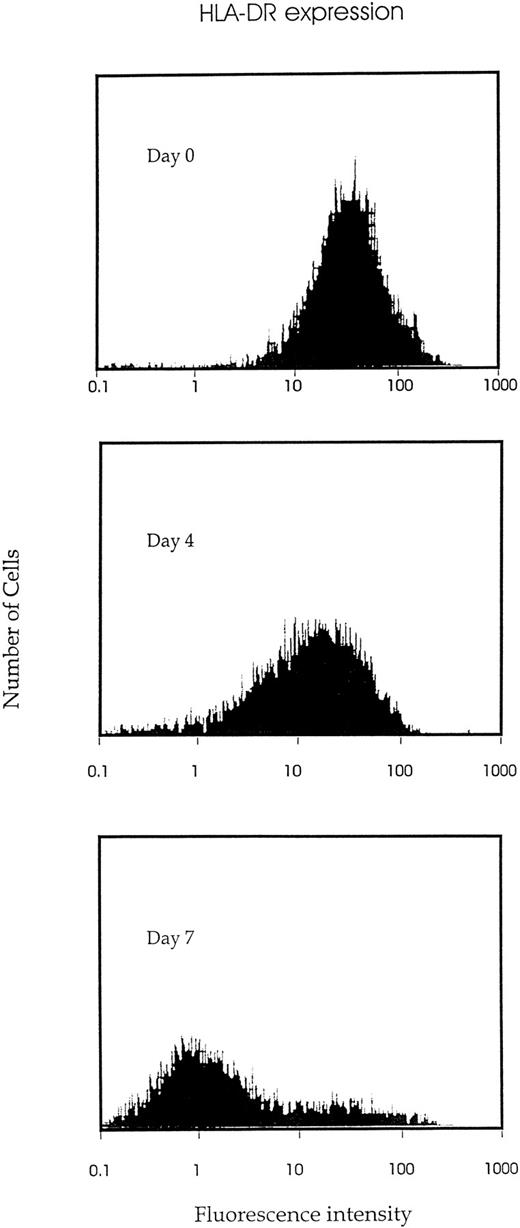
IK expression is associated with growth factor–induced proliferation and differentiation of CD34+ cells.We analyzed the time course of IK expression by CD34+ cells undergoing proliferation and differentiation in liquid cultures containing a combination of seven growth factors (IL-1, IL-3, IL-6, SCF, G-CSF, GM-CSF, and EPO). IK expression gradually increased with time and reached the highest level on day 6 of culture (Fig 3). Compared with freshly isolated CD34+ cells (day 0), IK expression was increased more than 15-fold on day 6 of culture (IK/GAPDH, 151.6% v 9.6%). This augmentation was correlated with a higher proportion of differentiated cells, since the percentage of promyeloblasts and monoblasts increased from 3% to 4% on day 0 to 25% on day 6. Furthermore, we show that HLA-DR antigen expression gradually decreased during proliferation and differentiation of CD34+ progenitors (Fig 4).
Kinetic study of IK mRNA expression during growth factor–induced CD34+ proliferation and differentiation. Isolated CD34+ cells were cultured in IMDM containing 10% FCS and a combination of 7 cytokines (IL-1, IL-3, IL-6, SCF EPO, G-CSF, and GM-CSF ). At the indicated times from day 0 to day 8, cells were harvested to study IK expression by semiquantitative RT-PCR. The ratio of IK to GAPDH signals for each sample was 9.6%, 11.2%, 35.9%, 103.6%, 151.6%, and 72.8% from day 0 to day 8.
Kinetic study of IK mRNA expression during growth factor–induced CD34+ proliferation and differentiation. Isolated CD34+ cells were cultured in IMDM containing 10% FCS and a combination of 7 cytokines (IL-1, IL-3, IL-6, SCF EPO, G-CSF, and GM-CSF ). At the indicated times from day 0 to day 8, cells were harvested to study IK expression by semiquantitative RT-PCR. The ratio of IK to GAPDH signals for each sample was 9.6%, 11.2%, 35.9%, 103.6%, 151.6%, and 72.8% from day 0 to day 8.
Kinetic study of HLA-DR antigen expression during growth factor–induced CD34+ proliferation and differentiation. Isolated CD34+ cells were cultured in IMDM containing 10% FCS and 7 cytokines (IL-1, IL-3, IL-6, SCF, EPO, G-CSF, and GM-CSF ). And the indicated time (days 0, 4, or 7), cells were harvested, stained by FITC-conjugated MoAb to HLA-DR, and examined for HLA-DR antigen expression by flow cytometric analysis.
Kinetic study of HLA-DR antigen expression during growth factor–induced CD34+ proliferation and differentiation. Isolated CD34+ cells were cultured in IMDM containing 10% FCS and 7 cytokines (IL-1, IL-3, IL-6, SCF, EPO, G-CSF, and GM-CSF ). And the indicated time (days 0, 4, or 7), cells were harvested, stained by FITC-conjugated MoAb to HLA-DR, and examined for HLA-DR antigen expression by flow cytometric analysis.
IK expression is decreased by IK antisense oligonucleotide treatment.Our results demonstrating that IK was expressed in CD34+ cells and that its expression was increased during differentiation suggested that IK might play a role in hematopoiesis. This hypothesis was evaluated by an oligonucleotide antisense method that has been previously used to address the function of autocrine or paracrine cytokines or other hematopoietic regulators in immunomodulation and hematopoiesis regulation.19,20 In the present study, we used partially phosphorothioated antisense oligonucleotides instead of the fully modified ones because the former have a lower resistance to nucleases than the latter and the partially modified oligonucleotides present less sequence-independent cytotoxicity than the fully modified ones.16 17 After 6 days in liquid culture containing growth factors, even at a final concentration of oligonucleotides as high as 20 μmol/L, cell viability assessed by the trypan blue exclusion test was higher than 95%. These data confirm that the oligonucleotides were not toxic to CD34+ cells.
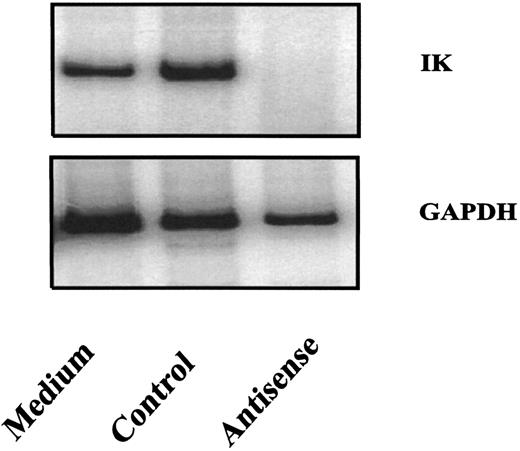
Several mechanisms are believed to be involved in the effects of antisense oligonucleotides in cells.21 22 One of these mechanisms is that the formation of oligonucleotide and target mRNA duplexes results in a degradation of the target mRNA by RNase H present in the cytoplasm. To determine whether IK antisense oligonucleotides used in this study could decrease IK expression, purified CD34+ cells were exposed to antisense or control oligonucleotides in liquid cultures containing IL-1, IL-3, and SCF. IK expression in purified CD34+ cells was inhibited after a 24-hour treatment with IK antisense oligonucleotides, but not with control oligonucleotides (Fig 5). Data are presented for AS1 and NS1.
Inhibition of IK mRNA expression by IK antisense oligonucleotides. Purified cord blood CD34+ cells were cultured in the presence or absence of IK antisense or control oligonucleotides (20 μmol/L). After 24 hours of culture, the cells were collected for analysis of IK mRNA expression by semiquantitative RT-PCR.
Inhibition of IK mRNA expression by IK antisense oligonucleotides. Purified cord blood CD34+ cells were cultured in the presence or absence of IK antisense or control oligonucleotides (20 μmol/L). After 24 hours of culture, the cells were collected for analysis of IK mRNA expression by semiquantitative RT-PCR.
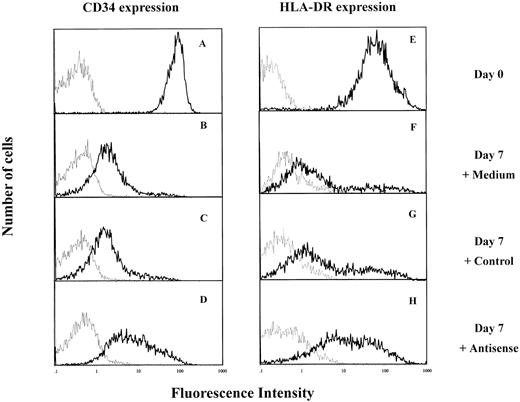
IK antisense oligonucleotides prevented the decrease of HLA-DR and CD34 antigen expression during CD34+ cell differentiation.Since IK was an inhibitor of HLA-DR expression, the possibility that the latter could be modulated by IK antisense oligonucleotides was examined. Purified CD34+ cells express HLA-DR (Figs 1B and 6E). When these cells are cultured in medium containing IL-1, IL-3, and SCF, they gradually lose CD34 and HLA-DR antigen expression with cell proliferation and differentiation11 (and from our results). After 7 days of culture in these experimental conditions, the majority of cells express low levels of CD34 antigen (Fig 6B and C) and HLA-DR antigen (Fig 6F and G) whether they are untreated (control medium) or treated with control oligonucleotides. However, in the presence of IK antisense oligonucleotides, most of the cells express high levels of CD34 and HLA-DR antigens (Fig 6D and H).
Effect of IK antisense oligonucleotides on HLA-DR and CD34 antigen expression by cord blood CD34+ cells. Isolated CD34+ cells were cultured in IMDM containing 5% FCS and a combination of 3 cytokines, IL-1, IL-3, and SCF, in the presence of IK antisense or control oligonucleotides. After 7 days of culture, cells were collected and stained either with antihuman FITC-conjugated anti-HLA-DR MoAb or PE-conjugated anti-CD34+ MoAb (━) or with negative control antibodies (─). (A and E) Freshly isolated CD34+ cells (day 0) CD34+ cells cultured for 7 days either in the absence of oligonucleotide (B and F ) or in the presence of control (20 μmol/L) (C and G) or antisense (20 μmol/L) (D and H) oligonucleotides.
Effect of IK antisense oligonucleotides on HLA-DR and CD34 antigen expression by cord blood CD34+ cells. Isolated CD34+ cells were cultured in IMDM containing 5% FCS and a combination of 3 cytokines, IL-1, IL-3, and SCF, in the presence of IK antisense or control oligonucleotides. After 7 days of culture, cells were collected and stained either with antihuman FITC-conjugated anti-HLA-DR MoAb or PE-conjugated anti-CD34+ MoAb (━) or with negative control antibodies (─). (A and E) Freshly isolated CD34+ cells (day 0) CD34+ cells cultured for 7 days either in the absence of oligonucleotide (B and F ) or in the presence of control (20 μmol/L) (C and G) or antisense (20 μmol/L) (D and H) oligonucleotides.
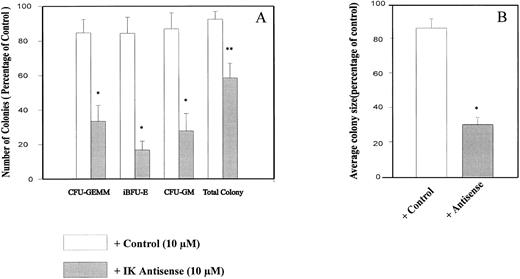
IK antisense oligonucleotides impaired growth factor–dependent CD34+ colony formation in semisolid medium and CD34+ differentiation in short-term liquid culture.To determine the possible role of endogenous IK in regulating normal hematopoiesis, we plated cord blood CD34+ cells in semisolid medium containing a combination of seven growth factors plus either IK antisense or control oligonucleotides or control medium. In our experimental conditions, the cloning efficiency of untreated control CD34+ cells purified from cord blood was 40.2% ± 2.9%. Indeed, from 500 plated CD34+ cells, we could obtain 10.3 ± 2.1 CFU-GEMM, 50.1 ± 9.1 iBFU-E, 21.2 ± 3.1 mBFU-E, 39.8 ± 5.9 large CFU-GM, and 70.0 ± 7.4 small CFU-GM. The total colony number was decreased by antisense oligonucleotide (AS1) in comparison to control treatment (MS1) (41.5% ± 8.5% v 7.8% ± 4.3%, P = .0420, n = 5; Fig 7A). This reduction is mainly due to the inhibition of colony formation from primitive progenitors, since the number of CFU-GEMM, iBFU-E, and large CFU-GM was decreased by IK antisense oligonucleotide treatment in comparison to the control (66.5% ± 9.3% v 15.3% ± 7.7%, P = .0195, n = 5; 83.2% ± 5.0% v 16.9% ± 8.4%, P = .0043, n = 5; 72.3% ± 9.8% v 13.5% ± 9.0%, P = .0079, n = 5). We obtained similar results with AS2 and MS2 oligonucleotides. These results showed that addition of IK antisense oligonucleotides (AS1 or AS2) but not of control oligonucleotides (MS1 or MS2) to purified CD34+ cell cultures resulted in an inhibition of colony formation, especially multilineage, early erythroid, and granulomonocytic colonies. No significant change in the percentage of small granulocytic or erythroid colonies was observed after IK antisense oligonucleotide treatment in comparison to the control. Interestingly, we found that antisense oligonucleotide treatment mainly inhibited the granulocytic component of CFU-GM, but had no obvious effect on the macrophage component (data not shown). Whatever the cytologic type of the colony (CFU-GEMM, CFU-GM, or BFU-E), the mean size was also remarkably decreased by antisense oligonucleotide treatment from 16.1 ± 5.7 × 103 and 14.8 ± 6.59 × 103 in the presence of medium alone or control oligonucleotides, respectively, to 4.38 ± 1.83 × 103 in the presence of IK antisense oligonucleotide (P = .0067; Fig 7B).
Inhibition of CD34+ colony formation by IK antisense oligonucleotides. CD34+ cells at 500/mL/dish were plated in semisolid media containing appropriate growth factors in the presence or absence of antisense/control oligonucleotides (10 μmol/L/mL). After 14 days of culture, colonies were scored. Results are expressed as a percentage of the control, ie, in the absence of oligonucleotide, for colony count (A) and colony size (B). The number of colonies obtained with the control culture were 10.3 ± 2.1 CFU-GEMM, 50.1 ± 9.1 iBFU-E, 21.2 ± 3.1 mBFU-E, 39.8 ± 5.9 large CFU-GM, and 70.0 ± 7.4 small CFU-GM. In control medium, the mean colony size was 16.1 ± 1.9 × 103 cells. Student's t test for paired samples *P < .05, **P < .001 (n = 5).
Inhibition of CD34+ colony formation by IK antisense oligonucleotides. CD34+ cells at 500/mL/dish were plated in semisolid media containing appropriate growth factors in the presence or absence of antisense/control oligonucleotides (10 μmol/L/mL). After 14 days of culture, colonies were scored. Results are expressed as a percentage of the control, ie, in the absence of oligonucleotide, for colony count (A) and colony size (B). The number of colonies obtained with the control culture were 10.3 ± 2.1 CFU-GEMM, 50.1 ± 9.1 iBFU-E, 21.2 ± 3.1 mBFU-E, 39.8 ± 5.9 large CFU-GM, and 70.0 ± 7.4 small CFU-GM. In control medium, the mean colony size was 16.1 ± 1.9 × 103 cells. Student's t test for paired samples *P < .05, **P < .001 (n = 5).
When CD34+ cells (5 × 103) were cultured in liquid medium supplemented with 20% FCS plus the seven factors, the total number of cells recovered after 6 days of culture was fivefold lower in the presence of antisense oligonucleotides (1.8 ± 0.3 × 104) as compared with untreated CD34+ cells (9.4 ± 1.2 × 104) or CD34+ cells treated with control oligonucleotides (8.1 ± 0.9 × 104). Furthermore, the percentage of differentiated cells (mainly promyelocytes) is also significantly decreased in the presence of antisense oligonucleotides (9% ± 1%) in comparison to untreated or mock-treated CD34+ cells, in which the percentage of promyelocytes reached 25% ± 6% on day 6 of culture. However, when CD34+ cells were plated in semisolid medium after oligonucleotide pretreatment for 2 weeks in liquid medium containing seven growth factors, the number of high proliferative potential colony-forming cells (HPP-CFC) obtained was higher in culture pretreated with IK antisense oligonucleotide (21.5 ± 4.9) compared with control oligonucleotide (10.5 ± 2.1) or control cultures (8.5 ± 0.5).
DISCUSSION
Although the role of MHC molecules in antigen presentation is well defined, their function in hematopoiesis is less well understood. CD34+ lymphohematopoietic progenitors express the three MHC class II molecules (HLA-DR, HLA-DQ, and HLA-DP) with different intensities, with HLA-DR showing the highest expression on these progenitor cells.15 It has been suggested that expression of HLA-DR is associated with the regulation of hematopoietic progenitor cell proliferation and differentiation.13,14 By using neutralizing MoAbs anti–HLA-DR, Mascle et al23 have shown that fixation of the antibody on the HLA-DR molecule induced an augmentation of thymidine uptake by bone marrow cells and increased formation of CFU-GM colonies in semisolid medium. More recently, Hong et al24 have demonstrated that the addition of anti-MHC class II MoAbs resulted in a dose-dependent decrease of CFU-GM precursors in long-term bone marrow culture but had no effect on CFU-GM culture in agar, suggesting that additional factors including stromal cells and cytokines might also be involved in the anti-MHC class II–mediated hematopoiesis inhibition. Although these two studies might seem contradictory, they showed a correlation between the presence of HLA-DR and the ability for colony formation by hematopoietic progenitors. However, until now, there has not been any direct evidence linking HLA-DR expression and hematopoietic differentiation.
Previous results from our group have shown that IK mRNA could be detected in a hematopoietic cell line5; we presently report that IK is expressed in mononuclear cells and in CD34+ progenitors purified from either cord blood or bone marrow (Fig 2A). Furthermore, our data indicate that IK transcription also occurs in a subpopulation of primitive CD34+ cells expressing a low level of HLA-DR antigen (CD34highDRlow, Fig 2B). IK expression is inversely correlated with HLA-DR antigen expression on both CD34+ progenitors and mature hematopoietic cells. Analysis of IK mRNA and HLA-DR antigen expression during the in vitro growth factor–induced hematopoietic proliferation and differentiation process showed that IK expression is gradually increased (Fig 3) while HLA-DR expression is progressively lost (Fig 4). These results are in agreement with the fact that CD34+ cells express a lower level of IK and a higher level of HLA-DR than mononuclear cells, which are mainly composed of differentiated cells.
To further investigate the correlation between increased IK and decreased HLA-DR expression and its possible role in hematopoiesis, we used the antisense oligonucleotide (AS1) corresponding to the region of the translation initiation codon of IK mRNA. We showed that AS1 oligonucleotide treatment induced a specific inhibition of IK transcripts (Fig 5). Treatment of CD34+ cells by the same antisense oligonucleotide, which was not toxic for CD34+ cell growth, also impaired colony formation, suggesting that IK is required for the in vitro growth factor–dependent CD34+ progenitor growth.
The sequence-dependent effects of the IK antisense oligonucleotide AS1 are supported by the following results. First, sequence-specific degradation of IK mRNA is observed in the presence of the antisense oligonucleotide, but not in the presence of control oligonucleotide (Fig 5); it therefore likely results from RNAse H action as a common mechanism of the inhibition of mRNA expression.25 Second, antisense oligonucleotide AS1 treatment prevented, at least partially, the decrease of HLA-DR during hematopoietic differentiation of CD34+ progenitors, in contrast to control oligonucleotide NS1 (Fig 6); this result is in agreement with the previously reported effect of IK on the inhibition of both IFN-γ–induced and constitutive expression of HLA class II.6 7 Third, antisense oligonucleotide AS1 has a significant effect on colony formation, especially from primitive hematopoietic progenitors, in comparison to control oligonucleotide NS1 (Fig 7). Fourth, antisense oligonucleotide AS2, corresponding to another site (position +169, +194) of IK mRNA, has an effect on HLA-DR expression (data not shown) and colony formation similar to the effect of AS1, whereas no effect was found with NS2.
Our results clearly show that IK downregulates HLA class II expression during in vitro growth factor–induced hematopoietic proliferation and differentiation, and is involved in hematopoietic differentiation. This leads us to raise the question of whether these two effects are linked. Although it is still too early to validate this hypothesis, several lines of evidence support the assumption that IK might be a key molecule in the hematopoietic differentiation process: (1) during growth factor–induced differentiation of CD34+ cells, IK expression is increased and is associated with a gradual loss of HLA-DR expression; (2) in preliminary experiments using purified recombinant IK fusion protein (1 μg/mL), we have been able to induce differentiation of the myeloid HL60 cell line as shown by an increased percentage of CD14 antigen–expressing cells (from 1% to 35%) 6 days after IK treatment, (3) IK antisense oligonucleotide treatment delayed the decrease of both HLA-DR and CD34 antigen expression, which both can be considered differentiation markers; (4) after 7 days in liquid culture containing growth factors, control untreated CD34+ cells showed a more differentiated cytology than cells treated with IK antisense oligonucleotides, and (5) since differentiation is correlated with the progenitor proliferative capacity, at least in the progenitor cell compartment, the decrease in CD34+ colony formation by IK antisense oligonucleotides could result from an inhibition of CD34+ differentiation; our result showing that the number of HPP-CFC is higher in IK antisense oligonucleotide–treated culture supports this hypothesis.
Taken together, our results provide evidence that IK is a molecule implicated, via HLA class II downregulation, in vitro CD34+ progenitor proliferation and differentiation. However, the molecular mechanism supporting this effect is not yet understood. According to the traditional view of autocrine or paracrine cytokine binding, fixation of the ligand to its extracellular receptor induces a cascade of downstream transduction signals responsible for activation of transcription factors and subsequent mitogenic responses. It would therefore be expected that addition of exogenous IK could abrogate the effect of IK antisense oligonucleotides. However, addition of recombinant IK, even at a high concentration (10 μg/mL), at the same time as IK antisense oligonucleotide did not reverse the inhibition of colony formation induced by antisense oligonucleotide treatment (data not shown). For this reason, it is more likely that IK acts on CD34+ progenitor differentiation by a synergistic or agonistic effect with exogenous growth factors. We have recently demonstrated that overexpression of IK inhibits HLA class II and CIITA class II transactivator expression.7 Thus, IK may also act on hematopoietic differentiation by intracellular modulation of constitutive HLA class II via inhibition of CIITA expression. Therefore, in CD34+ progenitors, the maintenance of HLA class II expression resulting from IK antisense oligonucleotide treatment may impair HLA class II–mediated signals involved in the hematopoietic differentiation and proliferation process.
ACKNOWLEDGMENT
We are greatly indebted to Dr Masse for the continuous supply of bone marrow samples and to Dr Charpentier for the cytological analysis.
Supported by INSERM, ARC (6288), and ANRB.
Address reprint requests to Marie-Caroline Le Bousse-Kerdilès, PhD, INSERM U268, Hôpital Paul Brousse, 14 Avenue Paul Vaillant Couturier, 94807 Villejuif, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal