Abstract
The X-linked form of chronic granulomatous disease (X-CGD), an inherited deficiency of the respiratory burst oxidase, results from mutations in the X-linked gene for gp91phox, the larger subunit of the oxidase cytochrome b. The goal of this study was to evaluate the impact of retroviral-mediated gene transfer of gp91phox on host defense against Aspergillus fumigatus in a murine model of X-CGD. Retrovirus vectors constructed using the murine stem cell virus (MSCV) backbone were used for gene transfer of the gp91phox cDNA into murine X-CGD bone marrow cells. Transduced cells were transplanted into lethally irradiated syngeneic X-CGD mice. After hematologic recovery, superoxide production, as monitored by the nitroblue tetrazolium (NBT) test, was detected in up to ≈80% of peripheral blood neutrophils for at least 28 to 35 weeks after transplantation. Neutrophil expression of recombinant gp91phox and superoxide production were significantly less than wild-type neutrophils. However, 9 of 9 mice with ≈50% to 80% NBT+ neutrophils after gene transfer did not develop lung disease after respiratory challenge with 150 to 500 A fumigatus spores, doses that produced disease in 16 of 16 control X-CGD mice. In X-CGD mice transplanted with mixtures of wild-type and X-CGD bone marrow, ≥5% wild-type neutrophils were required for protection against A fumigatus challenge. These data suggest that expression of even low levels of recombinant gp91phox can substantially improve phagocyte function in X-CGD, although correction of very small percentage of phagocytes may not be sufficient for protection against A fumigatus.
CHRONIC granulomatous disease (CGD) is a group of recessive inherited disorders in which neutrophils and other phagocytic leukocytes are unable to generate superoxide and derivative microbicidal oxidants.1 In the absence of this essential pathway, affected patients develop recurrent and often life-threatening bacterial and fungal infections, beginning in early childhood. The disease results from mutations in any one of four subunits of the phagocyte respiratory burst oxidase (NADPH-oxidase), including a cytochrome b heterodimer that functions as the redox center in the transfer of electrons from NADPH to oxygen.1 Two thirds of cases are caused by mutations in the X-linked gene for gp91phox, a 91-kD membrane glycoprotein that is the large subunit of the oxidase cytochrome b. A rare autosomal recessive form of CGD results from defects in the gene encoding p22phox, the 22-kD subunit of cytochrome b. The remaining autosomal recessive cases are caused by a genetic deficiency of either p47phox or p67phox, two soluble proteins in the oxidase complex. Current management of CGD includes the prophylactic use of antibiotics and interferon-γ,2,3 but serious infections still occur, particularly in those patients with X-linked CGD (X-CGD).4 5
CGD has been considered as a candidate disease for gene therapy targeted at hematopoietic stem cells. Transfer of a functional gene into hematopoietic stem cells should correct the defect in respiratory burst oxidase in circulating and tissue phagocytes, and provide a life-long cure of the disease. Complete correction of respiratory burst oxidase activity in even a minority of circulating neutrophils may abrogate the defect in host defense, based on the observation that female carriers with as few as 5% to 10% normal neutrophils are healthy.6 The relative level of phagocyte superoxide production may also be an important determinant in host resistance to microbial infection. Rare “variant” patients with X-linked CGD with low levels of residual phagocyte superoxide-generating activity can have a milder clinical course, yet others have numerous infectious complications that are indistinguishable from those patients with total absence of the respiratory burst.7 8
Gene transfer using retroviral and plasmid vectors has been successful in reconstituting phagocyte respiratory burst activity in CGD phagocytes cultured in vitro. In experiments using an X-CGD myeloid cell line generated by targeted disruption of the gp91phox gene, expression of only modest amounts of recombinant gp91phox led to a considerable reconstitution of superoxide production, with full restoration of enzyme activity seen with levels of gp91phox estimated at only ≈20% of wild-type levels.9-12 Retroviral-mediated gene transfer into peripheral blood (PB) or bone marrow (BM) progenitors obtained from CGD patients with either gp91phox-, p47phox-, or p22phox-deficiency can reconstitute the respiratory burst oxidase in granulocyte-monocyte progeny differentiated in vitro, although the relative level of enzyme activity could not be measured directly in these studies.13-15 In a recent clinical trial involving p47phox-deficient CGD patients, circulating neutrophils with respiratory burst activity were seen after infusion of autologous PB stem/progenitor cells that had been transduced with a retroviral vector containing the p47phox cDNA.16 The percentage of these corrected cells represented only 0.002% to 0.05% of the circulating neutrophils, reflecting both the relatively inefficiency of retroviral-mediated gene transfer into human hematopoietic cells and the fact that transduced cells were infused into nonablated recipients.
The goal of the present study was to determine whether retroviral-mediated gene transfer of gp91phox into hematopoietic precursor cells could improve phagocyte function in vivo in a murine model of X-CGD. X-CGD mice have a null allele for gp91phox, and, similar to patients with CGD, manifest impaired resistance to infection with Staphylococcus aureus and to Aspergillus fumigatus,17 an opportunistic fungus that is an important cause of serious infections in CGD patients.18,19 Hence, this animal model provides an opportunity to investigate the efficacy of gene replacement therapy in correcting the symptoms of an inherited disorder in vivo, which to date has been accomplished with limited success in only a handful of genetic diseases.20,21 In the present study, retroviruses based on the murine stem cell virus (MSCV) vector backbone22 were used for gene transfer of the gp91phox cDNA into X-CGD BM cells, which were then transplanted into lethally irradiated syngeneic X-CGD mice. Although neutrophil expression of recombinant gp91phox and superoxide production were significantly less than wild-type neutrophils, mice with rescue of respiratory burst activity in 40% to 80% of circulating PB neutrophils were resistant to respiratory challenge with A fumigatus. In contrast, in X-CGD mice transplanted with mixtures of wild-type and X-CGD BM cells, at least 5% of circulating wild-type neutrophils were required for protection against A fumigatus challenge.
MATERIALS AND METHODS
Cell lines.Retroviral packaging lines for GP+E8623 and GP+envAM1224 were provided by Dr A. Bank (Columbia University, New York, NY). They and derivative cell lines were maintained in 50% Hams F12 and 50% Dulbecco's modified Eagles medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (Sigma, St Louis, MO), 100 U/mL penicillin (GIBCO), 100 mg/mL streptomycin (GIBCO), 2 mmol/L glutamine (GIBCO), and 15 mmol/L HEPES Buffer Solution (GIBCO). NIH 3T3 fibroblasts were obtained from Dr D. W. Clapp (Indiana University School of Medicine, Indianapolis).
Mice.X-CGD mice with a null allele for gp91phox had previously been generated by targeted disruption of the gp91phox locus in murine embryonic stem cells derived from the 129-SV strain.17 Successive backcrossing of female carriers of the mutant gp91phox allele to C57B1/6J males was used to introduce the mutant gp91phox allele into the C57B1/6J background. X-CGD and wild-type mice studied in this report were obtained from litters generated after ≥7 generations of backcrossing. These included female mice homozygous for the mutant gp91phox allele that were generated by breeding X-CGD males and X-CGD females. No differences have been observed in the X-CGD phenotype in male versus female mice (unpublished observations, 1996). Genotyping of mice was performed by polymerase chain reaction (PCR) of tail blood as previously described, and confirmed by nitroblue tetrazolium (NBT) testing of PB neutrophils.17 Mice were maintained under specific pathogen-free conditions and fed autoclaved food and acidified water, as previously described.17
Retroviral vectors and virus-producing cells.Recombinant MSCV-91Neo retroviruses for expression of murine (m91) and human (h91) gp91phox were previously constructed using a murine stem cell virus (MSCV) vector backbone,22 such that the gp91phox cDNA is downstream of a modified myeloproliferative sarcoma virus long terminal repeat (LTR) followed by a phosphoglycerokinase (PGK) promoter-neomycin phosphotransferase cassette.11,12 A high titer ecotropic packaging line for the MSCV-m91Neo retrovirus was prepared by cross-infection (“ping-pong”) of previously described ecotropic GP+E86 and amphotrophic GP+envAM12 lines producing MSCV-m91neo virus particles.11 The ecotropic and amphotrophic lines were cocultured in the presence of Polybrene (Aldrich Chemical Co, Milwaukee, WI) at 4 μg/mL for 2 weeks, followed by isolation of individual clones by limiting dilution. Clones were split into duplicate fractions, one of which was grown in the presence of Hygromycin B (Calbiochem, San Diego, CA) at 200 mg/mL to identify ecotropic producer clones, which are hygromycin-sensitive. Ecotropic producer clones identified in this manner were evaluated for quantitative titers by G418-resistance of supernatant-infected 3T3 cells10 and the clone which had the highest titer (≈1 × 106 infectious particles/mL) was used for transduction of murine BM cells by cocultivation. In some experiments, a previously reported ecotropic packaging line for the MSCV-91Neo vector (titer ≈2 × 105 infectious particles/mL),12 which contains the human gp91phox cDNA, was used for transduction of murine BM.
Retroviral infection and BM transplantation.Isolation, transduction, and transplantation of murine X-CGD BM cells was essentially as previously described.12,25 Briefly, BM was obtained from femurs and tibias of 6- to 8-week-old X-CGD male mice17 2 days after intraperitoneal injection of 5-FU (Fluorouracil; SoloPak Laboratories Inc, Elk Grove Village, IL) 150 mg/kg body weight. BM cells were prestimulated for 48 hours in the presence of 100 U/mL recombinant human interleukin-6 (rhIL-6; Pepro Tech Inc, Rocky Hill, NY), and 100 ng/mL recombinant rat stem cell factor (rrSCF; Amgen, Thousand Oaks, CA). Cells were then overlaid for 48 hours onto Mitomycin-C–treated (Sigma) producer cells in the presence of 100 U/mL rhIL-6, 100 ng/mL rrSCF, and 4 μg/mL Polybrene. Marrow cells were obtained using 1× phosphate-buffered saline (PBS) and Cell Dissociation Buffer (GIBCO) according to the manufacturer's recommendations and 2 to 3 × 106 cells/mouse injected intravenously into lethally irradiated male X-CGD recipients (cesium 137, 11 Gy, split dose, with about 3 hours between doses). Beginning at 4 to 5 weeks after transplantation, PB counts and NBT testing were performed on tail blood samples (see below).
For some experiments, total BM was obtained as above from male or female X-CGD and wild-type mice without prior injection of 5-FU, and used to prepare mixtures of total BM cells containing different ratios of X-CGD to wild-type cells. These mixtures were injected intravenously into lethally irradiated X-CGD recipients of the same sex (2 × 106 BM cells per animal).
Experimental infection with A fumigatus. Mice were infected with A fumigatus conidia as previously described,17 using doses of 150 or 500 conidia per animal. Briefly, the trachea was exposed in mice anesthetized with ketamine, acepromazine, and atropine and the inoculum was instilled through a 24G angiocath (Becton Dickinson Vascular Access, Sandy, UT) in 35 μL of sterile saline containing 5% colloidal carbon (Eberhard Faber, Inc, Lewisburg, TN) to allow localization of the inoculum in each lung. As prophylaxis against secondary bacterial infection, mice were given intramuscular injection of Ceftriaxone (Rocephin; Hoffman-La Roche, Nutley, NJ) 1.25 mg per animal immediately before infection, and again 24 hours later, followed by oral Tetracycline (Polyotic; American Cyanamid Co, Wayne, NJ) 5 mg/mL in the drinking water for the remainder of the experiment. Mice were examined daily and were electively killed by cervical dislocation 17 to 21 days after challenge with A fumigatus. Lungs were removed and inflated and fixed in neutral buffered formalin for histologic examination of paraffin-embedded sections obtained from carbon-stained regions of lung. Sections were stained with hematoxylin and eosin for assessment of pathological changes or Grocott methamine silver for assessment of hyphae.
Isolation of neutrophil-enriched BM cells.Transplanted X-CGD mice and wild-type and X-CGD control mice were killed by cervical dislocation and BM cells obtained from femurs and tibias. To enrich BM cells for neutrophils, one of two different protocols was used. In some experiments, nonadherent BM cells were isolated by plating the entire BM harvest on a 10-cm tissue culture dish and incubating at 37°C for 1 hour. Nonadherent cells were collected and centrifuged at ∼1,250 rpm for 10 minutes at 4°C. Red blood cells (RBCs) were lysed in 3 mL ACK-buffer for 4 minutes on ice, 7 mL 1× Hanks' balanced salt solution (1× HBSS without Ca2+, Mg2+ and phenol red) + 1% bovine albumin (BSA), 1% glucose, pH 7.2, added and cells centrifuged as above. The cell pellet was then washed in 1× HBSS, 1% BSA, 1% glucose, pH 7.2. These cells contained 50% to 60% mature neutrophils as determined by examination of Wright's-stained cytospin preparations. In the second method, murine BM neutrophils were purified using discontinuous Percoll density gradients essentially as described,26 with the following modifications. BM cells were obtained and RBCs lysed as described above. The stock solution was diluted to working solutions of 81%, 62%, 55%, 50%, and 45% Percoll (Pharmacia Biotech AB, Uppsala, Sweden) concentration. Two milliliters of each of the 62%, 55%, and 50% Percoll solutions were layered successively onto 3 mL of the 81% solution. Finally, the cell suspension, diluted to 3 mL in a 45% Percoll Solution, was layered on top of the gradient. After centrifugation, the cell band between the 81% and 62% layer was collected and washed twice in 10 mL 1× HBSS, 1% BSA, 1% glucose, pH 7.2 (∼1,250 rpm for 10 minutes at 4°C). The final cell preparation contained between 70% and 90% mature neutrophils. For both nonadherent BM cell and Percoll preparations, the final cell preparation was maintained on ice in 1× HBSS with 1% glucose and 1% BSA until further processing (for assay of superoxide production and/or extraction of protein or DNA) (see below).
DNA, RNA, and immunoblot analysis.DNA was extracted using Isoquick (ORCA Research Inc, Bothell, WA) according to the manufacturer's instructions. Genomic DNA samples were cut with KpnI (Boehringer-Mannheim) before electrophoresis in a 1% agarose gel. Serial dilutions of a plasmid bearing the provirus digested with KpnI were also loaded on the same gel for estimation of provirus copy number. Total RNA was extracted using RNAzol B (TEL-TEST, Inc, Friendswood, TX) according to the manufacturer's recommendations and separated on a formaldehyde agarose gel as described.9 Southern and Northern transfers to Magnacharge nylon membranes (Micron Separations, Inc, Westborough, MA) were performed using standard protocols supplied by the manufacturer. Membranes were probed with random prime-labeled full-length murine gp91phox, actin, or neomycin phosphotransferase cDNAs, as previously described.10 Triton X-100 extracts and cellular membranes of either purified murine BM neutrophils or nonadherent murine BM cells were prepared as previously described.9,10 Protein concentration was measured by BCA protein assay (Pierce, Rockford, IL). Immunoblot analysis was performed as previously described,9 using polyclonal rabbit antibodies for gp91phox or p22phox, as described,17 or a polyclonal rabbit antibody for p47phox (kindly provided by Dr D. J. Uhlinger, Emory University School of Medicine, Atlanta, GA) as probes.
PB counts and measurement of respiratory burst oxidase activity.PB counts (hematocrit, white blood cell counts, and differential) were determined on blood obtained from the tail vein.27 The NBT assay was performed on PB neutrophils allowed to adhere to a glass slide for 15 to 20 minutes or on BM-derived neutrophils that were allowed to adhere to a chamber slide (Nunc, Inc, Naperville, IL) for 1 hour before the respiratory burst oxidase was activated with phorbol myristate acetate (PMA), as described.17 Slides were incubated for 20 to 30 minutes at 37°C before fixation and counterstaining with safranin. A continuous cytochrome c assay was used for quantitative determination of superoxide formation in PMA-stimulated BM-derived neutrophils in the presence or absence of superoxide dismutase.9 28
RESULTS
Recombinant MSCV-91Neo retrovirus vectors expressing either the human (MSCV-h91Neo) or murine (MSCV-m91Neo) gp91phox cDNA were used to transduce murine X-CGD BM cells by cocultivation with ecotropic packaging cells. Transduced cells were then transplanted into lethally irradiated syngeneic X-CGD recipient mice. These vectors have been shown to confer expression of modest amounts of recombinant gp91phox in an X-CGD myeloid cell line, leading to high-level reconstitution of respiratory burst oxidase activity that was comparable to wild-type cells in the majority of transduced clones.11,12 The murine and human gp91phox protein sequences are highly similar,11 and exhibit cross-species complementation of respiratory burst oxidase activity in human and murine X-CGD phagocytes cultured in vitro.11 12
After hematologic recovery of transplanted X-CGD mice, reconstitution of respiratory burst activity in circulating neutrophils was monitored using the NBT assay. Neutrophils from control X-CGD mice, which do not express gp91phox due to targeted disruption of the corresponding gene,17 are unable to generate superoxide and are hence NBT−. Thus, the presence of NBT+ neutrophils was used as a marker for the functional expression of recombinant gp91phox derived from the MSCV-91Neo provirus. The proportion of NBT+ neutrophils in recipients of retroviral-transduced X-CGD marrow was related to the titer of the retroviral producer line. In 6 recipients of marrow transduced with MSCV-h91Neo, which had a titer of ≈2 × 105 colony-forming units (cfu)/mL, the percentage of NBT+ neutrophils during weeks 4 through 14 after transplantation ranged between 1% and 3% in 4 mice and between 6% and 19% in the remaining 2 mice. The percentage of NBT+ cells in the latter 2 mice has fluctuated between 0% and 17% in weeks 20 through 35 after transplantation, with 6% and 13% NBT+ neutrophils detected at week 35. In 22 recipients of marrow transduced with the MSCV-m91Neo virus, which had a titer of ≈1 × 106 cfu/mL, the fraction of NBT+ neutrophils ranged between ≈50% and 80% during the first 10 to 12 weeks after transplantation. This percentage of NBT+ neutrophils has remained stable in a cohort of 9 mice followed for 28 weeks after transplantation. The peripheral hematocrit and white blood cell count in transplanted animals have been comparable to control X-CGD and wild-type values during this period of observation (data not shown).
A subgroup of X-CGD mice transplanted with MSCV-91Neo-transduced marrow were challenged with A fumigatus 10 to 14 weeks after transplantation, as described below. Mice were killed at 17 to 21 days after fungal challenge. DNA and RNA was isolated from total spleen tissue for analysis of proviral copy number and vector-derived gp91phox mRNA. The percentage of NBT+ neutrophils in the BM cells in each mouse at time of sacrifice was very similar to the percentage of NBT+ neutrophils in the PB. In mice where the high-titer MSCV-m91Neo vector was used, neutrophil-enriched cell preparations were obtained from BM by removal of adherent cells or by Percoll density fractionation, and used for analysis of gp91phox protein expression, and, in some cases, quantitative measurements of superoxide production. Because of the low rates of gene transfer, studies of gp91phox mRNA and protein expression were not performed on mice transduced with the MSCV-h91Neo vector.
Provirus copy number in spleen genomic DNA was studied by Southern blot, and was estimated by comparison of signal intensity of the neomycin phosphotransferase sequence to serial dilutions of a plasmid containing the provirus (not shown). In recipients of MSCV-h91Neo–transduced marrow, who had 1% to 2% NBT+ neutrophils, the viral copy number was estimated to be ≈0.1 copy per cell. In contrast, the viral copy number in spleen DNA isolated from recipients of MSCV-m91Neo–transduced marrow, who had ≈50% to 80% NBT+ cells, was estimated to be 1 to 2 copies per cell. Hence, the transduction efficiencies for each vector, as estimated both by the percentage of NBT+ neutrophils and by proviral copy number, correlated with their titers on 3T3 cells.
The expression of retrovirus gp91phox transcripts was examined in spleen tissue in mice transplanted with the MSCV-m91Neo–transduced BM, which contains a heterogenous mixture of lymphoid and myeloid cells derived the transplanted marrow. Expression of gp91phox is normally restricted to mature phagocytes, and was detected at only low levels in wild-type spleen (Fig 1). In mice transplanted with transduced marrow, two gp91phox transcripts of ≈5 kb and ≈4 kb were detected in spleen tissue, which are the sizes predicted for the unspliced and spliced transcripts driven by the viral 5′-LTR. The abundance of vector-derived transcripts in spleen was somewhat less than the endogenous ≈4.7-kb gp91phox transcript present in wild-type nonadherent BM, which contains 50% to 60% neutrophils. Because the spleen and nonadherent BM contain different cell populations, it is difficult to compare the relative level of retrovirus-derived gp91phox mRNA expression with the endogenous transcript. However, in this study mRNA analysis was not performed on nonadherent BM cells, which were used instead for analysis of gp91phox protein expression and superoxide formation.
Northern blot analysis of RNA extracted from mouse BM and spleen. Total RNA was extracted from nonadherent wild-type BM and from spleens obtained from wild-type (WT) mice, X-CGD (CGD) mice, and X-CGD mice 13 to 15 weeks after transplantation with MSCV-m91Neo–transduced X-CGD BM. Total cellular RNAs (20 μg per lane) were probed with a radiolabeled murine gp91phox cDNA (top), and then reprobed with radiolabeled actin cDNA (bottom). The position of the 28S and 18S ribosomal RNAs (top) and actin RNA (bottom) are indicated. The arrows indicate the position of the unspliced and spliced LTR-driven transcripts.
Northern blot analysis of RNA extracted from mouse BM and spleen. Total RNA was extracted from nonadherent wild-type BM and from spleens obtained from wild-type (WT) mice, X-CGD (CGD) mice, and X-CGD mice 13 to 15 weeks after transplantation with MSCV-m91Neo–transduced X-CGD BM. Total cellular RNAs (20 μg per lane) were probed with a radiolabeled murine gp91phox cDNA (top), and then reprobed with radiolabeled actin cDNA (bottom). The position of the 28S and 18S ribosomal RNAs (top) and actin RNA (bottom) are indicated. The arrows indicate the position of the unspliced and spliced LTR-driven transcripts.
To examine the relative levels of expression of gp91phox protein and other oxidase subunits, extracts of neutrophil-enriched BM preparations were analyzed by immunoblotting with specific antibodies (Fig 2). The endogenous gp91phox in wild-type murine neutrophils is ≈58 kD (Fig 2), which is smaller than the ≈91-kD human gp91phox due to differences in N-linked glycosylation sites.11 As previously reported,17 neutrophils from control X-CGD mice lacked gp91phox (Fig 2). X-CGD mice also had markedly reduced levels of the p22phox subunit of cytochrome b compared with wild-type samples, because each cytochrome subunit is unstable in the absence of its partner. In X-CGD mice transplanted with marrow transduced with the high-titer MSCV-m91Neo vector, recombinant gp91phox was detected only at low levels (Fig 2). A small increase in expression of the p22phox cytochrome subunit was seen in some samples obtained from the transplanted X-CGD mice compared with control X-CGD mice. As a control for protein loading, the same blot was also probed with an antibody for the p47phox oxidase subunit, whose expression is normal in X-CGD mice (Fig 2).
Immunoblot analysis of murine neutrophil-enriched BM. Neutrophil-enriched BM preparations were obtained from wild-type (WT) mice, X-CGD mice (CGD), and X-CGD mice 13 to 15 weeks after transplantation with MSCV-m91Neo–transduced X-CGD BM. Cellular proteins were extracted from either nonadherent BM cells (lanes 1 through 9) or a neutrophil-enriched Percoll fraction of marrow cells (lanes 10 through 13), and analyzed for expression of gp91phox, p22phox, and p47phox by immunoblotting with specific antibodies. Ten micrograms of extracted cellular protein was loaded for each sample. The immunoreactive bands in the control X-CGD samples probed with the gp91phox antibody are presumed to represent proteins that bind nonspecifically because they are not detected with other gp91phox-specific antibodies.
Immunoblot analysis of murine neutrophil-enriched BM. Neutrophil-enriched BM preparations were obtained from wild-type (WT) mice, X-CGD mice (CGD), and X-CGD mice 13 to 15 weeks after transplantation with MSCV-m91Neo–transduced X-CGD BM. Cellular proteins were extracted from either nonadherent BM cells (lanes 1 through 9) or a neutrophil-enriched Percoll fraction of marrow cells (lanes 10 through 13), and analyzed for expression of gp91phox, p22phox, and p47phox by immunoblotting with specific antibodies. Ten micrograms of extracted cellular protein was loaded for each sample. The immunoreactive bands in the control X-CGD samples probed with the gp91phox antibody are presumed to represent proteins that bind nonspecifically because they are not detected with other gp91phox-specific antibodies.
Respiratory burst oxidase activity, as measured using a quantitative assay for superoxide formation, was studied in nonadherent BM cells obtained from wild-type mice, control X-CGD mice, and X-CGD mice transplanted with marrow transduced with the high-titer vector for murine gp91phox expression (Table 1). No superoxide formation was detected in control X-CGD mice, as previously reported,17 but superoxide formation was partially restored after transplantation with MSCV-m91Neo–transduced cells. The relative level of superoxide generation in transplanted X-CGD mice, after taking into account the fact that only 62% to 82% of neutrophils were NBT+ in these samples, was estimated to be approximately one third of wild-type levels.
Superoxide Generation by Nonadherent Murine BM Cells
| Mouse Group . | N . | Vmax (nmol O−2/min/ . | Cumulative O−2 . |
|---|---|---|---|
| . | . | 107 cells) . | (nmol/30 min/107 cells) . |
| Wild-type mice | 7 | 21 ± 10 | 352 ± 83 |
| X-CGD mice | 5 | 0 | 0 |
| X-CGD mice after transplantation with MSCV-m91Neo–transduced BM | 5 | 5.4 ± 2.8 | 67 ± 35 |
| Mouse Group . | N . | Vmax (nmol O−2/min/ . | Cumulative O−2 . |
|---|---|---|---|
| . | . | 107 cells) . | (nmol/30 min/107 cells) . |
| Wild-type mice | 7 | 21 ± 10 | 352 ± 83 |
| X-CGD mice | 5 | 0 | 0 |
| X-CGD mice after transplantation with MSCV-m91Neo–transduced BM | 5 | 5.4 ± 2.8 | 67 ± 35 |
Superoxide dismutase-inhibitable superoxide production was measured in nonadherent BM cells (which contain ≈50% to 60% mature neutrophils) using a continuous cytochrome c reduction assay, as described in Materials and Methods. Data are the mean ± SD. Greater than 95% of wild-type nonadherent BM neutrophils were NBT+, whereas the percentage of NBT+ neutrophils in X-CGD mice after transplantation with MSCV-m91Neo–transduced BM ranged from 62% to 82% (mean, 68% ± 7%). Two of the transplanted X-CGD mice had been challenged with A fumigatus 10 weeks after transplantation; the remaining three were studied 6 weeks after transplantation and were not challenged with A fumigatus.
To determine if retroviral-mediated gene transfer could improve phagocyte function and restore the defect in host defense in X-CGD mice, we examined the response to a respiratory challenge with A fumigatus. This opportunistic fungus causes infection in immunocompromised hosts, and is an important cause of life-threatening pneumonia and other infections in CGD patients.1,29 Respiratory burst-derived oxidants appear to be of particular importance in neutrophil-mediated killing of hyphae.29,30 We have previously shown that X-CGD mice develop a fatal, necrotizing bronchopneumonia after respiratory challenge with 3 × 106A fumigatus conidia (spores), a dose which fails to establish disease in wild-type mice.17 More recent studies in our laboratory have shown that even as few as 50 conidia instilled into the respiratory tract of X-CGD mice can produce chronic pulmonary infection and occasional deaths.31 Challenge with small numbers of A fumigatus conidia are more likely to mimic naturally occurring exposures, and hence doses of 150 to 500 spores were used in the present study.
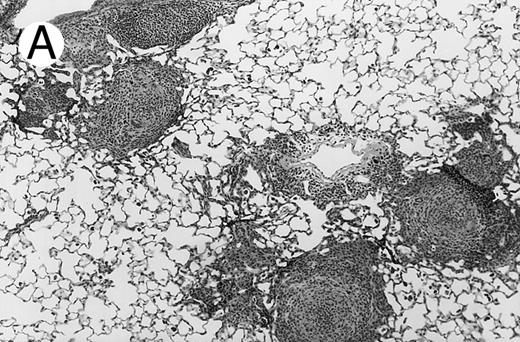
Three groups of X-CGD mice were studied: (1) control X-CGD mice; (2) X-CGD mice challenged 10 to 14 weeks after transplantation with MSCV-91Neo–transduced X-CGD BM; and (3) X-CGD mice challenged 5 to 8 weeks after transplantation with mixtures of wild-type and X-CGD BM cells. The results are summarized in Table 2. There were no deaths during the 17 to 21 days after challenge except for one mouse in the second group who died of complications of anesthesia on day 1. All 16 control X-CGD mice that were not transplanted developed lung disease after respiratory exposure to A fumigatus conidia. A common finding in lung tissue was the presence of multiple foci of granulomatous microabscesses that also contained occasional giant cells (Fig 3A). Rare hyphae were also seen. Larger inflammatory nodules containing numerous neutrophils and hyphae were seen in some mice (Fig 3B). Large abscesses with necrotic centers or regional bronchopneumonia were also occasionally observed (not shown). In 4 of 4 X-CGD mice transplanted with marrow transduced with the low-titer MSCV-h91Neo vector, who had only 1% to 2% NBT+ PB neutrophils at time of challenge, lung disease similar to that seen in untransplanted X-CGD mice was seen (Table 2). However, only minimal, if any, inflammatory changes were seen in 9 of 9 X-CGD mice with ≈50% to 80% NBT+ circulating neutrophils after transplantation with BM transduced with the high-titer vector (Table 2). Lungs appeared either normal (except for macrophages containing colloidal carbon that was administered along with conidia to mark the site of delivery) or contained occasional collections of mononuclear cells, typically in perivascular or peribronchial locations (Fig 3C). These findings were similar to those seen in lungs of wild-type mice after challenge with these numbers of A fumigatus conidia (unpublished observations, 1996).
Pulmonary Disease in Transplanted X-CGD Mice After Respiratory Challenge With A fumigatus Conidia
| Transplant Group . | N . | % NBT+ PMNs . | A fumigatus Lung Disease* . |
|---|---|---|---|
| None | 16 | 0 | 16/16 |
| MSCV-91Neo-transduced X-CGD BM† | |||
| MSCV-h91Neo (2 × 105 cfu/mL) | 4 | 1-2 | 4/4 |
| MSCV-m91Neo (1 × 106 cfu/mL) | 9 | 53-82 | 0/9 |
| Wild-type X-CGD BM‡ | |||
| Group I | 4 | 2-3 | 4/4 |
| Group II | 6 | 5-8 | 0/6 |
| Group III | 3 | 10-20 | 0/3 |
| Group IV | 4 | 20-35 | 0/4 |
| Group V | 4 | 90-98 | 0/4 |
| Transplant Group . | N . | % NBT+ PMNs . | A fumigatus Lung Disease* . |
|---|---|---|---|
| None | 16 | 0 | 16/16 |
| MSCV-91Neo-transduced X-CGD BM† | |||
| MSCV-h91Neo (2 × 105 cfu/mL) | 4 | 1-2 | 4/4 |
| MSCV-m91Neo (1 × 106 cfu/mL) | 9 | 53-82 | 0/9 |
| Wild-type X-CGD BM‡ | |||
| Group I | 4 | 2-3 | 4/4 |
| Group II | 6 | 5-8 | 0/6 |
| Group III | 3 | 10-20 | 0/3 |
| Group IV | 4 | 20-35 | 0/4 |
| Group V | 4 | 90-98 | 0/4 |
X-CGD mice were transplanted with either mixtures of wild-type and X-CGD BM or X-CGD BM transduced with MSCV-91Neo retroviruses. At the indicated times after transplant, X-CGD mice (along with control X-CGD mice that did not receive BM transplants) received a respiratory challenge with A fumigatus conidia, as described in Materials and Methods. All mice survived, and at 17 to 21 days after challenge, lungs were removed for histologic examination. The percentage of circulating NBT+ neutrophils was determined within 4 days before A fumigatus challenge, which also was similar to the percentage observed at time of sacrifice.
Granulomas, purulent bronchopneumonia, and/or abscesses.
Mice were challenged with 150 to 500 A fumigatus conidia 10 to 14 weeks after transplant.
Mice were challenged with 150 A fumigatus conidia 5 to 8 weeks after transplant, except for group V, which received 1 × 106 conidia.
Pulmonary findings 17 to 21 days after intratracheal instillation of A fumigatus conidia in X-CGD mice. Lung tissue was obtained 17 to 21 days after intratracheal challenge with 150 to 500 A fumigatus conidia, and stained with hematoxylin and eosin (A and C) or Grocott methamine silver (B). Representative photomicrographs obtained from three different mice are shown (original magnification × 100). (A) Untransplanted X-CGD mouse. Granulomatous microabscesses are seen, with occasional giant cells. (B) Untransplanted X-CGD mouse. Large inflammatory nodules with a central collection of neutrophils and numerous hyphae are seen. (C) X-CGD mouse transplanted with marrow transduced with the high-titer MSCV-m91Neo retrovirus. Occasional mononuclear cells in perivascular spaces and alveoli are seen, along with macrophages containing colloidal carbon (arrows) that was administered with the conidia to mark the site of delivery.
Pulmonary findings 17 to 21 days after intratracheal instillation of A fumigatus conidia in X-CGD mice. Lung tissue was obtained 17 to 21 days after intratracheal challenge with 150 to 500 A fumigatus conidia, and stained with hematoxylin and eosin (A and C) or Grocott methamine silver (B). Representative photomicrographs obtained from three different mice are shown (original magnification × 100). (A) Untransplanted X-CGD mouse. Granulomatous microabscesses are seen, with occasional giant cells. (B) Untransplanted X-CGD mouse. Large inflammatory nodules with a central collection of neutrophils and numerous hyphae are seen. (C) X-CGD mouse transplanted with marrow transduced with the high-titer MSCV-m91Neo retrovirus. Occasional mononuclear cells in perivascular spaces and alveoli are seen, along with macrophages containing colloidal carbon (arrows) that was administered with the conidia to mark the site of delivery.
To determine the minimum number of wild-type neutrophils required for protection against respiratory challenge with 150 A fumigatus conidia, X-CGD mice were transplanted with varying proportions of wild-type and X-CGD BM. After hematologic recovery, these mice were chimeric for NBT+ and NBT− neutrophils, in proportion to the mixture used for transplantation. The chimeric X-CGD mice were then challenged with A fumigatus conidia, and the results are summarized in Table 2. In 4 of 4 mice with only 2% to 3% circulating wild-type neutrophils, lung disease similar to that seen in untransplanted X-CGD mice was seen. However, X-CGD mice chimeric for ≥5% wild-type neutrophils were protected against exposure to low numbers of conidia. X-CGD mice that were transplanted solely with wild-type marrow, who had ≥90% NBT+ neutrophils after hematologic recovery, were also resistant to challenge with a 1 × 106A fumigatus conidia, a dose that is fatal in X-CGD mice within 1 week of challenge (unpublished data, 1996).
DISCUSSION
In this study, we show that retroviral-mediated transfer of the X-CGD gene product, gp91phox, can reconstitute respiratory burst oxidase activity in vivo in a murine model of X-CGD and protect against respiratory challenge with A fumigatus. This is one of the first studies to show the efficacy of gene therapy in correcting the phenotype of an inherited disorder, using an animal model that closely resembles the human disease.
The retroviral vectors used in this study were based on the murine stem cell virus vectors described by Hawley et al,22 in which expression of gp91phox is driven by a derivative of the myeloproliferative sarcoma virus LTR.11 12 The MSCV LTR contains modifications intended to prevent its transcriptional inactivation after transduction of stem cells. The expression of recombinant gp91phox for least 6 months after transplantation, as shown by the persistence of NBT+ neutrophils, indicates that the MSCV-91Neo vectors remain functionally active after transduction of murine hematopoietic stem cells. The expression of gp91phox is normally restricted to mature phagocytic cells. However, to date there have been no obvious deleterious effects of its constitutive expression in other hematopoietic lineages after retroviral-mediated gene transfer.
The abundance of recombinant gp91phox in neutrophils obtained from X-CGD mice transplanted with MSCV-91Neo–transduced BM was very low, but resulted in reconstitution of approximately one third of wild-type neutrophil respiratory burst oxidase activity. This finding confirms previous observations in the X-CGD PLB-985 myeloid cell line,9-12 which had suggested that gp91phox is not normally the rate-limiting component in superoxide production, and extends these observations to primary cells in vivo. The MSCV-91Neo vector conferred overall lower levels of gp91phox expression and superoxide production in murine X-CGD neutrophils compared with the human X-CGD PLB-985 line. The underlying reasons for this observation are unknown, but highlight the importance of analyzing expression in primary hematopoietic cells. In this study, the level of vector-derived gp91phox mRNA expression relative to that observed for recombinant gp91phox protein expression cannot be directly compared, because two different tissues (spleen and BM neutrophils, respectively) were used for analysis.
A fumigatus is an opportunistic fungus that is a frequent cause of serious pneumonia and other infections in patients with CGD. Alveolar macrophages are the first line of defense against inhaled A fumigatus conidia, which are ingested and killed by oxygen-independent mechanisms. In contrast, respiratory burst-derived oxidants are essential for neutrophil-mediated killing of hyphae which germinate from conidia escaping macrophage surveillance.29,30 In the current study, X-CGD mice were found to develop pulmonary disease when exposed to A fumigatus conidia in a dose range selected to mimic naturally occurring exposures.32 33 However, X-CGD mice with partial reconstitution of superoxide generation after retroviral-mediated gene transfer were resistant to A fumigatus challenge.
The minimum percentage of neutrophils with partial respiratory burst oxidase activity required for protection against A fumigatus cannot be determined from these experiments. However, as few as 5% to 10% of wild-type neutrophils were protective in X-CGD mice transplanted with mixtures of wild-type and X-CGD marrow. Thus, this aspect of the murine model also resembles observations made in humans, in that female carriers of X-CGD who have at least 5% to 10% normal neutrophils do not appear to be susceptible to A fumigatus infection.6 However, Aspergillus pneumonia has been seen in a female carrier with fewer than 5% NBT+ neutrophils.6 Similarly, lung disease produced by A fumigatus in chimeric X-CGD mice with very low numbers of wild-type neutrophils or X-CGD neutrophils corrected by gene transfer was indistinguishable from control X-CGD mice, suggesting that gene therapy strategies should aim at restoring respiratory burst activity to ≥5% of neutrophils.
In conclusion, our results suggest that expression of small amounts of recombinant gp91phox in murine X-CGD neutrophils after retroviral-mediated gene transfer can reconstitute a significant degree of antimicrobial activity, but that correction of only a small percentage of phagocytes may not be sufficient for protection against A fumigatus. In addition, the MSCV-based vector used for gene transfer of gp91phox was capable of restoring long-term in vivo correction of respiratory burst oxidase activity. These findings have important implications for designing approaches to gene therapy of human CGD.
ACKNOWLEDGMENT
We thank David Morgenstern for assistance with A fumigatus pneumonia experiments and densitometry, Atilio Orazi and Karla John for preparation of histologic specimens, and Donna Fischer for manuscript preparation.
Supported by the National Heart, Lung and Blood Institute (P01 HL53586) and a Clinical Research Award from the March of Dimes. The Wells Center for Pediatric Research is a Center for Excellence in Molecular Hematology funded by National Institute of Diabetes and Digestive and Kidney Diseases (P50 DK49218).
Address reprint requests to Mary C. Dinauer, MD, PhD, Herman B Wells Center for Pediatric Research, James Whitcomb Riley Hospital for Children, Room 2600, 702 Barnhill Dr, Indianapolis, IN 46202.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal