Abstract
Erythropoietin (Epo) induces a dose-dependent increase in intracellular free Ca2+ ([Ca2+]i ) in human erythroblasts, which is dependent on extracellular Ca2+ and blocked by high doses of nifedipine or Ni2+. In addition, pretreatment of human erythroblasts with mouse antihuman erythropoietin receptor antibody but not mouse immunopure IgG blocked the Epo-induced [Ca2+]i increase, indicating the specificity of the Ca2+ response to Epo stimulation. In this study, the erythropoietin-regulated calcium channel was identified by single channel recordings. Use of conventional whole cell patch-clamp failed to detect Epo-induced whole cell Ca2+ current. To minimize washout of cytosolic constituents, we next used nystatin perforated patch, but did not find any Epo-induced whole cell Ca2+ current. Using Ba2+ (30 mmol/L) as charge carrier in cell-attached patches, we detected single channels with unitary conductance of 3.2 pS, reversal potential of +72 mV, and whose unitary current (at +10 mV) increased monotonically with increasing Ba2+ concentrations. Channel open probability did not appreciably change over the voltage range (−50 to +30 mV) tested. Epo (2 U/mL) increased both mean open time (from 4.27 ± 0.75 to 11.15 ± 1.80 ms) and open probability (from 0.26 ± 0.06 to 2.56 ± 0.59%) of this Ba2+-permeable channel. Our data strongly support the conclusion that the Epo-induced [Ca2+]i increase in human erythroblasts is mediated via Ca2+ entry through a voltage-independent Ca2+ channel.
ERYTHROPOIETIN (Epo) is a glycoprotein growth factor that is obligatory for the proliferation and differentiation of erythroid progenitor and precursor cells. Both the murine1 and human2,3 erythropoietin receptor (Epo-R) have been cloned and found to belong to a new superfamily of receptors, which includes receptors for interleukin-2 (IL-2), IL-3, IL-4, IL-5, IL-7, granulocyte macrophage-colony stimulating factor (GM-CSF ), and G-CSF.4,5 The mechanism of signaling through the cytokine receptor family is currently a major focus of investigation.6 With respect to Epo-R, following Epo binding, increased [Ca2+]i7-9and cAMP levels,10,11 stimulation of protein kinases12including tyrosine kinases13,14 and serine and threonine kinases,15,16 activation of the ras pathway17 and of nuclear protein kinase C,18 and change in protooncogene expression19-21 have been reported to occur. Focusing on Epo-induced increase in [Ca2+]i , using digital video imaging of single, fura-2–loaded human burst forming unit-erythroid (BFU-E)-derived erythroblasts,7 we have previously demonstrated that the [Ca2+]i increase is specific for stage of differentiation,8 blocked by pertussis toxin11 or the tyrosine kinase inhibitor genistein,14 dependent on the presence of extracellular Ca2+, inhibited by high doses (10 to 50 μmol/L) of nifedipine or the nonspecific Ca2+ channel blocker Ni2+,22 not associated with increases in inositol-1,4,5-trisphosphate (IP3 ) or inositol-1,3,4,5-tetrakisphosphate (IP4 ),22 and that the Ca2+ signal is transmitted to the nucleus.23 The characteristics of the Epo-induced [Ca2+]i increase are most consistent with Ca2+ entry via a voltage-independent Ca2+ channel rather than IP3 mobilization of intracellular sequestered Ca2+.22 However, earlier attempts to detect an Epo-induced increase in whole cell Ca2+ current were not successful.22 Considering that important cytosolic constituents may have been washed out by internal dialysis (unavoidable in the conventional whole cell configuration) or that whole cell patch-clamp may not have the required sensitivity to detect low magnitude whole cell Ca2+ currents (especially in a small human erythroblast of diameter ≈ 10 μm), in the current study, we applied, for the first time, a perforated patch technique24 and single channel recording25 26 to human BFU-E–derived erythroblasts.
MATERIALS AND METHODS
Preparation of BFU-E–derived erythroid precursors.Adult peripheral blood was obtained according to a protocol approved by the Milton S. Hershey Medical Center Committee on Clinical Investigation. Adult blood BFU-E were partially purified by 2 aminoethylisothiouronium bromide hydrobromide-treated sheep red blood cell (RBC) rosetting, adherence to plastic, and panning, as described previously.7,8 Partially purified mononuclear cells were cultured in 0.9% methylcellulose media containing 30% fetal calf serum (FCS), 9.0 mg/mL deionized bovine serum albumin (BSA) (Cohn fraction V; Sigma, St Louis, MO), 1.4 × 10−4mol/L β-mercaptoethanol and 2 U/mL recombinant Epo (>100,000 U/mg; Cat #287-TC, R & D Systems, Minneapolis, MN). Single BFU-E, when cultured in methylcellulose, proliferate and differentiate over 14 days to large colonies containing 1 to 5 × 104 mature erythroblasts. Cells from maturing BFU-E–derived colonies were plucked from culture on day 10. Day 10 cells are partially hemoglobinized and proliferative capacity is decreased because minimal additional increase in colony size occurs subsequently. Day 10, but not day 7, cells respond to Epo with an increase in [Ca2+]i .8 22 Myeloid colonies represented less than 1% of hematopoietic colonies cultured from partially purified peripheral blood mononuclear cells prepared as described.
Measurement of [Ca2+]i by digital video imaging.BFU-E–derived cells were removed from culture on day 10, labeled with antihuman β2 microglobulin (Boehringer Mannheim, Indianapolis, IN), and bound to antimouse Ig-coated glass coverslips as described.8,22 Cell viability, as judged by trypan blue exclusion, was greater than 98%. Erythroblasts were incubated in phosphate-buffered saline (PBS) (in mmol/L: NaCl 137, KCl 2.7, MgCl2 0.49, CaCl2 0.68, Na2HPO4 4.3, KH2 PO4 1.5, pH 7.4) containing glucose (11.1 mmol/L), and exposed to fura-2 acetoxymethyl ester (2 μmol/L; Molecular Probes, Inc, Eugene, OR) for 20 minutes at 37°C. Total time lapse from removal of cells from culture to completion of fura-2 loading was 3 to 5 hours. Fura-2–loaded cells in PBS (37°C) were visualized with the digital video imaging system previously described,7,8,11,14,22,23 except that the light source was changed from the Spex dual-wavelength spectrofluorometer (Spex Industries, Edison, NJ) to a much more compact, filter-based (± 10 nm bandpass) system (Ionoptix, Milton, MA). To minimize photobleaching, excitation light was applied only during data acquisition. Paired 350- and 380-nm epifluorescence (emission filter 505 ± 20 nm) cell images (collected by Nikon Fluor 100x/1.30 NA oil objective) were obtained before and at 1, 5, 10, 15, and 20 minutes after Epo (2 U/mL) addition. The dose of Epo (2 U/mL) used in this study has been shown to elicit maximal [Ca2+]i increase and optimally support human erythroblast maturation in vitro.8 In this series of experiments, we did not convert fura-2 fluorescence intensity ratio (F350/F380) into [Ca2+]i . We have previously demonstrated that the fluorescence properties of fura-2 salt in solution and intracellular fura-2 (loaded by ester permeation) were similar.8 In addition, it can be shown mathematically that at low [Ca2+]i (20 to 300 nmol/L) typically measured in human erythroblasts, ▵[Ca2+]i is proportional to ▵F350/F380 (see Appendix).
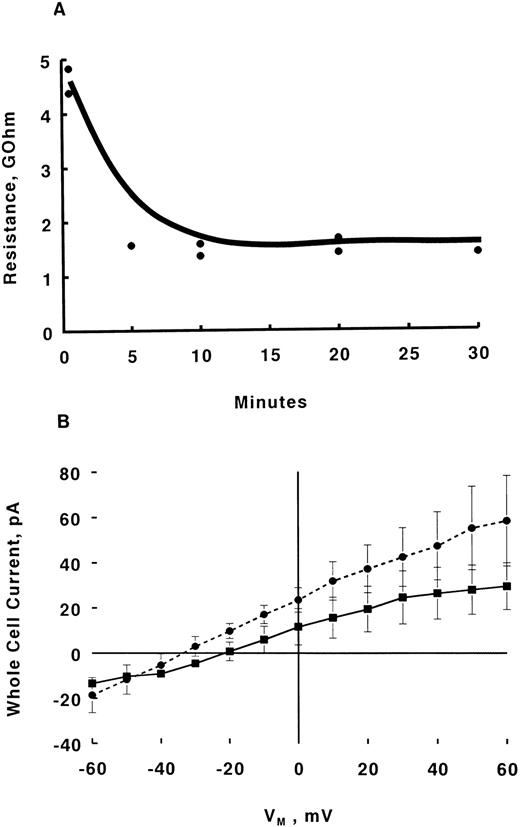
Simultaneous measurement of [Ca2+]i and whole cell current in early erythroid precursor cells. Day 10 BFU-E–derived cells bound to glass coverslips were bathed in PBS containing glucose. Whole cell patch-clamp recordings were performed at 22°C as described by Hamill et al25 and adapted by us for human erythroblasts.22 Briefly, borosilicate glass (No. 1B150-4; World Precision Instruments, Sarasota, FL) micropipettes (1 to -2 μm diameter) were fabricated, fire-polished, and filled with pipette solution A (containing in mmol/L: NaCl 5, KCl 125, MgCl2 2, adenosine triphosphate (ATP) 5, HEPES 20, fura-2 pentapotassium salt 0.05, pH 7.0), after which they showed resistances of ≈ 15 MΩ. After patch formation and subsequently gaining access to the cell interior, the cell was voltage-clamped at -10 mV and internally perfused with pipette solution A for 1 to 4 minutes. Whole cell current at −10 mV was then measured continuously, both before and after addition of Epo (2 U/mL). With voltages referenced to bath, Epo-induced whole cell Ca2+ current would be detected as a downward deflection (inward current). Similarly, outward currents would be detected as upward deflections (Fig 1B). For voltage-clamping and current amplification, a patch-clamp amplifier (Axopatch-1C; Axon Instruments, Foster City, CA) with CV-4 1/100 headstage was used. For acquisition and analysis of whole cell currents (both open configuration and perforated patch), an IBM PC/AT computer interfaced with an IBX A/D and D/A converter (Indec Systems, Sunnyvale, CA) was used in conjunction with BASIC-FASTLAB software (Indec Systems).
Simultaneous measurement of [Cai] and whole cell current in human erythroblasts. GΩ seal was attained in day 10 BFU-E–derived erythroblasts bathed in PBS. After break in, Vm was set at resting potential of −10 mV22 and the erythroblast was internally dialyzed for 1 (2 cells) or 4 minutes (2 cells) with pipette solution A (Materials and Methods) containing 50 μmol/L fura-2. There was no difference in stability of F350/F380 ratio (R) whether the erythroblast was dialyzed for 1 or 4 minutes. Baseline R was taken, Epo (2 U/mL) added and R was followed for 2 minutes before the addition of ionomycin (1 μmol/L). Our previous experience indicates that R significantly increased in intact erythroblasts 1 minute following Epo addition.8 Simultaneously whole cell current was measured at −10 mV for the duration of fluorescence measurements. (A) F350/F380 ratio (mean ± SE) of 4 erythroblasts before and after Epo or ionomycin addition. Error bars are not shown if they fall within boundaries of the symbol. (B) Whole cell current of one of the four erythroblasts shown in (A). If Epo induced a measurable inward whole cell Ca2+ current, it would be detected as a downward deflection in the current tracing, similar to what we observed in vasopressin-treated rat hepatocytes (Fig 5 in Duszynski et al42 ). No such downward deflection is apparent. By contrast, addition of ionomycin caused a large outward (positive) whole cell current.
Simultaneous measurement of [Cai] and whole cell current in human erythroblasts. GΩ seal was attained in day 10 BFU-E–derived erythroblasts bathed in PBS. After break in, Vm was set at resting potential of −10 mV22 and the erythroblast was internally dialyzed for 1 (2 cells) or 4 minutes (2 cells) with pipette solution A (Materials and Methods) containing 50 μmol/L fura-2. There was no difference in stability of F350/F380 ratio (R) whether the erythroblast was dialyzed for 1 or 4 minutes. Baseline R was taken, Epo (2 U/mL) added and R was followed for 2 minutes before the addition of ionomycin (1 μmol/L). Our previous experience indicates that R significantly increased in intact erythroblasts 1 minute following Epo addition.8 Simultaneously whole cell current was measured at −10 mV for the duration of fluorescence measurements. (A) F350/F380 ratio (mean ± SE) of 4 erythroblasts before and after Epo or ionomycin addition. Error bars are not shown if they fall within boundaries of the symbol. (B) Whole cell current of one of the four erythroblasts shown in (A). If Epo induced a measurable inward whole cell Ca2+ current, it would be detected as a downward deflection in the current tracing, similar to what we observed in vasopressin-treated rat hepatocytes (Fig 5 in Duszynski et al42 ). No such downward deflection is apparent. By contrast, addition of ionomycin caused a large outward (positive) whole cell current.
For simultaneous [Ca2+]i measurements in voltage-clamped erythroblasts, excitation light (350 and 380 nm, 10 nm bandpass) derived from a second dual wavelength light source (Ionoptix) was directed to erythroblasts via a dichroic mirror (Zeiss FT395) only during data acquisition. Cell epifluorescence (505 ± 20 nm) was collected by Zeiss Plan-Neofluar 100x/1.30NA oil objective, passed through a pinhole (1.6 mm) and captured by a photomultiplier (Hamamatsu R928P). The photomultiplier output was digitized on-line (1 kHz for 2,000 ms) by a multichannel scaler (EG & G Ortec ACE-MCS System; Oak Ridge, TN) situated in a second IBM PC/AT computer. Bias voltage for the photomultiplier was chosen so that (1) autofluorescence of unloaded cells or background was negligible, and (2) photomultiplier was operating in the linear response region. Linearity of the photometric system was verified by us previously.27 Paired 350- and 380 nm fluorescence data were obtained before and 1 to 2 minutes after Epo (2 U/mL) addition. The fluorescence intensity ratios (F350/F380) were calculated for each time point and are shown in Fig 1A.
Measurement of whole cell currents by perforated patch.Micropipettes, electronics, and software used were similar to those used for whole cell configuration. Erythroblasts were bathed in Iscove's modified Dulbecco's medium (IMDM) ([Ca2+ ] = 1.5 mmol/L) containing FCS (1%) and HEPES (1%). Filling solution (pipette solution B) contained (in mmol/L): KCl 55, K2SO4 25, MgCl2 1, NaHEPES 10, pH 7.2. The concentrations of Cl−, Na+, K+ were designed to simulate the intracellular concentrations found in human RBC28 and finally arrived at empirically so that there was no visible shrinking or swelling of the cell after the membrane patch was perforated by nystatin. Inclusion of K2SO4 in pipette solution B was necessary to counter osmotic forces due to Donnan's equilibrium effects.24 On day of use, nystatin (5 mg) was added to 100 μL of dimethyl sulfoxide (DMSO), to which was added 900 μL of filtered (0.22 μm; Millipore Corp, Bedford, MA) pipette solution B and sonicated for 1 minute. Forty microliters of this nystatin stock solution (5 mg/mL) was added to 1 mL of filtered pipette solution B, sonicated for 1 minute, and used in experiments (final nystatin concentration 200 μg/mL). Both nystatin solutions (stock and experimental) were discarded 2 to 3 hours after initial mixing with DMSO.
The pipette tip was first dipped into pipette solution B and then backfilled with the nystatin-containing solution. Positive pressure was applied as the pipette passed through air-water interface and immediately released after the pipette tip entered the bath. Formation of GΩ seal proceeded in the usual manner and seal resistances were measured every 5 minutes. Steady-state seal resistances were usually achieved within 5 to 20 minutes (Fig 2A), after which whole cell currents were measured before and after addition of Epo (2 U/mL) (Fig 2B).
Effects of Epo on whole cell currents measured with nystatin perforated patch method. GΩ seals were formed in day 10 BFU-E–derived erythroblasts in IMDM containing FCS and HEPES (both 1%). Without nystatin in pipette solution, typical values for seal resistance were 20 to 50 GΩ. (A) Representative time course of decrease in seal resistance for two erythroblasts under nystatin perforated clamp. Voltage steps (between −40 and +80 mV, 10-mV increments, 100 ms) were applied at the times indicated and seal resistance was calculated. Mean steady-state seal resistance with nystatin-perforated patch was 2.4 ± 0.6 GΩ (n = 9). (B) Whole cell currents were measured only after attainment of minimal steady-state seal resistance, usually at 15 to 20 minutes after initial GΩ seal formation (A). IV relationships in human erythroblasts under nystatin-perforated patch-clamp before (▪, n = 5) and 15 minutes after Epo (2 U/mL) addition (•, n = 6) are shown (mean ± SE). Error bars are not shown if they fall within the boundaries of the symbol. If Epo induced a measurable inward whole cell current, it would be detected as a greater downward deflection at more negative voltages in the IV relationship, similar to what we observed in vasopressin-stimulated rat hepatocytes (Fig 6 in Duszynski et al42 ). This is not the case in Epo-stimulated erythroblasts. IV relationships at 1, 5, 10, and 20 minutes after Epo addition (n = 6 except for 20 minutes where n = 3) are similar and not shown.
Effects of Epo on whole cell currents measured with nystatin perforated patch method. GΩ seals were formed in day 10 BFU-E–derived erythroblasts in IMDM containing FCS and HEPES (both 1%). Without nystatin in pipette solution, typical values for seal resistance were 20 to 50 GΩ. (A) Representative time course of decrease in seal resistance for two erythroblasts under nystatin perforated clamp. Voltage steps (between −40 and +80 mV, 10-mV increments, 100 ms) were applied at the times indicated and seal resistance was calculated. Mean steady-state seal resistance with nystatin-perforated patch was 2.4 ± 0.6 GΩ (n = 9). (B) Whole cell currents were measured only after attainment of minimal steady-state seal resistance, usually at 15 to 20 minutes after initial GΩ seal formation (A). IV relationships in human erythroblasts under nystatin-perforated patch-clamp before (▪, n = 5) and 15 minutes after Epo (2 U/mL) addition (•, n = 6) are shown (mean ± SE). Error bars are not shown if they fall within the boundaries of the symbol. If Epo induced a measurable inward whole cell current, it would be detected as a greater downward deflection at more negative voltages in the IV relationship, similar to what we observed in vasopressin-stimulated rat hepatocytes (Fig 6 in Duszynski et al42 ). This is not the case in Epo-stimulated erythroblasts. IV relationships at 1, 5, 10, and 20 minutes after Epo addition (n = 6 except for 20 minutes where n = 3) are similar and not shown.
In some experiments in which it was desirable to eliminate K+ currents, K+ in pipette solution B was replaced with Cs+. External solution used under these (K+-free) conditions contained (in mmol/L): NaCl 140, CsCl 4, NaHEPES 20, MgCl2 0.5, CaCl2 5, tetraethylammonium (TEA) 10, pH 7.4 and 0.5 μmol/L apamin (Sigma). In these experiments, after minimal seal resistance was achieved, the erythroblasts (n = 5) were held at -10 mV and whole cell currents continuously monitored before and after addition of Epo (2 U/mL), similar to that depicted in Fig 1B. In this configuration, Epo-induced Ca2+ inward current will be detected as a downward deflection.
Whole cell currents (both conventional and perforated patch) were filtered at 2 kHz and data acquired at 10 kHz. Voltages were referenced to the bath, ie, positive voltage was depolarizing and negative voltage was hyperpolarizing. Outward current (positive current) represented flow of cations from cell to bath or anion flow from bath to cell.
Single channel recordings.Single channel recordings were performed at 29°C as described by Sakman and Neher26 in the cell-attached configuration. Briefly, patch-clamp pipettes were fabricated from glass capillaries (No. 1B150F-3; World Precision Instruments), fire-polished and the tapering shank coated with sylgard (184 silicone elastomer kit; Dow Corning, Midland, MI). Tip diameters were typically 1 to 1.5 μm. The same patch-clamp amplifier (Axopatch-1C) and headstage was used for voltage clamping and single channel current amplification. For data acquisition and analysis, an IBM PC/AT computer interfaced with an TL-1 DMA A/D and D/A converter was used in conjunction with pClamp 5.5 software (Axon Instruments).
For Ca2+ channel recordings using Ca2+ as charge carrier, external solution consisted of (in mmol/L): NaCl 140, CsCl 4, EGTA 1, MgCl2 0.5, NaHEPES 20, TEA 10, pH 7.4; while filling solution (pipette solution C) contained (in mmol/L): Cs+ glutamate 100, CsCl 20, Cs+ HEPES 20, CaCl2 6, pH 7.4. Both solutions contained glucose (25 mmol/L) and 100 nmol/L charybdotoxin (Sigma) was added to pipette solution C to block Ca2+-activated K+ currents.29 Using Ca2+ as the charge carrier resulted in an extremely low success rate: both in terms of totally silent sweeps (no openings were detected during the entire recording) or infrequent channel openings, which precluded meaningful analysis. Replacing NaCl or Cs+ glutamate with N-methyl-D-glucamine or choline chloride did not improve our chances of success. Even when Ca2+ in pipette solution C was increased to 110 mmol/L (by omitting Cs+ glutamate and CsCl), we did not obtain analyzable records. With Ba2+ as charge carrier, external solution consisted of (in mmol/L): choline Cl 140, CsCl 4, CaCl2 1, MgCl2 1, HEPES 20, glucose 11, pH 7.4; while filling solution (pipette solution D) contained (in mmol/L): choline Cl 90, BaCl2 30, CsCl 4, MgCl2 0.5, HEPES 20, pH 7.4. We were more successful in detecting Epo-regulated Ca2+ channels using these solutions. In experiments where [Ba2+] was varied, choline Cl concentrations were changed such that the calculated osmolarity of pipette solution D remained at 300 mOsm. In these experiments, pipette resistances were 8 to 12 MΩ and seal resistances were 20 to 40 GΩ. In contrast to using Ca2+ as the permeant ion, it should be noted that we were successful in obtaining single Ba2+ channel recordings suitable for analysis, whether Ba2+ was at 5, 10, or 30 mmol/L (Fig 5).
Anti-EpoR Antibody Blocks Epo-Induced [Ca2+]i Increase
| F350/F380 . | ||||
|---|---|---|---|---|
| Pretreatment . | Baseline . | Peak . | % Increase . | N . |
| IMDM | 0.38 ± 0.02 | 0.70 ± 0.07* | 185 ± 16 | 11 |
| Anti-EpoR antibody | 0.41 ± 0.02 | 0.42 ± 0.02 | 102 ± 1 | 14 |
| Mouse IgG | 0.39 ± 0.01 | 0.69 ± 0.10* | 175 ± 22 | 9 |
| F350/F380 . | ||||
|---|---|---|---|---|
| Pretreatment . | Baseline . | Peak . | % Increase . | N . |
| IMDM | 0.38 ± 0.02 | 0.70 ± 0.07* | 185 ± 16 | 11 |
| Anti-EpoR antibody | 0.41 ± 0.02 | 0.42 ± 0.02 | 102 ± 1 | 14 |
| Mouse IgG | 0.39 ± 0.01 | 0.69 ± 0.10* | 175 ± 22 | 9 |
Human day 10 BFU-E derived cells on coverslips were exposed to IMDM, anti-EpoR antibody (10 μg/mL), or mouse IgG (10 μg/mL) for 3 hours and during the 20 minutes of fura-2 loading. They were then washed with PBS and fluorescence intensity ratios (F350/F380) were measured with digital video imaging before and at 1, 5, 10, 15, and 20 minutes after Epo (2 U/mL) addition (Materials and Methods). Results are expressed as mean ± SE for baseline or peak F350/F380 or % increase above baseline.
Abbreviation: N, number of observations.
P < .05 compared with baseline.
Dependence of Ca2+ channel unitary current on Ba2+ concentration. Experiments were performed as in Fig 4 except that [Ba2+] in pipette solution D was varied as described in Materials and Methods. (A) Current tracings at 5, 10, 30, and 60 mmol/L Ba2+. Vp was at −20 mV. Each tracing represents a separate cell-attached patch. (B) Mean ± SE of unitary current amplitude at 5 mmol/L (n = 3), 10 mmol/L (n = 3), 30 mmol/L (n = 5), and 60 mmol/L (n = 3) Ba2+. Error bars are not shown if they fall within the boundaries of the symbol.
Dependence of Ca2+ channel unitary current on Ba2+ concentration. Experiments were performed as in Fig 4 except that [Ba2+] in pipette solution D was varied as described in Materials and Methods. (A) Current tracings at 5, 10, 30, and 60 mmol/L Ba2+. Vp was at −20 mV. Each tracing represents a separate cell-attached patch. (B) Mean ± SE of unitary current amplitude at 5 mmol/L (n = 3), 10 mmol/L (n = 3), 30 mmol/L (n = 5), and 60 mmol/L (n = 3) Ba2+. Error bars are not shown if they fall within the boundaries of the symbol.
For Ca2+ channel recordings, pipette potential (Vp) was set at −20 mV. Voltage was referenced to the bath as in whole cell current measurements. Positive single channel current (upward deflection) represented flow of cations from pipette to cell (thus inward current), while negative current (downward deflection) represented flow of cations from cell to pipette (thus outward current).
Data were filtered at 500 Hz, acquired at 1 kHz, and analyzed with pClamp 5.5 software. A channel was considered open if duration was ≥1 ms and half-amplitude threshold was reached.26 Channel open probability is defined as the sum total of time duration of all open events divided by total recording time. To qualify for detailed analysis and subsequent statistical comparisons, a minimum of 200 transitions per recording was required.
Data were presented as means ± standard error (SE). Statistical analysis was by paired or unpaired Student's t-test, as appropriate. A P ≤ .05 was considered statistically significant.
RESULTS
Antierythropoietin receptor antibody blocked [Ca2+]i increase to Epo in human erythroblasts.To confirm the specificity of [Ca2+]i increase in human erythroblasts to Epo stimulation, erythroblasts were pretreated for 3 hours with either mouse antihuman EpoR antibody (Protein A affinity purified mh2er/16.5.1; Genetics Institute, Cambridge, MA) or the nonspecific ImmunoPure mouse IgG, whole molecule (Cat #31204; Pierce, Rockford, IL) before stimulation with Epo. As shown in Table 1, pretreatment with antierythropoietin receptor antibody, but not mouse IgG, inhibited the Epo-induced [Ca2+]i rise, supporting the specificity of intracellular Ca2+ signaling in response to Epo stimulation.
Internal dialysis abolished [Ca2+]i response to Epo in human erythroblasts.Despite the fact that Epo has been repeatedly shown to increase [Ca2+]i in human erythroblasts7-9,11,14,22,23 and that the primary source of Ca2+ increase is extracellular,22 it has been difficult to unequivocally demonstrate the corresponding Ca2+ current using the conventional whole cell configuration.22 One possibility for the failure to detect Epo-induced Ca2+ current is that important cytosolic components/regulators may be washed away by internal dialysis with pipette filling solutions. This interpretation is consistent with the experimental results shown in Fig 1A in which the Epo-induced [Ca2+]i increase was totally abolished in four erythroblasts studied in the classic whole cell configuration. Simultaneous measurement of whole cell current (at −10 mV) also did not show any inward or outward currents on Epo addition (Fig 1B). In contrast, addition of ionomycin, which hyperpolarized human erythroblasts from −2.8 ± 1.9 to −71.5 ± 4.2 mV22 caused a large increase in intracellular fura-2 fluorescence intensity ratio (Fig 1A) with concomitant increase in outward current (Fig 1B), suggesting activation of Ca2+-activated K+ currents. To rule out the possibility that Ca2+ or K+ currents might be detectable at voltages other than −10 mV, in another seven erythroblasts internally dialyzed with 50 μmol/L fura-2, voltage steps (100 ms) from −80 to +40 mV in 10 mV increments elicited similar current-voltage relationships before and after Epo treatment (data not shown, but similar to Figs 4A and 5 in Cheung et al22).
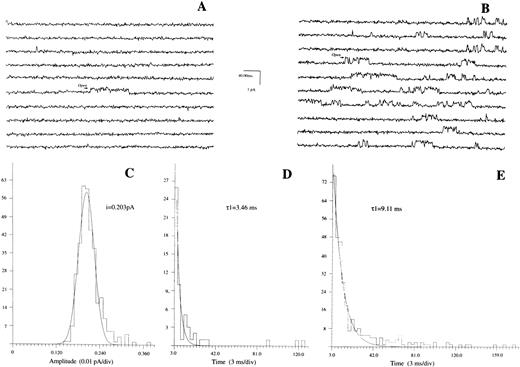
Erythropoietin increases Po and open time of Ca2+ channels. Patches were formed in day 10 BFU-E–derived erythroblasts bathed in media devoid of Na+ and K+ (Materials and Methods). Pipette solution contained 30 mmol/L Ba2+ as charge carrier and Vp was at −20 mV. (A) Baseline tracings. Ca2+ channel openings are indicated by upward transitions. (B) Same patch after bath addition of Epo (2 U/mL). (C) Amplitude histogram and Gaussian fit for channel shown in (B). (D) and (E) Life-time histograms and exponential fit for channels shown in (A) and (B), respectively. Composite data are shown in Table 2.
Erythropoietin increases Po and open time of Ca2+ channels. Patches were formed in day 10 BFU-E–derived erythroblasts bathed in media devoid of Na+ and K+ (Materials and Methods). Pipette solution contained 30 mmol/L Ba2+ as charge carrier and Vp was at −20 mV. (A) Baseline tracings. Ca2+ channel openings are indicated by upward transitions. (B) Same patch after bath addition of Epo (2 U/mL). (C) Amplitude histogram and Gaussian fit for channel shown in (B). (D) and (E) Life-time histograms and exponential fit for channels shown in (A) and (B), respectively. Composite data are shown in Table 2.
Whole cell perforated patch measurements.One way to minimize loss of intracellular constituents is the nystatin perforated patch method first described by Horn and Marty.24 Insertion of nystatin molecules into the patch of cell membrane under the pipette increases the local permeability to monovalent ions, but does not allow larger molecules to enter or leave the cell. With this technique, one waits until the steady-state seal resistance reaches a minimal value before performing whole cell current measurements. Figure 2A shows that attainment of minimal steady-state seal resistance in nystatin-perforated patches was achieved at 5 to 20 minutes. In nine erythroblasts studied with the perforated patch method, the mean steady-state seal resistance was 2.40 ± 0.56 GΩ. If Epo induces a measurable inward whole cell Ca2+ current, it would be detected as a greater downward deflection (more inward current) at more negative voltages in whole cell current-voltage relationships. In fact, whole cell currents obtained after attainment of minimal steady-state seal resistances showed no statistically significant differences in current-voltage relationships before and 15 minutes after Epo (2 U/mL) addition (Fig 2B). In addition, current-voltage relationships at 1, 5, 10, and 20 minutes after Epo addition were similar to that before Epo stimulation (data not shown) indicating no detectable Epo-induced whole cell inward current. In an additional five erythroblasts held at −10 mV with nystatin perforated voltage-clamp and whole cell currents continuously measured under K+-free solutions (Materials and Methods), addition of Epo (2 U/mL) did not elicit any inward or outward currents (data not shown, but similar to Fig 1B).
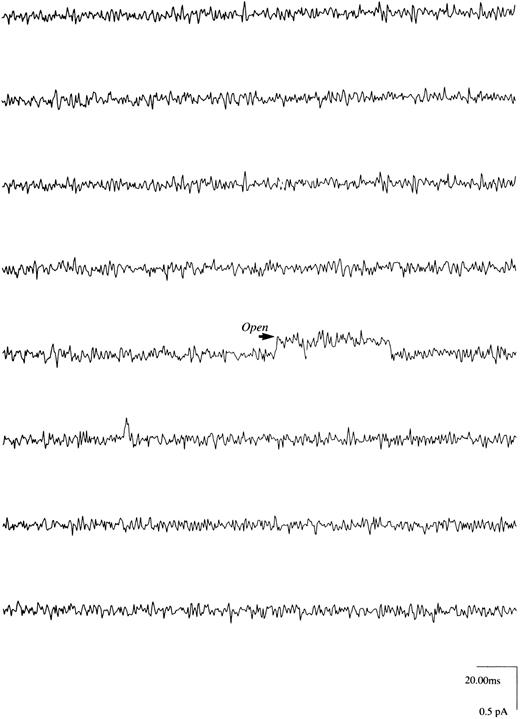
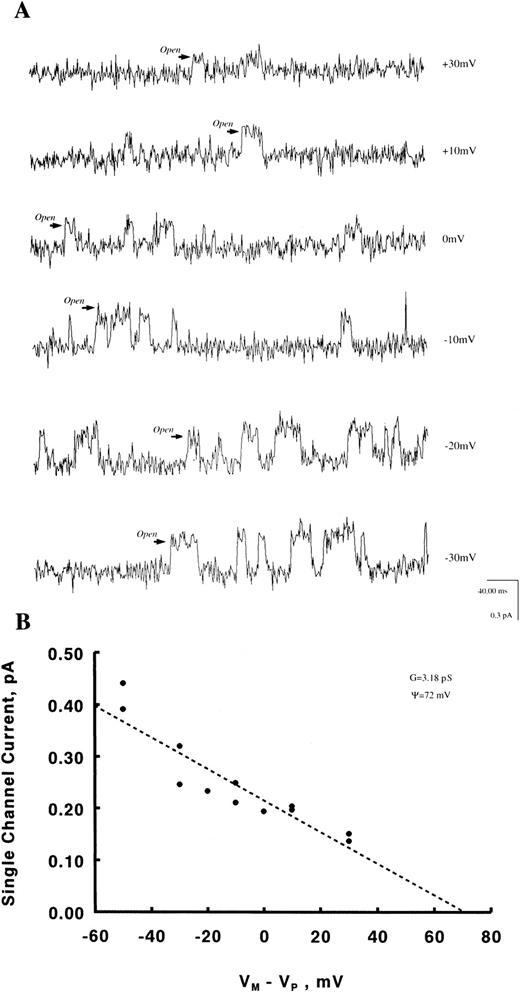
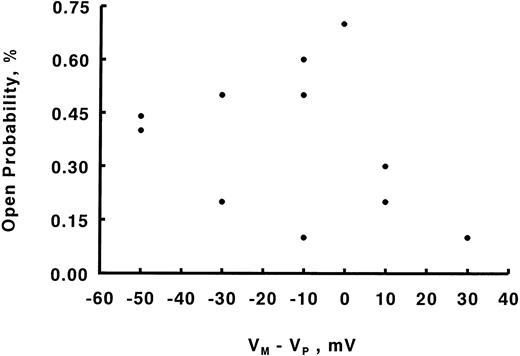
Regulation of Ca2+channels in human erythroblasts by erythropoietin.With Ca2+ (6 mmol/L) as the charge carrier, we observed channel openings carrying an inward current (Fig 3) in eight of 123 patches, giving an incidence rate of 6.5%. These channels were unlikely to be Cl− exit channels because with intracellular [Cl−] less than extracellular [Cl−]28 and at Vp of −20 mV, a Cl− current ought to be outward rather than inward as observed. The Ca2+ channel openings, however, were few with long silent intervals such that none of the eight patches was suitable for detailed analysis. Increasing pipette Ca2+ to 110 mmol/L did not increase our probability of obtaining analyzable single channel recordings. With Ba2+ (5 to 60 mmol/L) as the charge carrier, channel activity was much more easily observed. Figures 4A and B show raw single channel current tracings before and after Epo (2 U/mL) addition to the same erythroblast, respectively. Figure 4C shows amplitude histogram of the Epo-regulated Ca2+ channel. The life-time histograms of the Epo-regulated Ca2+ channel, both before and after Epo stimulation are shown in Figs 4D and E, respectively. As expected for a Ca2+ channel, increasing Ba2+ in pipette solution D monotonically increased the unitary current amplitude (Figs 5A and B), while progressively positive voltages decreased the unitary current amplitude (Fig 6A). The single channel conductance measured under our experimental conditions was 3.2 pS and the reversal potential was extrapolated to be +72 mV (Fig 6B). There was no apparent relationship between Po and voltage (Fig 7), indicating that this is a voltage-independent Ca2+ channel. Addition of Epo (2 U/mL) increased Po and mean open time, but had no effect on unitary current amplitude of this Ca2+ channel (Table 2).
Ca2+ channels in human erythroblasts. With Ca2+ (6 mmol/L) and charybdotoxin (100 nmol/L) in pipette solution and both external and pipette solutions K+-free (Materials and Methods), very few upward transitions (inward current) were observed as indicated. In eight of 123 GΩ seals, the average arithmetic unitary current amplitude was 0.287 ± 0.038 pA at Vp of −20 mV. We wish to emphasize that not enough channel openings (events) were present in these eight seals to allow us to perform meaningful fittings with pClamp 5.5.
Ca2+ channels in human erythroblasts. With Ca2+ (6 mmol/L) and charybdotoxin (100 nmol/L) in pipette solution and both external and pipette solutions K+-free (Materials and Methods), very few upward transitions (inward current) were observed as indicated. In eight of 123 GΩ seals, the average arithmetic unitary current amplitude was 0.287 ± 0.038 pA at Vp of −20 mV. We wish to emphasize that not enough channel openings (events) were present in these eight seals to allow us to perform meaningful fittings with pClamp 5.5.
Dependence of Ca2+ channel unitary current on voltage. Experiments were performed as in Fig 4 except that Vp was varied from −40 to +40 mV. In the cell-attached configuration, unitary current i is related to (Vm-Vp) according to the Goldman-Hodgkin-Katz equation.43 Vm was measured to be −10 mV in human erythroblasts.22 In human RBCs, it should be recalled that PCl is much higher than PK or PNa and that Vm is essentially identical to the Cl− equilibrium potential of −9 mV.28 Thus, absence of external Na+ or K+ in these experiments would be expected to have little effect on Vm . (A) Current tracings at (Vm-Vp) of −30 to +30 mV from a representative cell. (B) Scatter plot of i vs (Vm-Vp). Dotted line is a linear regression line42 from which the slope conductance and reversal potential were estimated to be 3.2 pS and +72 mV, respectively.
Dependence of Ca2+ channel unitary current on voltage. Experiments were performed as in Fig 4 except that Vp was varied from −40 to +40 mV. In the cell-attached configuration, unitary current i is related to (Vm-Vp) according to the Goldman-Hodgkin-Katz equation.43 Vm was measured to be −10 mV in human erythroblasts.22 In human RBCs, it should be recalled that PCl is much higher than PK or PNa and that Vm is essentially identical to the Cl− equilibrium potential of −9 mV.28 Thus, absence of external Na+ or K+ in these experiments would be expected to have little effect on Vm . (A) Current tracings at (Vm-Vp) of −30 to +30 mV from a representative cell. (B) Scatter plot of i vs (Vm-Vp). Dotted line is a linear regression line42 from which the slope conductance and reversal potential were estimated to be 3.2 pS and +72 mV, respectively.
The Epo-regulated Ca2+ channel is a voltage-independent channel. Experiments were performed as in Fig 6. Open probability Po was calculated for Ca2+ channels at various (Vm-Vp). There is no consistent relationship between voltage and Po .
The Epo-regulated Ca2+ channel is a voltage-independent channel. Experiments were performed as in Fig 6. Open probability Po was calculated for Ca2+ channels at various (Vm-Vp). There is no consistent relationship between voltage and Po .
Effects of Epo on Ca2+ Channels in Human Erythroblasts
| . | Control . | Epo . |
|---|---|---|
| Unitary current, pA | 0.197 ± 0.005 | 0.206 ± 0.010 |
| Mean open time, ms | 4.27 ± 0.75 | 11.15 ± 1.80* |
| Open probability, % | 0.26 ± 0.06 | 2.56 ± 0.59* |
| No. of observations | 5 | 5 |
| . | Control . | Epo . |
|---|---|---|
| Unitary current, pA | 0.197 ± 0.005 | 0.206 ± 0.010 |
| Mean open time, ms | 4.27 ± 0.75 | 11.15 ± 1.80* |
| Open probability, % | 0.26 ± 0.06 | 2.56 ± 0.59* |
| No. of observations | 5 | 5 |
Recordings of single channels in day 10 BFU-E–derived erythroblasts were obtained at 29°C and Vp-20 mV as described in Materials and Methods. Ba2+ (30 mmol/L) was the charge carrier for Ca2+ channels. Mean ± SEM are shown.
P < .02 compared with control.
All of the single channel records were obtained in the cell-attached patch configuration, because we were unable to acquire information about this Ca2+ channel in the excised patch mode. Loss of activity of Ca2+-permeable channels on patch excision has been reported.30
DISCUSSION
We have previously demonstrated that the Epo-induced [Ca2+]i increase was not IP3 mediated, but rather was exclusively due to extracellular Ca2+ influx.22 Attempts to detect the corresponding inward whole cell current were not fruitful.22 Our current results underscore two important reasons for the inability to measure whole cell Ca2+ current in human erythroblasts: (1) washout of important cytosolic components with the conventional whole-cell configuration (Fig 1); and (2) inadequate sensitivity of whole cell current method to detect small changes in Ca2+ current (Results; Fig 2B). Considering the small cytoplasmic volume, it is likely that cytosolic proteins are rapidly washed out from erythroblasts in conventional whole-cell configuration. This is supported by the observation that the ability to increase [Ca2+]i in response to Epo was lost within 1 to 2 minutes of break-in (Fig 1A).
The major finding of our current study is that using single channel recording analysis, we have detected in human erythroblasts an ion channel permeable to Ca2+ (Fig 3) and Ba2+ (Fig 4) whose open probability and mean open time were increased by Epo (Fig 4; Table 2). To our knowledge, this is the first demonstration of growth factor regulated, voltage-independent (Fig 7) Ca2+ channel in hematopoietic stem cells. Assuming that Epo-induced [Ca2+]i increase is due to increased openings of Ca2+ channels (based on our cumulative results from digital video imaging, whole cell patch-clamp, and single channel recording studies), the Epo-regulated Ca2+ channel in human erythroblasts is: (1) voltage-independent (Fig 7); (2) inhibited by Ni2+ or high doses (10 to 50 μmol/L) of nifedipine22; (3) inhibited by Pertussis toxin11; (4) activated after Epo receptor occupancy (Table 1) via Gi∝230a; (5) not activated by increase in IP3 or IP4 ;22 (6) inhibited by tyrosine kinase inhibitor genistein14; and (7) expressed differently during different stages of erythroblast maturation process.8 These characteristics do not differentiate the Epo-regulated voltage-independent Ca2+ channel between a receptor-operated calcium channel (ROCC) versus a second messenger-operated Ca2+ channel (SMOCC), although depletion-operated Ca2+ current (DOCC) can be ruled out.30-32 Our observation that bath addition of Epo stimulated Ca2+ channel activity recorded in the membrane patch not exposed to Epo (Table 2) strongly suggests that the Epo-regulated Ca2+ channel is of the SMOCC variety. The precise second messengers are unknown, but likely to be regulated by Giα ,11 most likely Gi∝2,30a and/or tyrosine kinases.14
Voltage-independent Ca2+ currents have been described in other hematopoietic cells such as cloned human helper T lymphocytes33 and DMSO-differentiated HL-60 myeloid cells.34 Despite similarities in slope conductance (≈ 7 pS with 110 mmol/L Ba2+, Kuno et al33 ), reversal potential (≈ +60 mV; Kuno et al33 ), and activation by bath application of mitogen/growth factor, activation of the voltage-independent Ca2+channel in human erythroblasts (conductance of ≈3 pS with 30 mmol/L Ba2+ and reversal potential of ≈+70 mV, Fig 6B) was not associated with appreciable increases in IP3 or IP4 .22 In contrast, chemoattractant-induced Ca2+ influx into myeloid cells was mediated via generation of IP3 ,34 while application of micromolar concentrations of IP3 to the cytoplasmic membrane surface enhanced Ca2+ channel openings in a dose-dependent manner during excised inside-out patch recordings from human Jurkat E6-1 T lymphocytes.35 These observations suggest that while both mitogen-activated Ca2+ channels in leukocytes and Epo-activated Ca2+ channels in human erythroblasts belong to the SMOCC family, the second messengers regulating voltage-independent Ca2+ channel activity may be very different in different cells.
The lack of measurable whole cell Ca2+ current in human erythroblasts precludes any accurate estimate of number of Ca2+ channels per erythroblast. However, based on a [Ca2+]i increase rate of 100 nmol/L/min,8 and an estimated erythroblast volume of 0.5 pL (assuming a perfect sphere with diameter 10 μm; Yelamarty et al23 ), the calculated Ca2+ current/cell would have been 1.7 × 10−4 pA. This estimated current amplitude represents the lower limit since [Ca2+]i increase rate as measured by fura-2 fluorescence (net Ca2+ flux minus intracellular organelle sequestration minus intracellular Ca2+ binding) is necessarily much lower than unidirectional Ca2+ influx. Assuming similar channel permeability to Ca2+ and Ba2+ and using the open probability measured with 30 mmol/L Ba2+ (Table 1), only one Ca2+ channel/erythroblast needed be present to more than adequately accommodate the 100 nmol/L/minute [Ca2+]i increase rate observed in Epo-treated erythroblasts. Another independent method of estimating the number of Ca2+ channels per cell takes advantage of the probability of detecting the channel with a pipette of 1 to 2 μm in diameter enclosing a patch of membrane of area A of 0.79 to 3.14 μm2. Under our experimental conditions, we detected Ca2+ channel openings in eight of 123 successful GΩ seals. If we assume that every channel enclosed within the pipette circumference would open and be detected, the probability of detection PD was 0.065. If the density of Ca2+ channels in human erythroblasts is 1 channel per n μm2, then A/n = PD . Substituting A and PD gives the range of Ca2+ channel density: between 1 in 48 μm2 to 1 in 12 μm2. The number of Ca2+ channels per erythroblast would be 6.5 to 26. This estimate of the number of Ca2+ channels per erythroblast corresponded to an Epo-induced whole cell Ca2+ current of only 0.07 to 0.28 pA, assuming single channel conductance of 3.2 pS (Fig 6), channel open probability of 2.56% (Table 1), and that the Epo-treated cell was stepped from +70 mV (reversal potential, Fig 6) to −60 mV. The low magnitude of whole cell Ca2+ current is below the limit of detection with conventional whole cell current measurements. Our crude arithmetic exercises are based on many assumptions, but serve to illustrate the reasons for our difficulty in measuring whole cell Ca2+ current, as well as our infrequent encounters with single Ca2+ channels in erythroblasts (Results). The low estimated number of Ca2+ channels per cell, when compared with the 1,100 Epo-receptors per BFU-E–derived erythroblast at similar stages of maturation,36 is also consistent with our conclusion that the Epo-regulated voltage-independent Ca2+ channel belongs to the SMOCC, rather than the ROCC family.
The increase in [Ca2+]i with Epo has been demonstrated to be related to cell differentiation8 and the Ca2+ signal is transmitted to the nucleus.23 Recent studies suggest that Ca2+ may regulate gene transcription by modulating transcription factor phosphorylation via protein kinase C37 or Ca2+-calmodulin–dependent protein kinases.38,39 More exciting is the very recent demonstration that Ca2+-loaded calmodulin was able to inhibit DNA binding of several basic-helix-loop-helix transcription factors40 and activate Ras through its influence on Ras-GRF exchange factor.41 It is intriguing to speculate on the role of Ca2+ channels in control of hematopoietic cell proliferation and differentiation because of the enticing possibility for future pharmacologic interventions in promoting cell maturation.
In summary, we have demonstrated by single channel recording that erythropoietin regulates a voltage-independent Ca2+ channel in human erythroblasts. We suggest that the Epo-regulated Ca2+ channel belongs to the family of second messenger-operated Ca2+ channels and may be involved in controlling erythroblast differentiation.
ACKNOWLEDGMENT
The authors thank Beverly Bell for assistance in the preparation of the manuscript; Laurie Bell and MaryBeth Elensky for their expert technical assistance. We also thank Drs Simon Jones and Alan D'Andrea for their advice regarding the antihuman erythropoietin receptor antibody.
APPENDIX
The equation relating [Ca2+]i to R is: [Ca2+]i = Kd (Sf2/Sb2 ) (R − Rmin )/(Rmax − R); where Kd is the apparent dissociation constant of Ca2+-fura-2; Sf2 and Sb2 are the fluorescence proportionality constants of fura-2 at zero and saturating Ca2+, respectively; and Rmax and Rmin are F350/F380 ratios at saturating and zero Ca2+, respectively.8 Taking first derivative, d[Ca2+]i /dR = K(Rmax − Rmin )/(Rmax − R)2, where K = Kd(Sf2/Sb2 ). At [Ca2+]i levels typically found in resting and Epo-stimulated human erythroblasts (20 to 300 nmol/L8,22), R ≪ Rmax . Under these conditions, and Rmax ≫ Rmin , d[Ca2+]i ≅ (K/Rmax ) dR or ▵R is linearly proportional to ▵[Ca2+]i .
Supported in part by National Institutes of Health Grants No. HL-41582, DK-40127, and DK46778. B.A.M. is the recipient of an American Cancer Society Faculty Award.
Address reprint requests to Joseph Y. Cheung, MD, PhD, Division of Nephrology, Milton S. Hershey Medical Center, PO Box 850, Hershey, PA 17033.

![Fig. 1. Simultaneous measurement of [Cai] and whole cell current in human erythroblasts. GΩ seal was attained in day 10 BFU-E–derived erythroblasts bathed in PBS. After break in, Vm was set at resting potential of −10 mV22 and the erythroblast was internally dialyzed for 1 (2 cells) or 4 minutes (2 cells) with pipette solution A (Materials and Methods) containing 50 μmol/L fura-2. There was no difference in stability of F350/F380 ratio (R) whether the erythroblast was dialyzed for 1 or 4 minutes. Baseline R was taken, Epo (2 U/mL) added and R was followed for 2 minutes before the addition of ionomycin (1 μmol/L). Our previous experience indicates that R significantly increased in intact erythroblasts 1 minute following Epo addition.8 Simultaneously whole cell current was measured at −10 mV for the duration of fluorescence measurements. (A) F350/F380 ratio (mean ± SE) of 4 erythroblasts before and after Epo or ionomycin addition. Error bars are not shown if they fall within boundaries of the symbol. (B) Whole cell current of one of the four erythroblasts shown in (A). If Epo induced a measurable inward whole cell Ca2+ current, it would be detected as a downward deflection in the current tracing, similar to what we observed in vasopressin-treated rat hepatocytes (Fig 5 in Duszynski et al42 ). No such downward deflection is apparent. By contrast, addition of ionomycin caused a large outward (positive) whole cell current.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.92/2/m_bl_0020f1.jpeg?Expires=1767810639&Signature=l-yFQ6tZKUIHLX9aw9Sq0czs8v136~H2OnM3329zhKnHrIQyxKA2xcYLDicjPP~Cf4meBbwO4EnyCOTl2d77BW-kvXZdVZN066C6sNinDWLGxaTQT1lfWInMdWOKhQlHJB8BpU8DGEjuEYVcWhjqA6WatkzCurzc~FYguFuXa8Hk1XD-tBvLPLDlKbSQv2bwkDxy1Dq6ey1d2UyI2GFQENG1uG2yHVk-i0jeD82w4OgFSXoDq~eqvkTyi1WKwF973eXwyumMbHbzUNOBpL2g50LnuiNpGuR5qoYyEVV~Q5apm0mnKA9inWba0nG2PtVX26IUBi4KAtlpxQMsb1k8eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Dependence of Ca2+ channel unitary current on Ba2+ concentration. Experiments were performed as in Fig 4 except that [Ba2+] in pipette solution D was varied as described in Materials and Methods. (A) Current tracings at 5, 10, 30, and 60 mmol/L Ba2+. Vp was at −20 mV. Each tracing represents a separate cell-attached patch. (B) Mean ± SE of unitary current amplitude at 5 mmol/L (n = 3), 10 mmol/L (n = 3), 30 mmol/L (n = 5), and 60 mmol/L (n = 3) Ba2+. Error bars are not shown if they fall within the boundaries of the symbol.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.92/2/m_bl_0020f5.jpeg?Expires=1767810639&Signature=f6gJhKQsbei4mvQgqExQ0wg7oXuZLlojX-nFQvNxXhQYLNA4-hn1LeH-PFNEvj19km93ogYmlKlnEkOkRZExEJOs-vyt6RnIHc1bd~Jtrt5wKK77FhltG5c9RxHLK3DI6R7SfwWRO3AD6v7oScUEof1dgk-yCha-qnFvTMUzkbe0aLuLegONlNFS3ach78QU3yAXgSFvCqK9a0w2Q9eIO-B96jKTz~SETdwR0j-6kFQPcUmCVSXr23hfGXXJE4QrkYV7h77VwHMSyckwBNydxtBiNncbzqwDCAE7tPeXFBoRinerddOtCo~iHLcPFKfial-DFWfpeVUOfluh0xV3vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal