Abstract
The biochemical signaling mechanisms involved in transducing the effects of interferon-γ (IFN-γ) on human leukemia-derived HL-60 cell differentiation are not completely understood. Recent studies established the existence of a sphyngomyelin (SM) cycle that operates in response to the action of IFN-γ on HL-60 cells, but the mechanisms by which IFN-γ induces the SM hydrolysis remain unexplored. In this study, biochemical events mediating IFN-γ effects on SM turnover and their specificity and role in HL-60 differentiation were investigated. The activation of the SM cycle by IFN-γ occurred rapidly, with a decrease of approximately 20% in the SM level observed after 60 minutes with a concomitant increase in ceramide level. Treatment of HL-60 cells with IFN-γ did not influence the 1,2-diacylglycerol concentration, intracellular Ca2+ concentration, or phospholipase D activity. IFN-γ stimulated a rapid release of arachidonic acid (AA) from HL-60 cells; the effect was abolished by the pretreatment of cells with pertussis toxin, suggesting a role for a pertussis-toxin–sensitive G protein in IFN-γ–mediated activation of phospholipase A2 (PLA2 ). At 4 to 120 hours after the stimulation of the cells with IFN-γ, a significant increase in the particulate and soluble PLA2 activity was observed, corresponding to an increase in the level of immunoreactive cPLA2 in both cytosol and membrane fractions. The treatment of cells with tyrosine kinase inhibitor herbimycin A completely abolished the effect of IFN-γ on PLA2 activity in membrane and cytosolic fractions, but had no effect on IFN-γ–mediated early AA release suggesting dual mechanism of PLA2 activation. Melittin, potent activator of PLA2 , and AA mimicked the effect of IFN-γ on SM hydrolysis. Pretreatment of HL-60 cells with the PLA2 inhibitor, bromophenacyl bromide (BPB), or pertussis toxin abolished the effect of IFN-γ on SM hydrolysis; exogenous addition of AA overcame the effects of BPB and pertussis toxin. Long-term exposure (5 days) of HL-60 cells to IFN-γ caused an increase in nitroblue tetrazolium (NBT)-reducing and nonspecific esterase (NSE) activity and induced expression of FcγRI (CD64) without significant effects on cell number, adherence, or fagocytic activity. The treatment of cells with AA or melittin induced NBT, NSE, and CD64 expression to the level similar to that observed with IFN-γ, and no further increase was observed with the combination of IFN-γ and AA or IFN-γ and melittin. Treatment of HL-60 cells with indomethacin, an inhibitor of cyclo-oxygenase, and nordihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase, had no effects on IFN-γ–mediated induction of CD64 expression. These studies indicate a key role for the phospholipase A2/AA pathway, as an early biochemical signal elicited by the occupation of IFN-γ–receptor, in mediating IFN-γ induction of the SM cycle and phenotypic changes associated with differentiation of HL-60 along monocytic lineage.
THE CAPACITY OF HL-60 promyelocytic leukemia cells to differentiate in vitro toward cells carrying granulocytic or monocytic markers offers a valuable model system for the dissection of biochemical mechanisms specific to each differentiation pathway. HL-60 cells differentiate into monocyte-like cells following exposure to interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and vitamin D3 . Dibutyryl cyclic adenosine monophosphate (dbcAMP) and retinoic acid (RA) induce maturation along neutrophilic pathway and phorbol 12-myristate 13-acetate (PMA) causes the cells to differentiate into a macrophage-like phenotype.1 Over the last few years, the sphingomyelin turnover, involving the hydrolysis of membrane sphingomyelin by an activated sphingomyelinase to generate ceramide, has emerged as a key pathway mediating the action of monocytic inducers.2 The sphingomyelin cycle appeared to be specific to the monocytic differentiation of HL-60 cells, as it was not activated by inducers of either granulocytic or macrophage-like differentiation.3 Vitamin D3 , IFN-γ and TNF-α cause an early and reversible hydrolysis of sphingomyelin, and addition of bacterial sphingomyelinase or synthetic ceramide mimics the action of agonists on the induction of nitroblue tetrazolium (NBT)-reducing activity and nonspecific esterase (NSE) levels.3,4 Vitamin D3-activated sphingomyelinase was partially purified from HL-60 cells, and it was shown to be a cytosolic (possibly translocated) Mg-independent enzyme.5 Sphingomyelinase catalyzes the parallel reaction to phospholipase C, generating ceramide and phosphocholine; ceramide is a candidate second messenger responsible for mediating the effects of agonists on cell growth and differentiation.2 Several distinct immediate targets for the action of ceramide have been recently described including a 97-kD ceramide-activated protein kinase, a ceramide activated protein phosphatase, and protein-kinase C-ζ.6 Although a number of downstream effects modulated by ceramide have been recognized, little is known concerning early biochemical events coupling receptor occupancy to sphingomyelinase activation.2,6 Recent evidence suggests that TNF-α may induce the activation of neutral sphingomyelinase in HL-60 cells through the activation of phospholipase A2 and the generation of arachidonic acid (AA).7 In U937 cells, TNF-α activates both neutral and acidic, lysosomal sphingomyelinase; the latter being activated by diacyl-glycerol generated by activation of PtdCho-specific phospholipase C.8 The mechanism by which other agonists of monocytic differentiation induce activation of sphingomyelinase has not been examined.
Treatment of HL-60 cells with IFN-γ increases the percentage of cells positive for nonspecific esterase, having Fc receptors (FcR), reducing NBT, and expressing surface markers associated with differentiation along monocytic lineage.9 The biological activity of IFN-γ is initiated by the interaction with high-affinity receptor composed of a ligand binding α-chain and an accessory factor required for signal transduction. Both chains have been cloned and neither of them possesses intrinsic tyrosine kinase activities, but differentially associate with Jak 1 and Jak 2 members of Janus family kinases.10 These kinases are required for the rapid phosphorylation of latent, cytosolic Stat1 (signal transducers and activators of transcription), which translocates to the nucleus and binds to the gamma-activated sequence (GAS) in IFN-γ–responsive genes.11,12 Jak-STAT signaling pathway is considered to be responsible for mediating IFN-γ activation of early response genes.12 IFN-γ–induced signals mediating delayed activation of late genes (ie, major histocompatibility complex [MHC] class II) involve multiple phosphorylation events that may be cell type specific; the involvement of protein kinase C (PKC), Na+/H+ antiporter or Ca2+-calmodulin complex has been implicated.13,14 Recent findings that IFN-γ stimulates receptor-coupled AA release in human neuroblastoma cell line,15 and increases synthesis and activation of cytosolic phospholipase A216 suggest a possible role of phospholipase A2-activation as an early event in signaling by IFN-γ . Biochemical and molecular mechanisms involved in coupling IFN-γ–receptor activation to sphingomyelinase regulation are presently unknown.
In the present study, we present evidence that sphingomyelin hydrolysis in response to IFN-γ is mediated by the activation of phospholipase A2 and AA release. Our results show that the treatment of HL-60 cells with either AA or melittin, an activator of PLA2 , is sufficient to induce expression of FcγRI surface protein, NSE, and NBT-reducing ability to the levels similar to those observed with IFN-γ.
MATERIALS AND METHODS
Materials.Reagents were obtained from the following sources: CHAPS, EGTA, EDTA, HEPES, interferon-γ, cytochalasin B, fura-2 AM, ionomycin, A23187, dithiothreitol (DTT), lipid standards, Triton X-100, leupeptin, phenylmethylsulfonyl fluoride (PMSF ), adenosine triphosphate (ATP), indomethacin, nordihydroguaiaretic acid (NDGA), AA, melittin, pertussis toxin, bromophenacyl-bromide, RPMI-1640, fetal bovine serum, penicillin, streptomycin, insulin, transferrin, NSE kit 91-1 and NBT kit 840-W from Sigma, St Louis, MO. Herbimycin A was purchased from Calbiochem, Nottingham, UK. Tyrphostine (AG-115) was a generous gift from Dr A. Levitzki, Department of Biological Chemistry, The Alexander Silberman Institute of Life Sciences, Jerusalem, Israel. [γ-32P] ATP, [3H] alkyl-lysophosphatidylcholine ([3 H] alkyl-lysoPtdCho), [arachidonyl-14C]PtdCho, [3H] AA, [3H] choline-chloride, and enhanced chemiluminescence kit from Amersham International, Amersham, Bucks, UK. Rabbit polyclonal antibodies raised against denatured human cPLA2 were obtained from Genetics Institute Inc, Cambridge, MA. Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAbs) HLA-DR, murine IgG2a, murine IgG1, and unconjugated murine IgG2a and IgG1 were purchased from Becton Dickinson, Immunocytochemistry Systems, San Jose, CA, FITC-conjugated MoAb versus CD14 from Sigma, and MoAb versus CD64, control murine IgG1 and rat F(ab)2-FITC against murine IgG from Serotec, Oxford, UK. All other chemicals were of analytical grade.
Cell culture. HL-60 cells (ECCACC no. 88112501) were obtained from the European Collection of Animal Cell Cultures, PHLS, Porton, Salisbury, UK, and maintained in exponential growth in RPMI 1640 medium with 15% heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 humidified atmosphere at 37°C. The cells were washed twice with phosphate-buffered saline (PBS) and resuspended in serum-free media containing insulin (5 mg/L) and transferrin (5 mg/L) before treatment with various compounds. If not stated otherwise, all incubations were performed with concentration of the cells adjusted to 0.5 × 106 cells/mL.
Sphingomyelin.HL-60 cells were incubated with [3H]choline chloride (0.5 μCi/mL; specific activity: 80 Ci/mmol) for 48 hours in serum-free medium containing insulin (5 mg/L) and transferrin (5 mg/L). Postlabeling, cells were washed with PBS and resuspended in serum-free medium for 2 hours. Cells were then treated as indicated and harvested. The lipids were extracted by method of Folch17 and dissolved in 50 μL of chloroform. A total of 20 μL was then applied on silica gel 60 TLC plates and chromatographed using the solvent system chloroform/methanol/acetic acid/H2O (60:30:8:5, by vol). Lipids were visualized using iodine vapor and the spot corresponding to SM standard was scraped off and 3H content determined by scintillation counting.
Mass assay of diacylglycerol (DAG).DAG was extracted from the cells using 1.5 mL of chloroform/methanol (1:2, vol/vol). Further extraction was performed as described by Folch et al.17 The lipid extract was dissolved in 0.5 mL of chloroform and loaded on a silicic acid column (0.5 mL; made in Pasteur pipette), eluted with 1 mL of chloroform, dried again, and the mass measurement for DAG was performed using DAG kinase whose purification was achieved in a single step from rat brain using diethyl aminoethyl (DEAE)-Sepharose column as described by Divecha and Irvine.18 The mass measurement for DAG was performed in the following manner. The dried lipid was dissolved by adding 20 μL of CHAPS (9.2 mg/mL) and sonicated at room temperature for 15 seconds. After addition of 80 μL of buffer (50 mmol/L Tris/acetate, 80 mmol/L KCl, 10 mmol/L magnesium acetate, 2 mmol/L EGTA, pH 7.4), the reaction was started by adding 20 μL of DAG kinase followed by 80 μL of buffer containing 20 μmol/L ATP and 1 μCi of [32P]ATP. After a 1-hour incubation at room temperature, the reaction was stopped by adding 750 μL of chloroform/methanol/HCl (80:160:1, by vol). Phosphatidic acid (PtdOH) was extracted as described by Folch et al17 and chromatographed on oxalate (1%)-sprayed TLC plates using the solvent system chloroform/methanol/25% ammonia/water (45:35:2:8, by vol). After autoradiography, the spots corresponding to PtdOH were scraped off and their 32P content determined by scintillation counting. The DAG mass content in each sample was adjusted to between 5 and 50 pmol, as the sensitivity of the mass assay is highest within this range.18
Mass assay of ceramide.Ceramide was extracted from the cells using 1.5 mL of chloroform/methanol (1:2, vol/vol). Further extraction was performed as described by Folch et al.17 Mass measurement for ceramide was performed using DAG kinase exactly as described for mass measurement of DAG. Ceramide phosphate was separated on TLC plates using the solvent system butanol/acetic acid/H2O (6/2/2, by vol).4 After autoradiography, the spots corresponding to ceramide phosphate were scraped off and their 32P content determined by scintillation counting.
Determination of phospholipase D (PLD) activity.Cells were labeled with 5 μCi/mL [3H]alkyl-lysoPtdCho for 30 minutes at 37°C in a buffer comprising 20 mmol/L HEPES, 137 mmol/L NaCl, 2.5 mmol/L KCl, 1 mmol/L CaCl2 , 1 mmol/L MgCl2 , 5.6 mmol/L glucose, and 1 mg/mL fatty acid-free bovine serum albumin (BSA) (pH 7.2), as described by Stuchfield and Cockcroft.19 The labeled cells were washed twice, resuspended in the same buffer and equilibrated for 30 minutes at 37°C. Cytochalasin B was added to the cells (5 μmol/L final concentration) and 100 μL samples were transferred to tubes containing IFN-γ with ethanol (2%, vol/vol, final concentration). After incubation, the cells were quenched with chloroform/methanol (1:2, vol/vol). At this stage, a mixture of unlabeled PtdOH and phosphatidylethanol (PtdEt) was added for localization of the reaction products by TLC. Phase separation was achieved by sequential addition of 2 mol/L KCl and chloroform. Lipids were extracted as described by Folch et al17 and chromatographed on oxalate (1%)-sprayed TLC plates using the solvent system chloroform/methanol/acetic acid/water (75:45:3:1, by vol). Lipids were visualized using iodine vapor and the spots corresponding to PtdOH and PtdEt standards were scraped off and their 3H content determined by scintillation counting.
Measurement of intracellular calcium concentration ([Ca2+]i ).HL-60 cells were incubated in Hanks' physiological salt solution with 2 mmol/L fura-2-AM at 37°C for 30 minutes, washed and resuspended in fresh solution. Cells (5 × 106) were transferred to a 3-mL cuvette for measurement of [Ca2+]i . The fluorescence was measured in a Perkin Elmer LS50 spectrofluorimeter with excitation at 340 and 380 nm and emission at 510 nm. [Ca2+]i was calculated from the fluorescence ratio, as described by Grynkiewicz et al.20 Maximum fluorescence was determined by adding 10 μmol/L ionomycin, and the minimum fluorescence was determined by adding 50 mmol/L EGTA.
Measurement of AA release.For the study of AA release, HL-60 cells were prelabeled at 37°C for 60 minutes with 5 μCi/mL of [3H] AA, washed twice, and resuspended in serum-free medium containing insulin and transferrin; 100-μL samples were transferred to tubes containing IFN-γ, melittin, and/or different inhibitors. After incubation, cells were pelleted and 50 μL of supernatant was counted for 3H radioactivity.
Determination of phospholipase A2 (PLA2 ) activity.After incubation, the cells were washed once with PBS and put into 0.5 mL homogenization buffer: 50 mmol/L HEPES (pH 7.4), 0.25 mol/L sucrose, 1 mmol/L EDTA, 1 mmol/L EGTA, 50 μmol/L NaF, 10 μmol/L leupeptin, 0.15 mmol/L PMSF and CaCl2 to give a free Ca2+ concentration of 150 nmol/L. The cells were homogenized with 10 strokes using a Potter-Elvehjem motor-driven Teflon pestle at 600 rpm. The homogenate obtained was then centrifuged at 104,000g for 60 minutes in a Beckman TL-100 ultracentrifuge. The resulting supernatant was designated the soluble and the pellet was designated the particulate fraction.
Determination of PLA2 enzymic activity was performed at 37°C for 60 minutes in 200 μL of buffer (0.1 mol/L Tris/HCl, pH 7.4, containing 1 mmol/L EDTA, 1 mmol/L EGTA, 10 μmol/L leupeptin, 0.15 mmol/L PMSF, CaCl2 to give a free Ca2+ concentration of 150 nmol/L and 5 mmol/L DTT to inactivate secretory PLA2 ) containing 50 μg of protein and 3 nmol of [arachidonyl-14C]PtdCho (the specific radioactivity was adjusted to 10,000 dpm/nmol by adding unlabeled PtdCho). The reaction was terminated by adding 4 μL of acetic acid and 800 μL of chloroform/methanol (2:1, vol/vol) containing 20 μg of AA. After centrifugation, the lower phase was concentrated and chromatographed on TLC plates using chloroform/methanol/water (65:25:3, by vol) as solvent system. Two radioactive spots corresponding to fatty acid and PtdCho were detected by autoradiography. The spots were scraped off and their 14C content was determined by scintillation counting.
Immunoblot analysis of cPLA2 . Particulate and soluble fractions from HL-60 cells were prepared as described above (for the determination of PLA2 activity). Proteins for electrophoresis were prepared so that the concentration of each sample was 50 μg/25 μL of sample loading buffer.21 Electrophoresis was performed using a Bio-Rad Minigel apparatus at an acrylamide concentration of 10% (wt/vol). After electrophoresis, the proteins were transferred to nitrocellulose using a Bio-Rad wet-blotting system. After blocking the nitrocellulose in 20 mmol/L Tris, 140 mmol/L NaCl, 0.05% (vol/vol) Tween 20 and 4% (wt/vol) low-fat dry milk, the blots were incubated with rabbit antihuman cPLA2 (1:5,000) for 2 hours, washed in the above buffer and incubated with horseradish peroxidase-conjugated goat antirabbit IgG antisera (1:2,000). Detection of immunoreactive bands was performed using the ECL Western blotting system (Amersham).
Assessment of cellular differentiation.For differentiation studies, the cells in the logarithmic growth phase were harvested by centrifugation and resuspended in a serum-free medium supplemented with insulin (5 mg/L) and transferrin (5 mg/L). The cells were seeded at an initial cell density of 2.5 × 105/mL in 6-well plates (Nunc, Roskilde, Denmark) and treated with different agents in a 5% CO2 humidified atmosphere at 37°C for 5 days. Cell growth was quantified using a hemocytometer. Viable cells were determined by trypan blue exclusion. Cytocentrifuge smears of treated cells were examined for NBT reduction (Sigma kit 840-W) and NSE activity (Sigma kit 91-A). For determination of cellular adherence, the density of cells in suspension was measured before and after scrapping of adherent cells from the culture plastic. The number of adherent cells was then calculated as a percentage of total cells in suspension. The phagocytic activity was determined by measuring the uptake of fluorescent microspheres (Fluoresbrite Carboxylate Microspheres, 1.75 mm in diameter, Polysciences), as described by Blair et al.22 Briefly, after incubation, HL-60 cells were resuspended in fresh medium at a density of 106 viable cells/mL and incubated overnight in the presence of 5.0 × 105 fluorescent microspheres per mL. After incubation, free microspheres were removed by three washes with PBS, and the proportion of cells showing phagocytized particles was determined by flow cytometry.
Flow-cytometric analysis of unstimulated and stimulated HL-60 cells included the expression of monocytic cell surface markers (CD14 and CD64/FcγRI) and the assessment of binding of FITC-conjugated mouse IgG2a to HL-60 cells. HL-60 cells were washed with cold PBS without Ca++ and Mg++ and 106 of viable cells were used for each labeling. All incubations were performed on ice for 30 minutes. For CD64-labeling, cells were incubated with anti-CD64 or an appropriate isotypic control (mouse IgG1) in a total volume of 100 μL PBS containing 1% BSA. After two washings, the cells were incubated in the same buffer with rat F(ab)2-FITC against murine IgG. For CD14-labeling, cells were incubated with FITC-conjugated mouse anti-CD14 or FITC-conjugated isotypic control (IgG2a), followed by two washings in PBS. For the indirect assessment of the expression of FcγRI, 106 of HL-60 cells were either preincubated with unlabeled mouse irrelevant IgG2a or were kept in PBS alone. Following washings with PBS, the cells were incubated with FITC-labeled mouse IgG2a, washed, and immediately analyzed on a flow cytometer. The flow-cytometric analysis was performed on the Ortho Cytorone Absolute (Ortho Diagnostic System Inc, Raritan, NJ) using Ortho Immunocount software. For each marker, a total of 5,000 events was collected from the gated population and the fluorescence displayed on a single FL-histogram with the cursor of the control samples set to include up to 1.0% of the fluorescence in the positive region. The results were collected as a percentage of events in the positive regions of the histogram.
RESULTS
When HL-60 cells were prelabeled for 48 hours with [3H]choline, washed, and incubated with IFN-γ, a significant decrease in the radioactivity incorporated into sphingomyelin compared with control was observed (Fig 1A). Time course studies with 2,000 U/mL IFN-γ showed that IFN-γ induced a decrease of the sphingomyelin level of approximately 10% as early as 15 minutes following treatment of the cells. Peak response of approximately 20% sphingomyelin hydrolysis was observed at 60 minutes, and the sphingomyelin level remained decreased at 2 hours. Extent of sphingomyelin hydrolysis resembles that seen with 100 U/mL of IFN-γ, although Kim et al3 described a later onset of sphingomyelin hydrolysis (1 hour) and later peak effect (2 hours) following exposure of HL-60 to 100 U/mL of IFN-γ. As shown in Fig 1B, the kinetics of the increase in the amount of ceramide produced in response to IFN-γ (2,000 U/mL) mirrored the observed decrease in sphingomyelin levels.
Effect of IFN-γ on sphingomyelin (A) and ceramide (B) levels in HL-60 cells. [3H]Choline-labeled or unlabeled cells were treated at time 0 with 2,000 U/mL of IFN-γ. At the indicated times, cells were harvested, lipids were extracted, and SM levels were determined by TLC, while ceramide levels were determined using a DAG kinase assay as described in Materials and Methods. The data (means ± standard deviation [SD]) are from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Effect of IFN-γ on sphingomyelin (A) and ceramide (B) levels in HL-60 cells. [3H]Choline-labeled or unlabeled cells were treated at time 0 with 2,000 U/mL of IFN-γ. At the indicated times, cells were harvested, lipids were extracted, and SM levels were determined by TLC, while ceramide levels were determined using a DAG kinase assay as described in Materials and Methods. The data (means ± standard deviation [SD]) are from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
To determine an early mechanism involved in coupling IFN-γ–R activation to regulation of sphingomyelin levels, a role for phospholipase C, intracellular calcium, and phospholipase D activation was examined. In U-937 cells, DAG, generated by a phosphatidylcholine-specific phospholipase C, was described to initiate ceramide generation via an acidic sphingomyelinase in response to TNF-α.8 We could not observe any increase in DAG or the intracellular calcium concentration [Ca2+]i in HL-60 cells treated with IFN-γ (2,000 U/mL) (results not shown). DAG concentration in unstimulated cells varied from 98 to 141 pmol/mg of protein (mean value 121 ± 13; number of cell preparations = 5) and did not change within 1 to 120 minutes following treatment with IFN-γ. Resting [Ca2+]i varied from 73 to 140 nmol/L (mean value 110.8 ± 14; number of cell preparations = 3), which is similar to the typical [Ca2+]i measured in unstimulated hematopoietic cells.23 To measure phospholipase D activity, the cells were prelabeled with [3H]alkyl-lysoPtdCho. Activation of PLD generates PtdOH, which can be converted into DAG in a time-dependent manner by the enzyme PtdOH phosphohydrolase. When the cells are stimulated in the presence of 2% (vol/vol) ethanol, PtdEt is formed at the expense of PtdOH, and as the former is a more stable product than PtdOH, the sensitivity of the assay will be increased. Despite using 2% ethanol in the assays, we did not observe any increase in radioactivity, either in PtdOH or in PtdEt, upon stimulation of HL-60 cells with IFN-γ (results not shown).
Evidence was provided that AA triggered sphingomyelin hydrolysis in HL-60 cells stimulated with TNF-α.7 To investigate a possible release of AA in response to IFN-γ, [3H]AA-labeled HL-60 cells were stimulated with different doses of IFN-γ and assessed for [3H]AA release. Time course studies with IFN-γ showed that IFN-γ induced release of AA within minutes of stimulation with maximal release at 2 minutes (Fig 2A). As shown in Fig 2B, there was a concentration-dependent enhancement of AA release, with maximal increase observed with 2,000 U/mL IFN-γ. As shown in Table 1, treatment of cells for 2 minutes with melittin (500 ng/mL), an activator of phospholipase A2 , mimicked the stimulatory effect of IFN-γ on AA release, and no further increase in release of radiolabel was observed when cells were treated with the combination of melittin (500 ng/mL) and IFN-γ (2,000 U/mL). The pretreatment of cells with the PLA2 inhibitor, bromophenacyl bromide (BPB, 10 μmol/L),16 markedly inhibited both basal and stimulated [3H]AA release, providing further evidence of PLA2 activation in response to IFN-γ. The pretreatment of cells with indomethacin (50 μmol/L), an inhibitor of cyclo-oxygenase, or NDGA (50 μmol/L), an inhibitor of lipoxygenase, had no effect on IFN-γ–mediated AA release. Protein tyrosine kinase phosphorylation cascade seemed not to be involved in the signaling pathway leading to arachidonate release in response to IFN-γ, since tyrphostine AG-115 (20 μmol/L)24 and herbimycin A (1 μg/mL) (Table 2),25 potent tyrosine kinase inhibitors, had no effect on IFN-γ–stimulated [3H]AA release. On the other hand, the stimulatory effect of IFN-γ on AA release was completely abolished by an 18-hour pretreatment of the cells with pertussis toxin (PTX) at 20 ng/mL, strongly suggesting the involvement of G-protein(s) in AA release by IFN-γ.
Time course and dose response curve of IFN-γ–stimulated AA release. HL-60 cells were prelabeled for 60 minutes with 5 μCi/mL [3H]AA, washed twice, and resuspended in the medium. After incubation with IFN-γ, cells were pelleted and the resultant supernatant was counted for 3H radioactivity. (A) Time course of AA release following treatment with 2,000 U/mL of IFN-γ. (B) Dose response of AA release at 2 minutes following IFN-γ–treatment. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Time course and dose response curve of IFN-γ–stimulated AA release. HL-60 cells were prelabeled for 60 minutes with 5 μCi/mL [3H]AA, washed twice, and resuspended in the medium. After incubation with IFN-γ, cells were pelleted and the resultant supernatant was counted for 3H radioactivity. (A) Time course of AA release following treatment with 2,000 U/mL of IFN-γ. (B) Dose response of AA release at 2 minutes following IFN-γ–treatment. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Effects of IFN-γ, Melittin, and Different Inhibitors on AA Release by HL-60 Cells
| Treatment . | Control . | IFN-γ . |
|---|---|---|
| None | 100 ± 13 | 661 ± 56* |
| Melittin | 561 ± 42* | 780 ± 67* |
| BPB | 30 ± 7† | 33 ± 3† |
| Indomethacin | 117 ± 14 | 590 ± 36* |
| NDGA | 113 ± 19 | 617 ± 53* |
| Tyrphostine | 112 ± 15 | 638 ± 26* |
| Pertussis toxin | 106 ± 15 | 113 ± 17 |
| Treatment . | Control . | IFN-γ . |
|---|---|---|
| None | 100 ± 13 | 661 ± 56* |
| Melittin | 561 ± 42* | 780 ± 67* |
| BPB | 30 ± 7† | 33 ± 3† |
| Indomethacin | 117 ± 14 | 590 ± 36* |
| NDGA | 113 ± 19 | 617 ± 53* |
| Tyrphostine | 112 ± 15 | 638 ± 26* |
| Pertussis toxin | 106 ± 15 | 113 ± 17 |
Cells were labeled with 5 μCi/mL of [3H]arachidonic acid for 60 minutes, washed, and treated with IFN-γ (2,000 U/mL), melittin (500 ng/mL) or the combination of IFN-γ (2,000 U/mL) and melittin (500 ng/mL) for 2 minutes. After pretreatment with BPB, 10 μmol/L for 30 minutes, indomethacin (50 μmol/L) for 15 minutes, NDGA (50 μmol/L) for 15 minutes, tyrphostine (20 μmol/L) for 60 minutes, and pertussis toxin (20 ng/mL) for 18 hours, cells were stimulated or not with 2,000 U/mL of IFN-γ for 2 minutes. After incubation, cells were pelleted and the resultant supernatant was counted to determine levels of released label. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate.
Significantly different (P < .05, Student's t-test) from controls.
Significantly different from both untreated and IFN-γ–stimulated cells.
Effects of Herbimycin A on IFN-γ–Induced PLA2 Activity and AA Release by HL-60 Cells
| Treatment . | PLA2 Activity (% control) . | AA Release (% control) . | |
|---|---|---|---|
| . | Membrane . | Cytosol . | . |
| None | 100 ± 14 | 100 ± 8 | 100 ± 12 |
| IFN-γ | 248 ± 24* | 241 ± 23* | 652 ± 43* |
| 12 hours | |||
| Herbimycin A | 102 ± 12 | 106 ± 13 | 104 ± 12 |
| Herbimycin A + IFN–γ | 98 ± 13 | 106 ± 12 | 637 ± 36* |
| 16 hours | |||
| Herbimycin A | 103 ± 14 | 107 ± 15 | 99 ± 11 |
| Herbimycin A + IFN–γ | 104 ± 12 | 111 ± 13 | 611 ± 65* |
| 20 hours | |||
| Herbimycin A | 109 ± 12 | 112 ± 15 | 103 ± 8 |
| Herbimycin A + IFN–γ | 102 ± 7 | 104 ± 13 | 656 ± 47* |
| Treatment . | PLA2 Activity (% control) . | AA Release (% control) . | |
|---|---|---|---|
| . | Membrane . | Cytosol . | . |
| None | 100 ± 14 | 100 ± 8 | 100 ± 12 |
| IFN-γ | 248 ± 24* | 241 ± 23* | 652 ± 43* |
| 12 hours | |||
| Herbimycin A | 102 ± 12 | 106 ± 13 | 104 ± 12 |
| Herbimycin A + IFN–γ | 98 ± 13 | 106 ± 12 | 637 ± 36* |
| 16 hours | |||
| Herbimycin A | 103 ± 14 | 107 ± 15 | 99 ± 11 |
| Herbimycin A + IFN–γ | 104 ± 12 | 111 ± 13 | 611 ± 65* |
| 20 hours | |||
| Herbimycin A | 109 ± 12 | 112 ± 15 | 103 ± 8 |
| Herbimycin A + IFN–γ | 102 ± 7 | 104 ± 13 | 656 ± 47* |
Cells were treated for 12, 16, and 20 hours with herbimycin A (1 μg/mL). When PLA2 activity was to be measured, cells were stimulated with 2,000 U/mL of IFN-γ during the last 4 hours of the incubation period with herbimycin A. PLA2 activity in particulate and soluble fractions was determined by hydrolysis of [arachidonyl-14C]PtdCho in the presence of 5 mmol/L DTT and 150 nmol/L Ca2+. When AA release was to be measured, cells were labeled with 5 μCi/mL of [3H]arachidonic acid for 60 minutes and stimulated with IFN-γ (2,000 U/mL) for 2 minutes at the end of incubation period with herbimycin A. After treatment, cells were pelleted and the resulting supernatant was counted to determine levels of released label. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate.
Significantly different (P < .05, Student's t-test) from controls.
The hormonally stimulated arachidonate release depends primarily on the activation of an AA-selective cytosolic phospholipase A2 (cPLA2 ). The PLA2 activity in cytosol and membrane fractions was measured in vitro using [arachidonil-14C]phosphatidyl choline as a substrate. The assays were performed in the presence of 5 mmol/L DTT to inactivate the secretory PLA2 .26 Because it is known that the free concentration of Ca2+ in the homogenization buffer can influence the intracellular distribution of PLA2 , the concentration of free Ca+ in both the homogenization and PLA2 assay buffers was adjusted to 150 nmol/L, which is similar to the measured [Ca2+]i . Figure 3 shows the time-dependent activation of membrane-bound (Fig 3A) and cytosolic (Fig 3B) PLA2 . The exposure of HL-60 cells to IFN-γ for up to 30 minutes had no effect on PLA2 activities in both fractions. IFN-γ significantly increased PLA2 activity in both fractions after a 4-hour incubation and it remained increased after 12, 48, and 120 hours. The treatment of cells with herbimycin A (1 μg/mL) for 12, 16, or 20 hours completely abolished the effect of the 4-hour incubation with IFN-γ on PLA2 activity in membrane and cytosolic fractions, but had no effect on IFN-γ–mediated early AA release (Table 2).
PLA2 activity in membrane and cytosol fractions of IFN-γ–stimulated HL-60 cells. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. PLA2 activity in particulate and soluble fractions was determined by hydrolysis of [arachidonyl-14C]PtdCho in the presence of 5 mmol/L DTT and 150 nmol/L Ca2+. (A) PLA2 activity in membrane fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. (B) PLA2 activity in cytosol fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
PLA2 activity in membrane and cytosol fractions of IFN-γ–stimulated HL-60 cells. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. PLA2 activity in particulate and soluble fractions was determined by hydrolysis of [arachidonyl-14C]PtdCho in the presence of 5 mmol/L DTT and 150 nmol/L Ca2+. (A) PLA2 activity in membrane fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. (B) PLA2 activity in cytosol fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
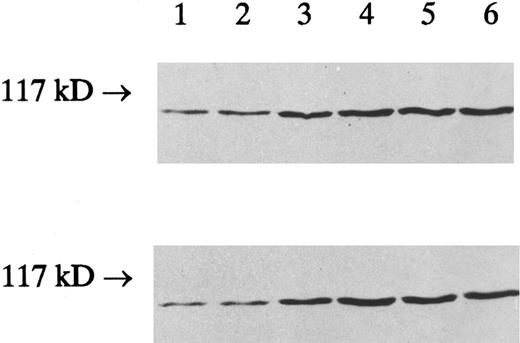
To determine whether the IFN-γ–enhanced cPLA2 activity was due to the increased amount of cPLA2 protein, an immunoblot of the membrane and cytosol fractions prepared from the control and IFN-γ–treated cells was performed using a specific antibody to cPLA2 . The increased cPLA2 protein levels correlated with the increased cPLA2 activity; the cPLA2 protein (110 kD) was increased in both the cytosol and the membrane fractions after treatment with IFN-γ for 4, 12, 48, and 120 hours (Fig 4).
The effect of IFN-γ on the amount of cPLA2 protein. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. Particulate and soluble fractions from HL-60 cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and immunoblotted with rabbit antihuman cPLA2 antibody. A representative Western blot of membrane pellet (upper) and crude cytosol (lower) from HL-60 cells is shown. The cPLA2 protein was shown from left as follows: control cells (lane 1), cells treated with IFN-γ (2,000 U/mL) for 30 minutes (lane 2), 4 hours (lane 3), 12 hours (lane 4), 48 hours (lane 5), and 120 hours (lane 6). The size of the molecular marker is indicated on the left side.
The effect of IFN-γ on the amount of cPLA2 protein. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. Particulate and soluble fractions from HL-60 cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and immunoblotted with rabbit antihuman cPLA2 antibody. A representative Western blot of membrane pellet (upper) and crude cytosol (lower) from HL-60 cells is shown. The cPLA2 protein was shown from left as follows: control cells (lane 1), cells treated with IFN-γ (2,000 U/mL) for 30 minutes (lane 2), 4 hours (lane 3), 12 hours (lane 4), 48 hours (lane 5), and 120 hours (lane 6). The size of the molecular marker is indicated on the left side.
To examine the role of phospholipase A2 activation and AA generation in mediating effects of IFN-γ on sphingomyelin hydrolysis, the effects of exogenous AA and melittin on sphingomyelin turnover were studied. Similar to previous findings,7 both AA (10 μmol/L) and melittin (500 ng/mL) stimulated a time-dependent sphingomyelin hydrolysis in [3H]choline-labeled HL-60 cells. Approximately 20% sphingomyelin hydrolysis was evident by 15 minutes, and maximal effects of up to 30% hydrolysis were observed 60 to 120 minutes after treatment with either 10 μmol/L AA (Fig 5A) or melittin (500 ng/mL) (Fig 5B). The time frame of the peak and the extent of sphingomyelin hydrolysis in response to AA and melittin were similar to those observed with IFN-γ. To further document the role of phospholipase A2 in mediating the effect of IFN-γ on sphingomyelin hydrolysis, cells were pretreated with bromophenacyl bromide (10 μmol/L) for 10 minutes and then stimulated with 2,000 U/mL of IFN-γ. As shown in Table 3, incubation of cells with either 2,000 U/mL of IFN-γ or 10 μmol/L AA for 120 minutes caused a significant decrease in sphingomyelin level and corresponding increase in the amount of ceramide. IFN-γ–mediated effects were completely abolished by the pretreatment of cells with 10 μmol/L BPB, and exogenous addition of AA overcame the inhibitory effect of BPB on sphingomyelin hydrolysis and ceramide production. As expected, herbimycin A, which had no effect on early AA release, did not inhibit IFN-γ–mediated effect on sphingomyelin turnover. On the other hand, 18 hours pretreatment of the cells with pertussis toxin at a concentration that was shown to be inhibitory on IFN-γ–stimulated AA release completely abolished IFN-γ–induced sphingomyelin hydrolysis, but had no effects on AA-induced decrease in the level of sphingomyelin and increase in the amount of ceramide.
Sphingomyelin levels in response to AA and melittin. [3H]Choline-labeled HL-60 cells were treated with either 10 μmol/L AA (A) or 500 ng/mL of melittin (B) and sphingomyelin levels were determined as described in Materials and Methods. The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Sphingomyelin levels in response to AA and melittin. [3H]Choline-labeled HL-60 cells were treated with either 10 μmol/L AA (A) or 500 ng/mL of melittin (B) and sphingomyelin levels were determined as described in Materials and Methods. The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Effects of IFN- γ , AA, BPB, Pertussis Toxin, and Herbimycin A on Sphingomyelin and Ceramide Levels in HL-60 Cells
| Treatment . | Sphingomyelin (% control) . | Ceramide (pmol/mg of protein) . | ||||
|---|---|---|---|---|---|---|
| . | Control . | IFN-γ . | AA . | Control . | IFN-γ . | AA . |
| None | 100 ± 3.7 | 74.2 ± 5.33-150 | 73.8 ± 5.13-150 | 18.3 ± 1.9 | 41.5 ± 3.93-150 | 44.2 ± 4.33-150 |
| BPB | 102.1 ± 5.3 | 97.6 ± 4.2 | 74.1 ± 4.23-150 | 17.9 ± 1.6 | 18.1 ± 1.7 | 43.7 ± 3.63-150 |
| Pertussis toxin | 103.2 ± 4.3 | 102.1 ± 3.7 | 73.7 ± 3.63-150 | 17.5 ± 2.2 | 19.1 ± 2.1 | 42.8 ± 5.13-150 |
| Herbimycin A | 101.5 ± 3.7 | 75.6 ± 4.23-150 | ND | 18.5 ± 2.1 | 42.1 ± 4.33-150 | ND |
| Treatment . | Sphingomyelin (% control) . | Ceramide (pmol/mg of protein) . | ||||
|---|---|---|---|---|---|---|
| . | Control . | IFN-γ . | AA . | Control . | IFN-γ . | AA . |
| None | 100 ± 3.7 | 74.2 ± 5.33-150 | 73.8 ± 5.13-150 | 18.3 ± 1.9 | 41.5 ± 3.93-150 | 44.2 ± 4.33-150 |
| BPB | 102.1 ± 5.3 | 97.6 ± 4.2 | 74.1 ± 4.23-150 | 17.9 ± 1.6 | 18.1 ± 1.7 | 43.7 ± 3.63-150 |
| Pertussis toxin | 103.2 ± 4.3 | 102.1 ± 3.7 | 73.7 ± 3.63-150 | 17.5 ± 2.2 | 19.1 ± 2.1 | 42.8 ± 5.13-150 |
| Herbimycin A | 101.5 ± 3.7 | 75.6 ± 4.23-150 | ND | 18.5 ± 2.1 | 42.1 ± 4.33-150 | ND |
[3H]Choline-labeled or unlabeled HL-60 cells were preincubated with BPB (10 μmol/L) for 10 minutes, pertussis toxin (20 ng/mL) for 18 hours or herbimycin A (1 μg/mL) for 14 hours and then treated or not with either IFN-γ (2,000 U/mL) or AA (10 μmol/L) for 2 hours. After incubation, cells were harvested, lipids were extracted, and SM levels were determined by TLC, while ceramide levels were determined using DAG kinase assay. The data (means ± SD) are from three independent experiments assayed in duplicate.
Abbreviation: ND, not determined.
Significantly different (P < .05, Student's t-test) from controls.
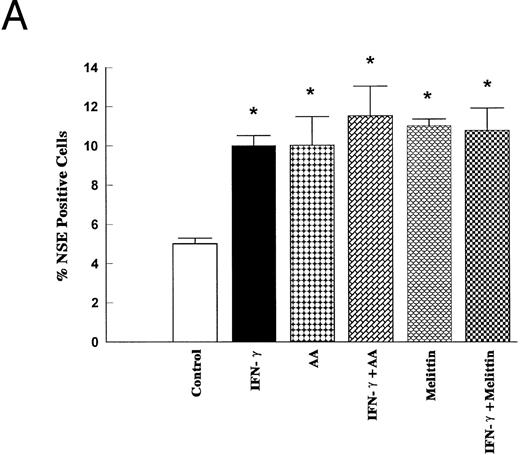
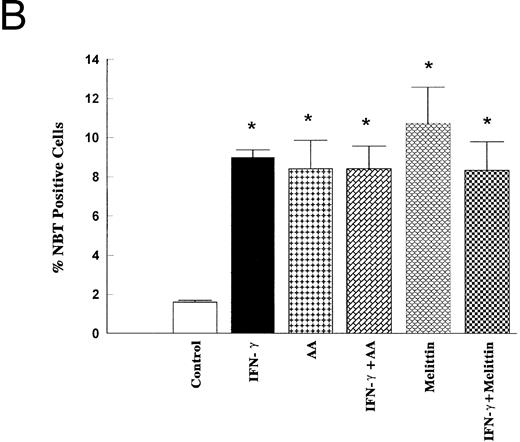
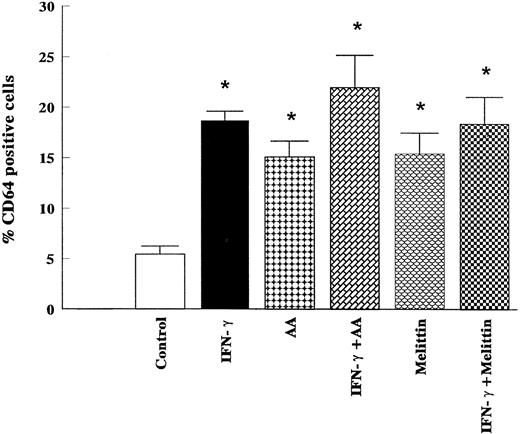
To further document a role of PLA2/AA signaling pathway in mediating IFN-γ–induced monocytic differentiation of HL-60 cells, the effects of a 5-day incubation of cells with IFN-γ (2,000 U/mL), AA (10 μmol/L), and melittin (500 ng/mL) were studied. The treatment of cells with IFN-γ for 5 days had no effect on cell proliferation, phagocytic ability, or the percentage of adherent cells compared with control (results not shown), but significantly increased the percentage of cells positive for NSE or NBT-staining (Fig 6). Cell surface analysis of HL-60 cells treated for 1 to 5 days with 2,000 U/mL of IFN-γ showed that IFN-γ had no effect on the expression of CD14 as a marker of mature monocytes, but significantly increased the binding of murine IgG2a after 5 days of incubation (results not shown). The binding of murine IgG2a to untreated or IFN-γ–treated HL-60 cells correlated with their expression of high-affinity FcRγI as measured by the binding of anti-CD64 (Fig 7). As shown in Figs 6 and 7, AA (10 μmol/L) and melittin (500 ng/mL) mimicked the effect of IFN-γ on NBT, NSE, and CD64 expression, and no further increase was observed when the cells were simultaneously treated with either combination of IFN-γ (2,000 U/mL) and AA (10 μmol/L) or IFN-γ and melittin (500 ng/mL). None of the groups tested had effects on cell proliferation, phagocytic ability, percentage of adherent cells, or increased expression of surface CD14 compared with control (results not shown).
Effects of IFN-γ, AA, and melittin on the NSE activity and the NBT reduction. HL-60 cells were cultured for 5 days in the presence of IFN-γ (2,000 U/mL), AA (10 μmol/L), melittin (500 ng/mL) or the combination of IFN-γ (2,000 U/mL) and AA (10 μmol/L) and the combination of IFN-γ (2,000 U/mL) and melittin (500 ng/mL). The cells were harvested and stained for α-NSE activity (A) and for the NBT reducing ability (B) The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Effects of IFN-γ, AA, and melittin on the NSE activity and the NBT reduction. HL-60 cells were cultured for 5 days in the presence of IFN-γ (2,000 U/mL), AA (10 μmol/L), melittin (500 ng/mL) or the combination of IFN-γ (2,000 U/mL) and AA (10 μmol/L) and the combination of IFN-γ (2,000 U/mL) and melittin (500 ng/mL). The cells were harvested and stained for α-NSE activity (A) and for the NBT reducing ability (B) The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.
Effects of IFN-γ, AA, and melittin on the levels of surface expression of CD64 in HL-60 cells. HL-60 cells were cultured for 5 days in the presence of IFN-γ (2,000 U/mL), AA (10 μmol/L), melittin (500 ng/mL), or the combination of IFN-γ (2,000 U/mL) and AA (10 μmol/L), and the combination of IFN-γ (2,000 U/mL) and melittin (500 ng/mL). Expression of CD64 was determined by flow cytometry. The data (means ± SD) represent the percentage of cells staining positive for the respective antigen obtained from six independent experiments. * Significantly different (P < .05, Student's t-test) from controls.
Effects of IFN-γ, AA, and melittin on the levels of surface expression of CD64 in HL-60 cells. HL-60 cells were cultured for 5 days in the presence of IFN-γ (2,000 U/mL), AA (10 μmol/L), melittin (500 ng/mL), or the combination of IFN-γ (2,000 U/mL) and AA (10 μmol/L), and the combination of IFN-γ (2,000 U/mL) and melittin (500 ng/mL). Expression of CD64 was determined by flow cytometry. The data (means ± SD) represent the percentage of cells staining positive for the respective antigen obtained from six independent experiments. * Significantly different (P < .05, Student's t-test) from controls.
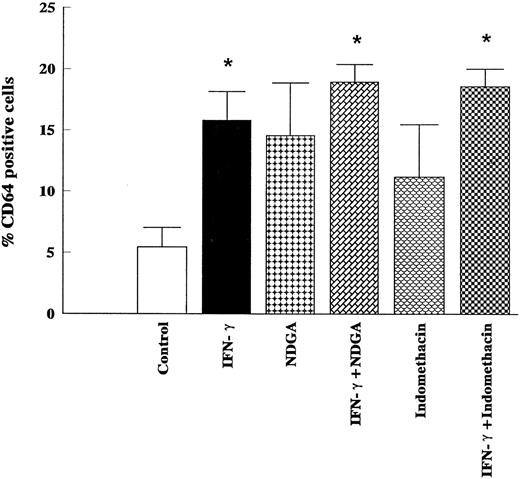
Because AA is enzymatically converted to leukotrienes by lipoxygenase and to prostaglandins by cyclo-oxygenase, there is a possibility that the action of IFN-γ on monocytic differentiation is mediated by one of those metabolites. Consequently, the cells were incubated by NDGA (50 μmol/L), a lipoxygenase inhibitor, or indomethacin (50 μmol/L), a cyclo-oxygenase inhibitor. Neither NDGA nor indomethacin affected the cell proliferation or adherence (results not shown). As shown in Fig 8, indomethacin (50 μmol/L) had no effect on the expression of CD64 on either control or IFN-γ–stimulated HL-60 cells. The treatment of cells with NDGA (50 μmol/L) had no effects on IFN-γ–stimulated CD64 expression, but slightly, although not significantly, increased the expression of CD64 in the control group (Fig 8). Results have to be interpreted with caution considering the potential of this concentration of NDGA to affect other pathways.27 Moreover, although HL-60 cells contain low levels of 5-lipoxygenase protein, the activity of enzyme in undifferentiated HL-60 cells and their capacity to generate leukotrienes are generally assumed to be negligible.28
Lack of effects of indomethacin and NDGA on the stimulation of surface expression of CD64 induced by IFN-γ in HL-60 cells. HL-60 cells were incubated for 5 days with IFN-γ (2,000 U/mL), indomethacin (50 μmol/L), NDGA (50 μmol/L), or the combination of IFN-γ (2,000 U/mL) and indomethacin (50 μmol/L) and the combination of IFN-γ (2,000 U/mL) and NDGA (50 μmol/L). Expression of CD64 was determined by flow cytometry. The data (means ± SD) represent the percentage of cells staining positive for the respective antigen obtained from six independent experiments. * Significantly different (P < .05, Student's t-test) from controls.
Lack of effects of indomethacin and NDGA on the stimulation of surface expression of CD64 induced by IFN-γ in HL-60 cells. HL-60 cells were incubated for 5 days with IFN-γ (2,000 U/mL), indomethacin (50 μmol/L), NDGA (50 μmol/L), or the combination of IFN-γ (2,000 U/mL) and indomethacin (50 μmol/L) and the combination of IFN-γ (2,000 U/mL) and NDGA (50 μmol/L). Expression of CD64 was determined by flow cytometry. The data (means ± SD) represent the percentage of cells staining positive for the respective antigen obtained from six independent experiments. * Significantly different (P < .05, Student's t-test) from controls.
DISCUSSION
Although the role of sphingomyelin turnover in IFN-γ–induced monocytic differentiation of HL-60 cells has been firmly established,3 the mechanisms operating in the regulation of IFN-γ–responsive sphingomyelinase are unknown. A delayed onset of sphingomyelin hydrolysis in response to IFN-γ3 or other monocytic inducers3,4,7 argues against a model of direct interaction of agonist-sensitive sphingomyelinase to activated receptor. A model has been proposed in which long-term cellular responses, such as differentiation, require a signaling network including sequential activation of phospholipases; the early activation of glycerophospholipid turnover is sufficient for mediating more prompt cellular responses and is followed by activation of sphingomyelinase and the sphingomyelin hydrolysis of a protracted course.29 The activation of neutral sphingomyelinase in HL-60 and promonocytic U937 cells in response to TNF-α is mediated by AA, while the activation of acidic sphingomyelinase in U937 requires prior activation of PC-PLC and generation of DAG.7 8 In the present study, data supporting a role for phospholipase A2 and AA in mediating effects of IFN-γ on sphingomyelin hydrolysis are strong and suggestive: (1) sphingomyelin hydrolysis with a concomitant increase in ceramide in response to IFN-γ is preceded by an early release of AA, (2) no increase in activity of phospholipase D or C was observed, (3) AA and melittin, an activator of phospholipase A2 , mimicked the effect of IFN-γ on sphingomyelin level, (4) the stimulatory effect of IFN-γ on sphingomyelin hydrolysis was abolished by the pretreatment of the cells with an inhibitor of phospholipase A2 , and this inhibitory effect was overcome by adding AA.
Our results show that IFN-γ increases PLA2 activity in HL-60 cells in a biphasic manner; an early release of AA from intact, prelabeled cells was observed within minutes of stimulation, and a later, sustained increase in PLA2 activity in vitro, which corresponded with the increase in cPLA2 expression, was observed 4 to 120 hours after the addition of IFN-γ. IFN-γ–stimulated AA release was suppressed by prior treatment of the cells with pertussis toxin, which ADP-ribosylates Gi-proteins and thereby blocks communications between receptors and effector enzymes. Pertussis-sensitive PLA2 activation was described in response to agonists that bind to the classical G-protein coupled receptor (having seven membrane spanning motifs) like ATP or thrombin, as well as in response to those agents (PDGF, basic fibroblast growth factor [FGF] that bind to receptors with tyrosine kinase activities, but the signal transduction system regulated by Gi that activates cPLA2 remains ill-defined.30-32 It has been proposed that the activation of cPLA2 by receptor-coupled G proteins may be mediated through either direct interaction of G proteins with cPLA2 or G protein activation of PLC, leading to increased levels of DAG and inositol trisphoshate/cytosolic Ca2+, activation of PKC, and phosphorylation and activation of cPLA2 .30,31 The latter has been ruled out in our model because no increase in the 1,2-diacylglycerol or intracellular Ca2+ concentration was observed in response to IFN-γ. Moreover, IFN-γ–induced AA release was not modified by herbimycin A or tyrphostine, further indicating that the PLA2 species involved is not regulated by tyrosine phosphorylation. Present results are similar to those described by Ponzoni et al15 in human neuroblastoma cells, suggesting the possibility that PLA2 is directly activated by the interaction between the IFN-γ-R and G-protein(s). Although it was observed that IFN-γ induced AA release in vivo, no increase in PLA2 activities in cell extracts was observed in vitro within minutes of stimulation. It is possible that in cell free extracts G-proteins are uncoupled from PLA2 , so their stimulatory effect on PLA2 activity in the intact cell could not be measured in vitro.33 A second possibility is that a major proportion of AA may be released by enzymes other than cPLA2 , such as secretory PLA2 , which is sensitive to the presence of disulfide reducing agents; immunochemical evidence for the presence of both cPLA2 and type II PLA2 in HL-60 cells was obtained.34 Although a contribution by sPLA2 of AA release cannot be completely ruled out, a role for sPLA2 seems less probable, as sPLA2 is a granule-associated, disulfide-containing enzyme released to the outside of the cell that requires millimolar concentrations of Ca2+ for activity,26,35 and our experiments with IFN-γ alone were conducted at physiological Ca2+ levels normally present in the growth medium. A final possibility is that inhibitory effects of pertussis toxin on AA release from cells are related to indirect effects of this toxin such as transient increases in cAMP.36 However, present data strongly support a role of cPLA2 in IFN-γ–stimulated increase in PLA2 activity observed at 4 to 120 hours after addition of the agonist; under our assay conditions, where 5 mmol/L DTT was present to inhibit any activity of sPLA2 , only the cPLA2 was measured, and an increase in cytosolic and membrane-bound PLA2 activity is directly proportional to an increase in cPLA2 protein in both fractions. The long-term effect on PLA2 activity is probably due to a sustained production of new cPLA2 , and the effect depends on tyrosine phosphorylation, as the increase could be inhibited by the pretreatment of the cells with herbimycin A, but the precise mechanisms responsible for the parallel increase in cPLA2 proteins remain unclear. Moreover, it is not yet clear whether the latter increase in cPLA2 activity and expression is directly related to IFN-γ–stimulated signaling events or through the effects of another cytokine induced by IFN-γ.
The key role of the sphingomyelin pathway in mediating the monocytic differentiation of HL-60 cells was proposed on the basis of results that show sphingomyelin hydrolysis occurs specifically in response to monocytic inducers and that the action of agonists on the induction of NBT-reducing ability and nonspecific esterase levels could be mimicked by the addition of bacterial sphingomyelinase or synthetic ceramide.2-4 However, a marked increase in the percentage of cells reducing NBT is regularly observed in response to retinoic acid 37,38or DMSO,39,40 which induce maturation of HL-60 cells along the neutrophilic pathway and have no effects on the sphingomyelin level. Although an increase in NSE level is a more specific marker of monocytic differentiation of HL-60 cells (1), it occurs in response to PMA,41 which exerts an opposite effect on sphingomyelin turnover since a significant increase in the level of sphingomyelin is observed in response to this inducer of macrophage-like phenotype.3 Our results show that monocytic differentiation in response to IFN-γ is somewhat incomplete; the cells exposed to IFN-γ for 5 days do not lose their ability to proliferate and do not express CD14 as a specific marker of mature monocytes. These results are in agreement with several studies in which the growth or proliferative ability was measured 3 to 6 days after the addition of a single dose of IFN-γ,42-44 although a marked inhibition of the growth of HL-60 by IFN-γ was also reported.45 It is highly probable that, even if 10% to 20% of HL-60 cells that express monocytic markers were arrested in Go/G1 on the fifth day of incubation, we could not detect the cessation of their proliferation by counting the cells in chambers. Vitamin D3 , an agonist that induces the sphingomyelin hydrolysis to the extent similar to that observed with IFN-γ,3,4 induced a marked growth arrest and expression of CD14.46 IFN-γ had no effect on CD14 expression, but induced a significant increase in the number of FcγRI or CD64. Obviously, the signaling mechanisms responsible for the induction of monocyte specific cell membrane antigens do not rely upon sphingomyelin turnover exclusively.
The ability of AA and melittin to mimic the effects of IFN-γ on NBT and NSE induction could be explained by their effect on sphingomyelin hydrolysis, but the effects on CD64 might occur independently. Previous studies have examined the possible role of AA metabolites in the induction of monocytic differentiation; the capability of HL-60 cells to generate prostaglandins or leukotrienes was shown to be upregulated during differentiation by PMA or vitamin D3 , respectively.27,47 The present results have demonstrated that the treatment of HL-60 cells with either indomethacin, an inhibitor of cyclo-oxygenase, or NDGA, an inhibitor of lipoxygenase, had no effects on IFN-γ–mediated induction of CD64 expression. Numerous studies have suggested that free fatty acids may mediate a variety of cellular processes independently of further metabolism to eicosanoids; a number of molecules have been defined as direct targets for the action of arachidonate in vitro, including ion channels, phospholipases, protein kinase C, and mitogen-activated protein (MAP) kinase.48,49 AA was found not only to regulate the transcription of genes coding for enzymes of fatty acid metabolism, but also to affect the transcription of distinct genes involved in cell differentiation.50-52 The expression of human FcγRI gene is restricted to myeloid cells and is specifically upregulated by IFN-γ53; a rapid and cycloheximide insensitive activation of transcription in response to IFN-γ appears to be mediated by JAK-Stat signaling pathways.11,12 Although it has been confirmed that the regulation of Fcγ-receptor expression depends on tyrosine phosphorylation,54 there is indirect evidence suggesting that lipid metabolism may be involved, as dexamethasone was reported to inhibit both basal and IFN-γ augmented FcγRI expression in U937 cells.55 AA might facilitate intracellular signaling involved in the differentiation response to IFN-γ, in analogy to the AA-dependent activation of the interferon-stimulated response element (ISRE) in cells treated with IFN-α.56
In summary, the data presented in this report support a role of the phospholipase A2 and AA, as an early event in the signaling pathway elicited by IFN-γ, in mediating IFN-γ–induced hydrolysis of sphingomyelin. The present studies have demonstrated that AA and melittin mimic the effects of IFN-γ on NBT-reduction, NSE level, and CD64 expression on HL-60 cells. Additional studies are necessary to define the downstream effector molecules of this PLA2/SM pathway that are capable of regulating the expression of phenotypic markers associated with the differentiation of HL-60 along the monocytic lineage.
ACKNOWLEDGMENT
We thank Robin F. Irvine, Leonard R. Stephens, Nullin Divecha, David Lander, Andrew Letcher, Matko Marušić, Branka Užarević, and Žaklina Ćavar for valuable help and advice and René Lui for editing the manuscript.
Supported by the Ministry for Science of the Republic of Croatia and by the Wellcome Trust.
Address reprint requests to Hrvoje Banfić, MD, PhD, Zavod za fiziologiju, Medicinski fakultet, Šalata 3, POB 978, 10000 Zagreb, Croatia.

![Fig. 1. Effect of IFN-γ on sphingomyelin (A) and ceramide (B) levels in HL-60 cells. [3H]Choline-labeled or unlabeled cells were treated at time 0 with 2,000 U/mL of IFN-γ. At the indicated times, cells were harvested, lipids were extracted, and SM levels were determined by TLC, while ceramide levels were determined using a DAG kinase assay as described in Materials and Methods. The data (means ± standard deviation [SD]) are from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f1a.jpeg?Expires=1769097847&Signature=GfAXy2~d43VnPCuUJMqQdpWA69~JIwOcsUoimjntqHOIZ~fA1h4xjnP4h1iKI3pnxQv-5IkfcA3lWpvgtYFxyiuaxXMZf5jPcsnO7fmTwfX~l41Vi8xNWbgcdCG-P0B7utN~EIEs8vODtGUj0lnKvEw0q7Iq4O5AgZ-pfVSGEv6yiAm6CxF1kPisVZL9uyGwNZ6p8Wa7xfPBNJY8nP22WWfr4TcZ0JhACK~GYUju7GWOiTsJKhkggiiY5WIeYxZYbWBWT9k4bXR3pVZbpMj4sI7NzriARIznC23U9wbls0rDGvidux3nWpQ9fB3qAMsYMVAZ0zbVEF2p2lOOy0jpSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of IFN-γ on sphingomyelin (A) and ceramide (B) levels in HL-60 cells. [3H]Choline-labeled or unlabeled cells were treated at time 0 with 2,000 U/mL of IFN-γ. At the indicated times, cells were harvested, lipids were extracted, and SM levels were determined by TLC, while ceramide levels were determined using a DAG kinase assay as described in Materials and Methods. The data (means ± standard deviation [SD]) are from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f1b.jpeg?Expires=1769097847&Signature=vvC~wabEQbG~ZGtenre1kfjsBUNaSyiAW4Vcne6h1SgLo2WOSPGMLNsDA4iuyjFwYLiS~aPdWweggum1lrwZrLQGy4xzh8pKSd3fy~jv7DVxmKs9ImvW9s3iNX4DDq9p9eKl5x1b2HD1J4aMrWjWAlB~phnjtb8LnIP5FfTapYKWc1AVqrwO0pSkEb4oK7C~unPC2ejLW1H80FGYkLb5cQQLS~2OutkzwvNweHLGyz-uTFqp-qNuwSqlJGJ6RrCz24Vkht5BmqenBPexGlx9ezjRTAqJKSG1oGOqem0LkIMbXWxBxOF8iucf99CpgPkcActfwa7Iyts3PdmQF9s6gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Time course and dose response curve of IFN-γ–stimulated AA release. HL-60 cells were prelabeled for 60 minutes with 5 μCi/mL [3H]AA, washed twice, and resuspended in the medium. After incubation with IFN-γ, cells were pelleted and the resultant supernatant was counted for 3H radioactivity. (A) Time course of AA release following treatment with 2,000 U/mL of IFN-γ. (B) Dose response of AA release at 2 minutes following IFN-γ–treatment. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f2a.jpeg?Expires=1769097847&Signature=c3HWVFS955flg0juW5kJoB2m3yf-m-KtSVyQGt9z2BwklhZgxjI1mReXDlHaHSrqZGYqYWoXblNw~9erJpjm5gv55BL8bF~qpgiS1UQJu9QX302wFtOq0baVJSnalNFLtpI86PJY0wRNg90epKHtNe9dGs94nSIFf0lqNR~Dp1ghLtv3or4AV1D5mxMbrzhOPkvK5J7Lpxroh5PJEyotBABQB4MQsvEDUJpiEzKu759ouUGdHPlaWoFDl98lfgp8SV7yP6otiC3EzFVeFJgyMOmKKnNXYpOsSXQWJnelgTSUQmd19IrmxsoBhcWVF9idVHEwVIZmxYVK3u8mAZ9ceQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Time course and dose response curve of IFN-γ–stimulated AA release. HL-60 cells were prelabeled for 60 minutes with 5 μCi/mL [3H]AA, washed twice, and resuspended in the medium. After incubation with IFN-γ, cells were pelleted and the resultant supernatant was counted for 3H radioactivity. (A) Time course of AA release following treatment with 2,000 U/mL of IFN-γ. (B) Dose response of AA release at 2 minutes following IFN-γ–treatment. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f2b.jpeg?Expires=1769097847&Signature=22gLIZ~c~Vf0oLOw~aSrzyRENZqY8UdQoUzcvl0Pq7GnG2dSN7qvli~WOXSUofXLbMGaq~~ELZl9zp723m5Q4yOBZNs8deLIX4hv2w~oRNAZggTSPulc~BVg6Ho-ezWe49ncm-H1JMFgPNgtQQgtDEPMgHzFbO1VIS6uK2~H6Q~dJx8HOqyhpks9QmqjgIB5uDR8KQnX7vqzoFO7Ec9AnNpL-iNviZZEKFGYBahzSRWChJ91Tbu79f3mwEg7Ffh6yxWkapBenzZ9GGM89lOwXtPL97wxwjf1bXQXtJKLTCiH-29f~eO5YxHLlQKp3rhXjye7U12nsp~o0h9MOwcs5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. PLA2 activity in membrane and cytosol fractions of IFN-γ–stimulated HL-60 cells. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. PLA2 activity in particulate and soluble fractions was determined by hydrolysis of [arachidonyl-14C]PtdCho in the presence of 5 mmol/L DTT and 150 nmol/L Ca2+. (A) PLA2 activity in membrane fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. (B) PLA2 activity in cytosol fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f3a.jpeg?Expires=1769097847&Signature=fX2OmBfsW0pjrJqs14PW1ye7w5lIZkZjtmCq7f~t-Szet9SoR9hfj1Ldqgg8pBrt2ZqeDYpX8GooZdQOQW-nWy1oqhAzmajgT9JMg2nHp7BoZ-RhGnYPT3JhrvFEz9uTVoa38lLas6jma~QB37IlPmJ5rJPiGdDGO8SYnMUCNa~MqeHEtBNYTgrBm9aKohcZ~uFSKlVE09rf8M~lxImMaCcy20bYE3ZME8f0BwjmwBIq4Q71bwJSE-nri9wHwwH7ULKqYlOesPKx~D-tvB5VTCfZsaVOKwe7JULjHjbUtTL29AYHQegzR2G6rTLgiuwUQ2YhNjqUgnVxSR0hEiirwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. PLA2 activity in membrane and cytosol fractions of IFN-γ–stimulated HL-60 cells. HL-60 cells were incubated with 2,000 U/mL of IFN-γ for indicated times and homogenized as described in Materials and Methods. PLA2 activity in particulate and soluble fractions was determined by hydrolysis of [arachidonyl-14C]PtdCho in the presence of 5 mmol/L DTT and 150 nmol/L Ca2+. (A) PLA2 activity in membrane fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. (B) PLA2 activity in cytosol fraction of HL-60 cells treated with 2,000 U/mL of IFN-γ for indicated times. The data (means ± SD) are expressed as percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f3b.jpeg?Expires=1769097847&Signature=bR7HibGWcQrA6bZdBchpAr8j1Un-doZENccaUY7oVq750MEoKg1pk1-qFWRgiJ-ry2MjVgNacBewgQVKz2Vov5nk5YekYSp308ByP7gHVdZ58oIZV1S1gweDZXdKG2FN-Hmkaf52ztTjLUo8JzHJ06m~o-Sktnux8u30hI4jPRVqGYJhOMhaOysZvxBu2cgmzuFaH6OiiNiwmMV-wBg~tVDS3Fnc~BG-Bw84SWluSR3k1nylOM3Uz3f5Rx6PCgnJuPgP6LIGDWiYzhlCApT6jbBPAbfr5aaeE17JjZf-AdGH-ZEe~25wk2iULayvWIERcGr~nMWYt~dMCUMWNbzVyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Sphingomyelin levels in response to AA and melittin. [3H]Choline-labeled HL-60 cells were treated with either 10 μmol/L AA (A) or 500 ng/mL of melittin (B) and sphingomyelin levels were determined as described in Materials and Methods. The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f5a.jpeg?Expires=1769097847&Signature=1JNycUKPml5ybCTybGe3BRk0xL0RTFAIOZscDXCC7OU95cA5MuaQcc3heuMIFUUHjTDveffx9Vm7plTOlsJVmXPmQhhtkmuDJang2kMsZV09EbEEGOIAj-zd~vxHXyl4mD5AcOsgPdxcpX1cyZllyH74Yw-zqOMxsz-91N2h7A0QdvfIQEZFAxDe8vQ-EOJqO1iGgIo-Nlnvm9etKLFR5iYR4k8NGyzTHsyTBIeBzE0i71aYGvRMNO8APc6voxFvs4cGGY7OvJN1IiYmxG8wC66imZJVYLxT52B-jbZCffJT4RmcT6iFhhjrKT958Fir0w3dQCfCM2qPj8u0xmGk0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Sphingomyelin levels in response to AA and melittin. [3H]Choline-labeled HL-60 cells were treated with either 10 μmol/L AA (A) or 500 ng/mL of melittin (B) and sphingomyelin levels were determined as described in Materials and Methods. The data (means ± SD) are expressed as a percentage of the control level obtained from three independent experiments assayed in duplicate. * Significantly different (P < .05, Student's t-test) from controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.81/2/m_bl_0028f5b.jpeg?Expires=1769097847&Signature=D-bq~l-YFQeEkBms-A~4Ws00C7-LnCiv12fsiKFpbjVUYmXvN5u6XRbfSUPMngL6bNzoV7xNfzbroV2iWynme1TPTttX8bRVyJkvoM2itVbMUY6OkaInzubo6eIF6V92vorTsuWdoEP7BKrNDMOOxtlKEbYL0P6gctjgGQNGBI-xBiHRkUNGQNcEsarkw9gZ-KXHVCDZmeZfyPrvv7u9-dfft53fwQaQ~oMTuSLOWSv1mQr8oRsMJE7UyQY~bBoPCH~20rDGQCNjDrD7uh6RBDyAa-4dA9-IjW2t96adoPys6PlKwqyr7qmU2GxHvsYdgAXthV4FdSaWvvPn0wzk0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal