Abstract
At sites of vessel wall damage, the primary hemostatic reaction involves platelet and fibrin deposition. At these sites, circulating leukocytes marginate and become activated. Adhered platelets can support leukocyte localization; however, the role of fibrin in this respect is not known. We studied the adhesion of human neutrophils (polymorphonuclear leukocytes [PMNs]) to endothelial extracellular matrix (ECM)-bound fibrin and platelets under flow conditions. ECM alone did not show PMN adhesion. ECM-coated cover slips were perfused with plasma to form a surface-bound fibrin network, and/or with whole blood to allow platelet adhesion. Unstimulated PMNs adhered to fibrin at moderate shear stress (20 to 200 mPa). ECM-bound platelets induced rolling adhesion and allowed more PMNs to adhere at higher shear (320 mPa). ECM coated with both platelets and fibrin induced more static and shear-resistant PMN adhesion. PMN adhesion to fibrin alone but not to platelet/fibrin surfaces was inhibited by soluble fibrinogen. Adhesion to fibrin alone was inhibited by CD11b and CD18 blocking antibodies. Furthermore, fibrin formed under flow conditions showed up to threefold higher PMN adhesion compared with fibrin formed under static conditions, due to structural differences. These results indicate that circulating PMNs adhere to fibrin in an integrin-dependent manner at moderate shear stresses. However, at higher shear rates (<200 mPa), additional mechanisms (ie, activated platelets) are necessary for an interaction of PMNs with a fibrin network.
PLATELET AND FIBRIN deposition occurs at sites of vascular damage, and thrombi often become infiltrated with inflammatory cells. Infiltration of granulocytes induced by vessel wall damage and platelet and fibrin deposition is considered to play a role in the pathogenesis of several diseases.1 Indeed, neutrophils participate in the propagation of deep venous thrombosis and atherosclerosis by adhesion to the fibrin- and platelet-rich layers within thrombi or atherosclerotic plaques.2,3 In vivo studies of a thrombus formed in an arteriovenous shunt in baboons showed colocalization of neutrophils, platelets, and fibrin deposition.4 In vitro, polymorphonuclear leukocytes (PMNs) were shown to adhere to thrombotic substrates such as activated platelets, fibrinogen, and fibrin networks.1 5
PMN and monocyte adhesion to fibrinogen is mediated by the β2-integrins (MAC-1 and p150.95)6-9 and by the β3-integrin–related leukocyte response integrin.10 Leukocyte response integrin recognizes arginyl-glycyl-aspartyl (RGD) sequences in soluble fibrinogen, which induces phagocytosis.11 MAC-1 (CD11b/CD18) binds to a region within the γ-chain of fibrinogen,12,13 whereas p150.95 (CD11c/CD18) recognizes the N-terminus of the α-chain.7 MAC-1 and p150.95 are constitutively expressed on resting PMNs, but have a low affinity for their ligands.14 Stimulation with various inflammatory agonists induces a transient increase of affinity of all three integrins and induces increased expression of MAC-1 as a result of redistribution from intracellular stores.14
Studies concerning the interaction of PMNs with fibrinogen are mainly performed under static conditions. However, shear forces, as occur under physiologic conditions, strongly influence adhesion to surface-coated proteins or cells.15 Moreover, PMN adhesion to thrombi formed at the extracellular matrix (ECM) under flow conditions has been even less studied. We investigated whether different types of surface-bound fibrin can support PMN adhesion under flow conditions, and addressed the role of the cellular adhesion molecules. Adhesion of neutrophils to fibrin surfaces was compared with adhesion to immobilized platelets. Our experiments provide new insights into the PMN adhesion to hemostatic surfaces that occurs in the damaged vessel wall.
MATERIALS AND METHODS
Monoclonal Antibodies (MoAbs)
The MoAb LAM1-3 (CD62l, anti–L-selectin, 5 μg/mL for blocking studies) was a kind gift from T. Tedder (Harvard Medical School, Boston, MA).16 The MoAb WASP12.2 (CD62p, anti–P-selectin, 1 μg/mL for blocking studies) was purchased from Endogen (Boston, MA). MoAbs 44a (CD11b, 10 μg/mL for blocking studies), IB4 (CD18, 10 μg/mL for blocking studies), and TS-1 (CD11a, 10 μg/mL for blocking studies) were isolated from the supernatant of hybridomas obtained from the American Type Culture Collection (Rockville, MD). The MoAb Leu-8 (anti–L-selectin, CD62l) was purchased from Becton Dickinson (San Jose, CA). The MoAb 80H3 (CD66b) was purchased from Immunotech (Marseille, France).
Reagents
Percoll was obtained from Pharmacia (Uppsala, Sweden). FMLP, thrombin, and 4β-phorbol, 12-myristate, 13-acetate (PMA) were purchased from Sigma (St Louis, MO). D-Phenylalanyl-L-propyl-L-arginine chloromethyl ketone (PPACK) was purchased from Calbiochem (Behring, La Jolla, CA). Fibrinogen was obtained from Enzyme Research Laboratories (South Bend, IN). Heparin (Thromboliquine) was obtained from Organon Teknika (Boxtel, The Netherlands). Neuraminidase was purchased from Behring (Behringwerke, Marburg, Germany). Tissue culture supplies (media, antibiotics, and trypsin) were purchased from GIBCO Biocult (Paisley, UK). Experiments were performed in HEPES buffer (20 mmol/L HEPES, 132 mmol/L NaCl, 6 mmol/L KCl, 1.2 mmol/L KH2PO4 , 1 mmol/L MgSO4⋅7H2O, 5 mmol/L glucose, and 1 mmol/L CaCl2 , pH 7.4). All other reagents were of reagent grade.
ECM Coating
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cord veins according to the method of Jaffe et al,17 with minor modifications.18 The cells were cultured in RPMI 1640 containing penicillin, streptomycin, and amphotericin B, with 20% (vol/vol) heat-inactivated human serum. Endothelial cells of the second passage were harvested by trypsin digestion, subcultured on glass 1% gelatin-coated cover slips, and grown to confluence in 5 to 7 days. Tissue factor–rich ECM was obtained as described previously.19 In short, HUVECs were stimulated with the phorbol ester PMA (20 ng/mL overnight) and removed from the cover slips by treatment with 0.1 mol/L NH4OH (5 minutes at room temperature [RT]). Isolated matrices were washed and kept in phosphate-buffered saline at 4°C for a maximum of 3 weeks.
Perfusion Surfaces
Fibrin and platelet coating of glass cover slips was performed under varying conditions. Table 1 and Fig 1 summarize some of the typical features of the different surfaces. To determine the percentage of surface covered with fibrin or platelets, some of the cover slips were fixed (1% paraformaldehyde) and stained with May-Grünwald Giemsa. Coverage was evaluated by light microscopy and image analysis using a Quantimet 570C (Leica/Cambridge, Cambridge, UK). All surfaces (except the ECM control) showed a homogeneous coverage with either platelets or fibrin(ogen).
Characteristics of Fibrin(ogen) and Platelet Surfaces
| Surface . | Procedure . | Fibrin/Fibrinogen . | Platelet Coverage (%) . | Plasma Proteins . |
|---|---|---|---|---|
| Fb-flow | PPP perfused over ECM (at shear stress 20 mPa) | Fibrin | <1 | + |
| Fb-static | ECM incubated with PPP | Fibrin | <1 | + |
| Platelets | Citrated whole blood perfused over ECM | — | 85 | + |
| Fb + platelets | Citrated whole blood perfused over a Fb-flow surface | Fibrin | 55 | + |
| Fb-flow (no platelets) | Filtered plasma perfused over ECM | Fibrin | 0 | + |
| Fibrinogen-pure | Fibrinogen coated on a glass cover slip | Fibrinogen | 0 | − |
| Fb-pure | Fibrinogen-pure incubated with thrombin | Fibrin | 0 | − |
| ECM-control | Tissue factor–rich ECM on a glass cover slip | — | 0 | + |
| Surface . | Procedure . | Fibrin/Fibrinogen . | Platelet Coverage (%) . | Plasma Proteins . |
|---|---|---|---|---|
| Fb-flow | PPP perfused over ECM (at shear stress 20 mPa) | Fibrin | <1 | + |
| Fb-static | ECM incubated with PPP | Fibrin | <1 | + |
| Platelets | Citrated whole blood perfused over ECM | — | 85 | + |
| Fb + platelets | Citrated whole blood perfused over a Fb-flow surface | Fibrin | 55 | + |
| Fb-flow (no platelets) | Filtered plasma perfused over ECM | Fibrin | 0 | + |
| Fibrinogen-pure | Fibrinogen coated on a glass cover slip | Fibrinogen | 0 | − |
| Fb-pure | Fibrinogen-pure incubated with thrombin | Fibrin | 0 | − |
| ECM-control | Tissue factor–rich ECM on a glass cover slip | — | 0 | + |
Glass cover slips coated with tissue factor–rich ECM of PMA-stimulated HUVECs were treated under different conditions. This pretreatment resulted in cover slip coating with fibrin, fibrinogen, platelets, and plasma factors in different combinations and quantities. The presence of fibrin(ogen) and the percentage of surface covered with platelets was evaluated by light microscopy and image analysis (see Fig 1).
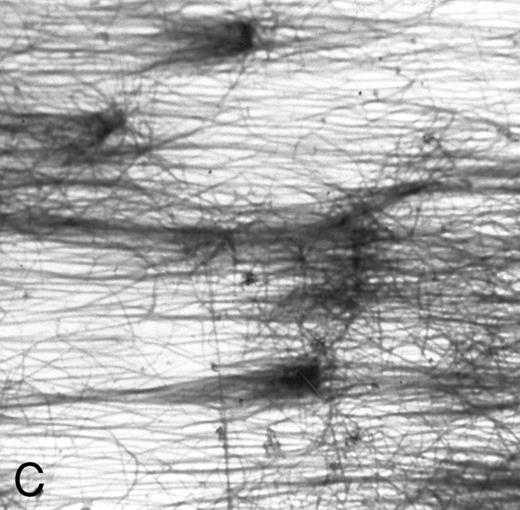
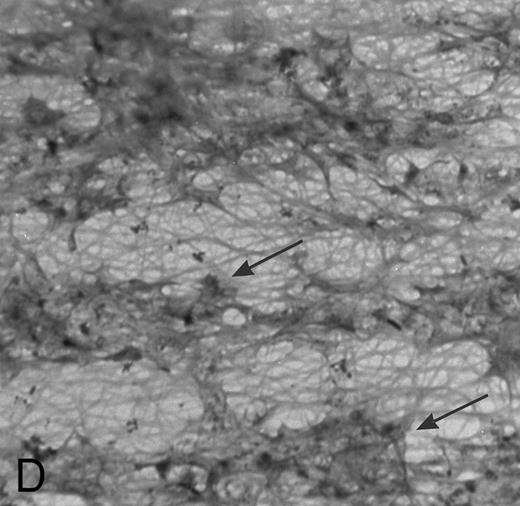
(A) Fibrin network formed under static conditions (Fb-static). Tissue factor–rich ECM-coated coverslips were incubated with PPP in a 6-well plate (10 minutes at 37°C). Arrows indicate neutrophils that have adhered after a perfusion with purified resting neutrophils. (B) Fibrin network formed at low shear. ECM-coated cover slips were perfused with PPP from heparinized blood at shear stress 20 mPa (10 minutes at 37°C). (C) Fibrin network formed at high shear stress. ECM-coated cover slips were perfused with PPP from heparinized blood at a shear stress of 80 mPa (10 minutes at 37°C). (D) Fibrin network with platelets (Fb + platelets). ECM-coated cover slips were perfused twice: first with PPP from heparinized blood at a shear stress of 20 mPa (10 minutes at 37°C), followed by perfusion with citrated whole blood for 7 minutes at a shear rate of 1,600 s−1 (ie, a shear stress of ∼7 Pa). As a result, platelets covered 30% to 40% of the fibrin network (arrows).
(A) Fibrin network formed under static conditions (Fb-static). Tissue factor–rich ECM-coated coverslips were incubated with PPP in a 6-well plate (10 minutes at 37°C). Arrows indicate neutrophils that have adhered after a perfusion with purified resting neutrophils. (B) Fibrin network formed at low shear. ECM-coated cover slips were perfused with PPP from heparinized blood at shear stress 20 mPa (10 minutes at 37°C). (C) Fibrin network formed at high shear stress. ECM-coated cover slips were perfused with PPP from heparinized blood at a shear stress of 80 mPa (10 minutes at 37°C). (D) Fibrin network with platelets (Fb + platelets). ECM-coated cover slips were perfused twice: first with PPP from heparinized blood at a shear stress of 20 mPa (10 minutes at 37°C), followed by perfusion with citrated whole blood for 7 minutes at a shear rate of 1,600 s−1 (ie, a shear stress of ∼7 Pa). As a result, platelets covered 30% to 40% of the fibrin network (arrows).
ECM control.Tissue factor–rich ECM of endothelial cells was used to coat cover slips as described. When used as a control surface, it was preincubated with plasma from citrate-anticoagulated blood (20 minutes at 37°C) to allow plasma protein coating without fibrin formation.
Fb-static.A fibrin network was obtained by incubating tissue factor–rich ECM-coated cover slips with platelet-poor plasma (PPP) from heparinized blood (10 minutes at 37°C). PPP (<104 platelets/μL plasma) was obtained by centrifugation (5 minutes at 3,500 rpm at 5°C) of whole blood of healthy donors, which was anticoagulated with 20 U/mL low–molecular-weight heparin (Fragmin; Kabi Vitrum, Stockholm, Sweden). Incubation of ECM cover slips with PPP under static conditions resulted in a homogeneous network of randomly oriented fibers covering 75% of the surface, and was designated Fb-static (Fig 1A).
Fb-flow.A fibrin network formed under flow conditions was formed by perfusion of PPP over tissue factor–rich ECM-coated cover slips. For surfaces designated Fb-flow, plasma perfusion was performed at a shear stress of 20 mPa (10 minutes at 37°C). For Fb-flow (shear 1) and Fb-flow (shear 80), shear stresses of 1 and 80 mPa were used, respectively. After perfusion, the cover slips were rinsed with HEPES buffer to remove plasma remnants. The fibrin network covered more than 75% of the surface and consisted of thick fibrin fibers, mostly oriented parallel to the direction of the flow (Fig 1B and C). Some single platelets were incorporated in the fibrin network (<1% coverage). A Fb-flow surface free of platelets was made by perfusing filtered plasma (instead of PPP) at a shear stress of 20 mPa. This surface was designated Fb-flow (no platelets).
Platelets.A platelet surface was made by perfusing whole blood (anticoagulated with 0.34% wt/vol trisodium-citrate) over a tissue factor–rich ECM-coated cover slip as described previously.20 In short, whole blood was perfused at a shear rate of 1,600 s−1 (ie, a shear stress of ∼7 Pa) in a recirculating system (7 minutes at 37°C). This resulted in a platelet coverage of the ECM-coated cover slips of 85% ± 4% (mean ± SE, n = 24). Under these conditions, no fibrin fiber deposition was present.
Fb + platelets.ECM cover slips were perfused twice to obtain a surface with a fibrin network together with platelets: a first perfusion was performed with PPP as described, followed by a perfusion with citrated whole blood. As a result, platelets adhered to the fibrin network covering 30% to 40% of the total surface (Fig 1D).
Fibrinogen-pure and Fb-pure.Purified fibrinogen was coated on a gelatin-coated glass cover slip. For this purpose a glass cover slip was coated with gelatin (1% wt/wt gelatin in phosphate-buffered saline, 10 minutes at RT), washed, and incubated in a fibrinogen solution (5 mg/mL in HEPES buffer, 30 minutes at RT). Additionally, some of the fibrinogen-coated cover slips were incubated with thrombin (1 U/mL HEPES buffer, 10 minutes at 37°C), resulting in fibrinogen cleavage and formation of pure fibrin. These surfaces were designated Fb-pure. In control experiments, remaining thrombin activity was antagonized by incubation with PPACK (1 μmol/L in HEPES buffer 20 minutes at RT).
Neutrophil Isolation
Blood was obtained from healthy volunteers from the Blood Bank, Utrecht, The Netherlands. Mixed granulocytes were purified from the buffy coat of 500 mL blood anticoagulated with 0.34% (wt/vol) trisodium-citrate (pH 7.4) as described previously.21 In short, mononuclear cells were removed by centrifugation over Ficoll-Paque (density, 1.077/μL). The remaining erythrocytes were lysed by incubation in isotonic 115-mmol/L ammonium chloride solution (pH 7.4) at 4°C for 20 minutes. Cells were washed twice, diluted in HEPES buffer (2 × 106 cells/mL), and kept at room temperature until the start of the perfusion. Neutrophil purity was greater than 95%, and viability measured with Trypan blue exclusion was greater than 98%; morphology was controlled by light microscopy. Before perfusion, neutrophils were always prewarmed for 5 minutes at 37°C. For blocking experiments, neutrophils were preincubated with MoAbs or fibrinogen for 30 minutes at room temperature.
Perfusion Chamber
Perfusions under steady flow were performed in a transparent rectangular perfusion chamber according to a modification of the original chamber described by Sakariassen et al.20 The chamber has a slit height of 0.3 mm and width of 6 mm, and contains two circular plugs on which mounted cover slips are exposed to blood, plasma, or cell suspensions. Neutrophils in suspension (2 × 106/mL in HEPES buffer at 37°C) were aspirated from a reservoir through plastic tubing, a valve, and the perfusion chamber by a Harvard syringe pump (Harvard Apparatus, South Natick, MA). In this way, the flow rate through the chamber could be precisely controlled. The wall shear stress (t) was calculated from the equation, t = (6Q⋅η)/(w⋅h2), in which Q is the flow rate, η the suspending-medium viscosity, w the slit width, and h the slit height.20
Evaluation of Adhesion and Rolling
Neutrophil perfusions were performed and evaluated as described previously.22 During the perfusion, the flow chamber was mounted on a microscope stage (DM RXE; Leica, Weitzlar, Germany) equipped with a B/W CCD video camera (Sanyo, Osaka, Japan) coupled to a VHS video recorder. Perfusion experiments were recorded on video tape. Video images were evaluated for the number of adhered cells with a Quantimet 570C image-analysis system (Leica/Cambridge). The number of surface-adhered neutrophils was measured after 5 minutes of perfusion at a minimum of 30 randomized high-power fields (total surface > 3 mm2) unless stated otherwise. The rolling velocity of cells was measured as described previously.22 In short, a sequence of images was digitally captured with a preset time interval (δt). At each image, the position of each cell was detected, and for all subsequent images the overlapping cells were added together. The distance moved by each cell and the number of images in which a cell appeared was measured. Cell velocities (v) in micrometers per second were calculated from the equation, v = L/δt⋅(x − 1), in which L is the distance (micrometers), δt is the time interval between images (seconds), and x is the number of images in which a cell appears. The cutoff value to distinguish between rolling and static adhesion was set at 1 μm/s. With this method, static adherent, rolling, and freely flowing cells (which were not in focus) could be clearly distinguished.
Statistical Analysis
Results are expressed as the mean ± SE. Statistical analysis of the data was performed using a paired Student's t-test for single measurements or repeated-measures analysis of variance (MANOVA) for series of measurements. P values less than .05 were considered significant.
RESULTS
The described results cover the adhesion of PMNs to fibrin(ogen)-coated surfaces under flow conditions. The experimental variables are wall shear stress and the presence of platelets and plasma proteins on the surface. The presence of inhibiting antibodies during the PMN perfusion was investigated also.
PMN Adhesion to Fibrin and Platelets
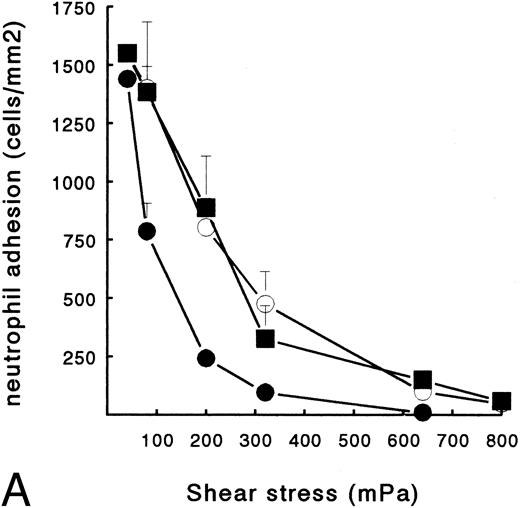
Human neutrophils can bind to platelets and fibrin(ogen) in suspension. In addition, previous studies show that surface-bound platelets support adhesion of circulating PMNs, and that shear stress is a key factor in this process.22,23 Here, we examined the effect of shear on PMN adhesion to platelets and to fibrin in the presence or absence of platelets. Therefore, ECMs with a fibrin network, platelets, or both were used for PMN perfusions at shear stresses ranging from 40 to 640 mPa. These shear stresses correlate in vivo with moderately large vessels. Figure 1B shows an ECM surface with a fibrin network formed under flow conditions (Fb-flow; Table 1). PMNs adhered specifically to the fibrin-covered areas and not to the ECM. Figure 2A shows the total number of adhered PMNs (static + rolling cells) after a perfusion of 5 minutes at different shear stresses. PMN adhesion to Fb-flow decreased with increasing shear stress, with some adhesion still left at shears up to 320 mPa (Fig 2A). Figure 1D shows a fibrin network with platelets (Fb + platelets; Table 1). After a perfusion with PMNs, no clear colocalization with loci containing platelets was observed. However, at all shear stresses tested, the presence of platelets at the surface induced more adhesion versus the surface with fibrin alone (Fig 2A). The relative difference was more evident with increasing shear. At a shear of 40 mPa, the difference was not statistically significant. At 640 mPa, no PMNs adhered to Fb-flow, whereas greater than 150 cells/mm2 still adhered to the platelet surface. No differences in the number of adhered PMNs were found between surfaces with platelets alone or Fb + platelets.
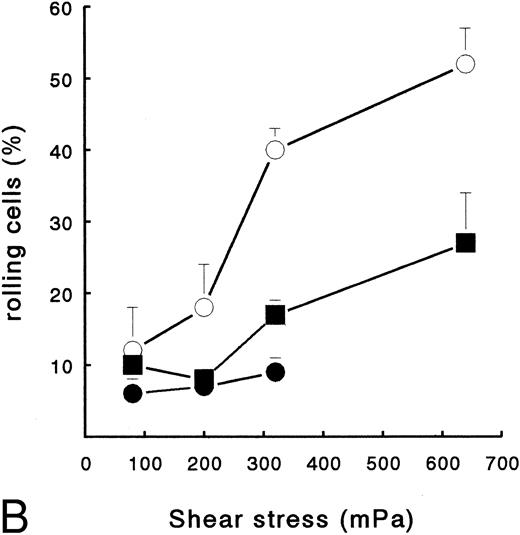
PMN adhesion to surface-bound platelets and fibrin. Isolated resting neutrophils (2 × 106 cells/mL HEPES buffer) were perfused at different shear stresses to determine adhesion to Fb-flow surface (•), Fb + platelets (▪), or ECM-bound platelets (○). (A) Total number of surface-interacting PMNs per mm2 measured after 5 minutes of perfusion (mean ± SE, n = 4). Fb-flow showed significantly less adhesion as determined by repeated-measures ANOVA (P < .05). (B) Rolling neutrophils calculated as a percentage of the total number of surface-interacting cells. ECM-bound platelets induced more rolling compared with the other surfaces as determined by repeated-measures ANOVA (P < .05).
PMN adhesion to surface-bound platelets and fibrin. Isolated resting neutrophils (2 × 106 cells/mL HEPES buffer) were perfused at different shear stresses to determine adhesion to Fb-flow surface (•), Fb + platelets (▪), or ECM-bound platelets (○). (A) Total number of surface-interacting PMNs per mm2 measured after 5 minutes of perfusion (mean ± SE, n = 4). Fb-flow showed significantly less adhesion as determined by repeated-measures ANOVA (P < .05). (B) Rolling neutrophils calculated as a percentage of the total number of surface-interacting cells. ECM-bound platelets induced more rolling compared with the other surfaces as determined by repeated-measures ANOVA (P < .05).
Although surfaces with platelets alone and Fb + platelets showed no difference in the total number of adhering cells, a marked difference in the type of adhesion was observed. In accordance with previous studies,22 23 PMNs showed a clear rolling adhesion on the platelet surface, especially at higher shear stress (Fig 2B). At a shear stress of 640 mPa, 52% ± 3% (n = 4) of the cells showed rolling adhesion at platelets (cells with velocity > 1 μm/s). However, at the same shear, PMNs on a Fb + platelets surface showed significantly less rolling adhesion (25% ± 7%, n = 4, P < .05). On the Fb-flow surface, little rolling was observed at all shear stresses tested (Fig 2B).
Different Surfaces and PMN Adhesion
Table 2 shows the number of PMNs adhering to different fibrin and platelet surfaces after perfusion at a shear stress of 80 or 200 mPa. The presence of fibrin/fibrinogen, platelets, and plasma proteins on the different surfaces is described in Table 1. To further examine the role of platelets in the interaction with PMNs, the complete absence of platelets in the fibrin network was obtained using filtered plasma instead of PPP [designated Fb-flow (no platelets)]. PMN adhesion to this surface at both shear stresses tested was similar to that for Fb-flow, which contains some platelets (Table 2). Apparently, residual platelets in the fibrin network did not influence adhesion.
PMN Adhesion to Fibrin(ogen) and Platelet Surfaces (total cells/mm2)
| Surface . | 80 mPa . | 200 mPa . |
|---|---|---|
| Fb-flow | 1,031 ± 99 | 315 ± 30 |
| Fb-static | 1,100 ± 40 | 161 ± 32* |
| Platelets | 1,300 ± 120 | 803 ± 124* |
| Fb + platelets | 1,251 ± 79 | 887 ± 157* |
| Fb-flow (no platelets) | 1,004 ± 111 | 263 ± 42 |
| Fibrinogen-pure | 1,049 ± 85 | 200 ± 54 |
| Fb-pure | 946 ± 107 | 213 ± 95 |
| ECM-control | 200 ± 40* | 19 ± 11* |
| Surface . | 80 mPa . | 200 mPa . |
|---|---|---|
| Fb-flow | 1,031 ± 99 | 315 ± 30 |
| Fb-static | 1,100 ± 40 | 161 ± 32* |
| Platelets | 1,300 ± 120 | 803 ± 124* |
| Fb + platelets | 1,251 ± 79 | 887 ± 157* |
| Fb-flow (no platelets) | 1,004 ± 111 | 263 ± 42 |
| Fibrinogen-pure | 1,049 ± 85 | 200 ± 54 |
| Fb-pure | 946 ± 107 | 213 ± 95 |
| ECM-control | 200 ± 40* | 19 ± 11* |
Glass cover slips coated with tissue factor–rich ECM of PMA-stimulated HUVECs were treated under different conditions. This pretreatment resulted in cover slip coating with plasma factors and platelets in different combinations and quantities (see Table 1). Subsequently, the surfaces were used in a PMN perfusion. Isolated neutrophils were perfused at a shear stress of 80 or 200 mPa, and the number of adhered PMNs was measured after 5 minutes. Data represent the total number of adhering and rolling neutrophils per mm2 (mean ± SE, n = 4 to 10).
Statistically significant difference compared with Fb-flow was determined by ANOVA (P < .05).
The effect of the presence of plasma proteins in the fibrin network on PMN adhesion was examined. Surfaces with purified fibrinogen and fibrin, designated fibrinogen-pure and Fb-pure, were used and compared with fibrin formed from plasma. PMN adhesion to these pure fibrin(ogen) surfaces was similar to the adhesion to fibrin formed from plasma (eg, Fb-static) (Table 2). In conclusion, at a shear stress of 80 mPa, PMNs adhered to all different surfaces in comparable amounts. Only the ECM control showed significantly less adhesion. At a higher shear stress of 200 mPa, differences in neutrophil adhesion to different surfaces became more pronounced. Here, four surfaces showed a significantly different adhesion (P < .05) when compared with the adhesion to a fibrin network formed under flow conditions from PPP (Fb-flow, 315 ± 30 PMN/mm2, n = 10) (Table 2). Cover slips coated with platelets alone or with Fb + platelets were more potent inducers of PMN adhesion at 200 mPa. Surprisingly, not only the ECM control but also Fb-static showed significantly less adhesion (19 ± 11 and 161 ± 32 PMN/mm2, respectively, n = 10, P < .05). The difference between PMN adhesion to Fb-flow and Fb-static was studied in more detail.
Fb-Flow Versus Fb-Static
A fibrin network formed under flow conditions has the unique feature of thick fibers orientated parallel to the flow direction and few unpolymerized fibrin(ogen) monomers (Fig 1C). Since fibrin structure was suggested to influence cellular adhesion,5,24 25 we examined the role of fibrin structure on PMN adhesion at different shear stresses. Tissue factor–rich ECM was exposed to PPP under flow conditions (at shear 20 mPa, Fb-flow) and under static conditions (Fb-static). Both surfaces were subsequently perfused with isolated PMNs at shear stresses 80, 200, and 320 mPa. At shear stresses of 200 and 320 mPa, Fb-flow induced twofold and fivefold more PMN adhesion, respectively, than Fb-static (Table 3). The effect of fibrin fiber alignment on PMN adhesion was examined in more detail. Different shear stresses, ranging from 0 (static) to 80 mPa, were used for fibrin formation on tissue factor–rich ECM. During the static incubation, a fibrin network consisting of randomly distributed fibers was formed (Fig 1A). Fibrin formed under shear conditions (at 1, 20, and 80 mPa) consisted of fibrin fibers that were increasingly aligned along the direction of flow (Fig 1B and C). Figure 3 shows PMN adhesion to these fibrin surfaces after a perfusion at a shear stress of 200 mPa. All fibrin surfaces formed under flow conditions showed increased PMN adhesion compared with Fb-static. Increasing the shear stress during fibrin formation, and thereby the extent of fiber alignment, resulted in increased PMN adhesion. Fibrin formed at a shear stress of 80 mPa showed a threefold increase compared with Fb-static. The orientation of fibrin fibers formed under flow conditions appeared to be important, since a 90° turn of the cover slip coated with fibrin formed at shear stress 80 mPa [Fb-flow (shear 80)] showed no significant increase of PMN adhesion compared with Fb-static (116% ± 26%, n = 8; results not shown).
Neutrophil Adhesion to Fibrin Formed Under Flow and Static Conditions (total cells/mm2)
| Surface . | 80 mPa . | 200 mPa . | 320 mPa . |
|---|---|---|---|
| Fb-flow | 1,031 ± 113 | 352 ± 32 | 101 ± 23 |
| Fb-static | 1,153 ± 126 (101%) | 163 ± 333-150 (46%) | 21 ± 153-150 (16%) |
| Surface . | 80 mPa . | 200 mPa . | 320 mPa . |
|---|---|---|---|
| Fb-flow | 1,031 ± 113 | 352 ± 32 | 101 ± 23 |
| Fb-static | 1,153 ± 126 (101%) | 163 ± 333-150 (46%) | 21 ± 153-150 (16%) |
Cover slips coated with tissue factor–rich matrix of HUVECs were either incubated with heparinized PPP (20 minutes at 37°C), forming Fb-static, or perfused with heparinized PPP at shear stress 20 mPa (20 minutes at 37°C), forming Fb-flow. Subsequently, the surfaces were rinsed and used for PMN perfusion at different shear stresses. Data represent the total number of adhering and rolling neutrophils per mm2 (mean ± SE, n = 4 to 10) and in parentheses the percentage of cells compared with Fb-flow at the same shear stress.
PMN adhesion to Fb-static was decreased significantly compared with Fb-flow as determined by paired Student's t-test (P < .01).
PMN adhesion to fibrin formed from plasma at different shear stresses. Heparinized PPP was perfused over cover slips coated with a tissue factor–rich matrix of HUVECs at different shear stresses ranging from 0 mPa (static) to 80 mPa (20 minutes at 37°C). Subsequently, the surfaces were rinsed and used for a PMN perfusion at 200 mPa. After 5 minutes of perfusion, the total number of surface-interacting PMNs per mm2 was measured. The mean ± SE of four experiments is shown. Statistically significant shear-dependency was determined by repeated-measures ANOVA (P < .05).
PMN adhesion to fibrin formed from plasma at different shear stresses. Heparinized PPP was perfused over cover slips coated with a tissue factor–rich matrix of HUVECs at different shear stresses ranging from 0 mPa (static) to 80 mPa (20 minutes at 37°C). Subsequently, the surfaces were rinsed and used for a PMN perfusion at 200 mPa. After 5 minutes of perfusion, the total number of surface-interacting PMNs per mm2 was measured. The mean ± SE of four experiments is shown. Statistically significant shear-dependency was determined by repeated-measures ANOVA (P < .05).
Inhibition of PMN-Fibrin Interaction With Antibodies
The role of several cellular adhesion molecules, such as β2-integrins, in PMN adhesion to fibrin was investigated using MoAbs. Table 4 shows the results using Fb-flow as the adhesive surface at shear stresses of 80 and 200 mPa. All antibodies induced similar results at both shear stresses. Incubation of PMNs with a CD18 antibody (clone IB4), which blocks adhesion through LFA-1, MAC-1, and p150.95, showed a decrease in PMN adhesion of 88% and 96% at 80 and 200 mPa, respectively (Table 4). A MAC-1 blocking antibody against CD11b (clone 44a) induced a similar inhibitory effect (85% inhibition at 80 mPa and 88% at 200 mPa). Functional blocking antibodies against LFA-1 (clone TS-1) did not affect PMN adhesion to fibrin. The possible role of selectins and their ligand sialyl-Lewis X–bearing proteins was determined by evaluation of the susceptibility for neuraminidase cleavage. After incubation of the surface with neuraminidase (0.25 U/mL for 15 minutes at 37°C), 913 PMNs/mm2 adhered, which is not different from the adhesion to untreated fibrin. Blocking antibodies against P-selectin (clone WASP 12.2) induced no significant inhibition of adhesion (Table 4). Using Fb-static as a surface, functionally blocking antibodies CD18 and CD11b, showed similar results compared with Fb-flow (Table 4). Blocking of the β2-integrin MAC-1 by these antibodies showed greater than 80% decreased adhesion at shear stress 80 mPa and greater than 90% at shear 200 mPa. Also, other surfaces, such as Fb-pure and fibrinogen-pure, showed similar results in CD11b- and CD18-blocking studies (data not shown).
Neutrophil Adhesion to Fibrin: Effect of MoAbs
| Condition . | Fibrin PMNs . | 80 mPa . | 200 mPa . | ||
|---|---|---|---|---|---|
| . | . | Total Cells per mm2 . | % . | Total Cells per mm2 . | % . |
| Surface: Fb-flow | |||||
| Control | 943 ± 98 | 100 | 287 ± 31 | 100 | |
| CD16 | X | 1,070 ± 137 | 115 | ND | |
| CD16 | X | 970 ± 80 | 105 | 283 ± 56 | 96 |
| CD18 | X | 119 ± 32 | 124-150 | 13 ± 6 | 44-150 |
| CD11a | X | 1,018 ± 125 | 108 | 277 ± 43 | 99 |
| CD11b | X | 147 ± 35 | 154-150 | 39 ± 18 | 124-150 |
| CD18 + CD11b | X | 65 ± 20 | 84-150 | ND | |
| P-selectin | X | 820 ± 174 | 85 | 315 ± 45 | 111 |
| Neuraminidase | X | 913 ± 137 | 95 | ND | |
| Surface: Fb-static | |||||
| Control | 1,168 ± 199 | 100 | 252 ± 40 | 100 | |
| CD16 | X | 1,334 ± 220 | 120 | ND | |
| CD18 | X | 177 ± 71 | 144-150 | 8 ± 1 | 44-150 |
| CD11b | X | 166 ± 54 | 134-150 | 12 ± 3 | 64-150 |
| Condition . | Fibrin PMNs . | 80 mPa . | 200 mPa . | ||
|---|---|---|---|---|---|
| . | . | Total Cells per mm2 . | % . | Total Cells per mm2 . | % . |
| Surface: Fb-flow | |||||
| Control | 943 ± 98 | 100 | 287 ± 31 | 100 | |
| CD16 | X | 1,070 ± 137 | 115 | ND | |
| CD16 | X | 970 ± 80 | 105 | 283 ± 56 | 96 |
| CD18 | X | 119 ± 32 | 124-150 | 13 ± 6 | 44-150 |
| CD11a | X | 1,018 ± 125 | 108 | 277 ± 43 | 99 |
| CD11b | X | 147 ± 35 | 154-150 | 39 ± 18 | 124-150 |
| CD18 + CD11b | X | 65 ± 20 | 84-150 | ND | |
| P-selectin | X | 820 ± 174 | 85 | 315 ± 45 | 111 |
| Neuraminidase | X | 913 ± 137 | 95 | ND | |
| Surface: Fb-static | |||||
| Control | 1,168 ± 199 | 100 | 252 ± 40 | 100 | |
| CD16 | X | 1,334 ± 220 | 120 | ND | |
| CD18 | X | 177 ± 71 | 144-150 | 8 ± 1 | 44-150 |
| CD11b | X | 166 ± 54 | 134-150 | 12 ± 3 | 64-150 |
Isolated neutrophils were perfused over cover slips covered with fibrin formed from plasma under flow conditions (Fb-flow) or static conditions (Fb-static). Neutrophils or fibrin were incubated with antibodies or neuraminidase as indicated. Cover slips with Fb-flow were incubated with control HEPES buffer, CD16 antibodies (CLB-FcR gran/1), or anti–P-selectin (Waps 12.2). The Fb-flow surface was also incubated with neuraminidase (0.25 U/mL for 15 minutes at 37°C). Neutrophils were incubated with control HEPES buffer, CD16 antibodies, CD18 antibodies (IB4), CD11a antibodies (TS-1), or CD11b antibodies (44a) for 30 minutes at room temperature. Then, the neutrophils (2 × 106/mL HEPES buffer) were perfused in the presence of the MoAbs at 37°C at shear stresses of 80 or 200 mPa. Data represent the total number of adhering and rolling neutrophils per mm2 and the percentage of the buffer control (mean ± SE, n = 4 to 10).
Abbreviation: ND, not determined.
Statistically significant effect of antibody treatment compared with control cells was determined by ANOVA (P < .05).
Effect of Soluble Fibrinogen
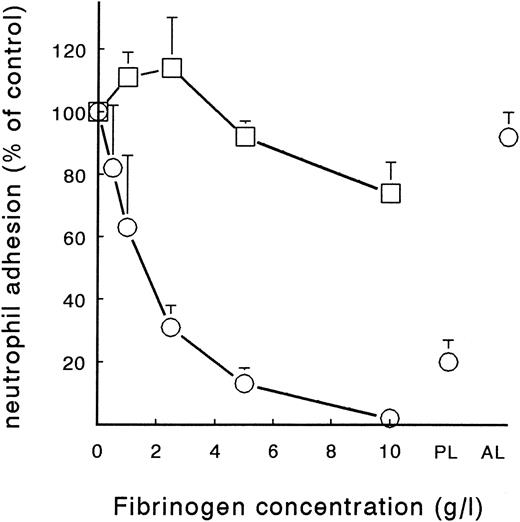
To characterize the possible effect of soluble fibrinogen on PMN adhesion to fibrin and platelets, purified fibrinogen was added at different concentrations during perfusion. Two different fibrin surfaces were used: fibrin formed from plasma under flow conditions with or without platelet deposition (Fb-flow and Fb + platelets). Addition of soluble fibrinogen resulted in a significant decrease in PMN adhesion to Fb-flow (Fig 4). Interestingly, soluble fibrinogen showed no effect on PMN adhesion to Fb + platelets. At a concentration of 2.5 g/L and higher, soluble fibrinogen significantly decreased PMN adhesion to Fb-flow in a concentration-dependent manner (P < .01 by repeated-measures ANOVA). At a concentration of 10 g/L, no PMN adhesion could be detected after 5 minutes of perfusion. The IC50 of fibrinogen-induced inhibition was approximately 1.8 g/L. When the cells were preincubated with 5 g/L fibrinogen (20 minutes at RT) and washed in fibrinogen-free medium before perfusion, PMN adhesion was restored to 80% ± 7% (n = 4) of the control value (results not shown). Loss of the inhibitory effect after washing could be explained by the low binding affinity of fibrinogen for unstimulated PMNs. When the neutrophils were washed and diluted in citrated plasma with a fibrinogen concentration of approximately 2 to 5 g/L, PMN adhesion decreased to 20% ± 7% (n = 4) of the control (Fig 4). In contrast, addition of purified human albumin (5 g/L wt/vol) to the neutrophil suspension did not induce a significant decrease in adhesion.
Effect of soluble fibrinogen on PMN adhesion to fibrin with or without platelets. Neutrophils were perfused over Fb-flow (○) and Fb + platelets (□) at a shear stress of 80 mPa in the presence of different concentrations of soluble fibrinogen. Neutrophils were also suspended in anticoagulated autologous plasma (PL) or a human albumin solution (AL, 5 g/L) and perfused over Fb-flow. Data represent the total number of adhering neutrophils per mm2 as a percentage of the control perfusion with no fibrinogen added. Absolute control values are 1,005 ± 75 cells/mm2 for Fb-flow and 1,209 ± 173 for Fb + platelets. The mean ± SE of four to six experiments is shown. A significant effect of soluble fibrinogen on PMN adhesion to Fb-flow was determined by repeated-measures ANOVA (P < .01).
Effect of soluble fibrinogen on PMN adhesion to fibrin with or without platelets. Neutrophils were perfused over Fb-flow (○) and Fb + platelets (□) at a shear stress of 80 mPa in the presence of different concentrations of soluble fibrinogen. Neutrophils were also suspended in anticoagulated autologous plasma (PL) or a human albumin solution (AL, 5 g/L) and perfused over Fb-flow. Data represent the total number of adhering neutrophils per mm2 as a percentage of the control perfusion with no fibrinogen added. Absolute control values are 1,005 ± 75 cells/mm2 for Fb-flow and 1,209 ± 173 for Fb + platelets. The mean ± SE of four to six experiments is shown. A significant effect of soluble fibrinogen on PMN adhesion to Fb-flow was determined by repeated-measures ANOVA (P < .01).
Time Dependency of PMN Adhesion to Fibrin
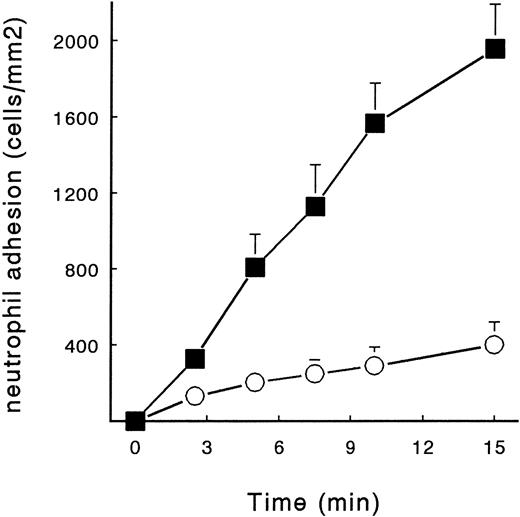
To show that at physiologic plasma concentrations of fibrinogen PMN adhesion to fibrin is still possible, we perfused neutrophils for 15 minutes in the presence and absence of 2.5 g/L fibrinogen. As a surface, we used a fibrin network formed under flow conditions (Fb-flow; Fig 1B). PMNs were perfused at a shear stress of 80 mPa. PMN adhesion increased at a rate of approximately 130 cells/mm2/min, whereas in the presence of soluble fibrinogen, 30 cells/mm2 adhered per minute (Fig 5). This constant increase persisted up to 15 minutes, showing 2,012 ± 210 and 400 ± 50 adhered cells/mm2 in the absence and presence of fibrinogen, respectively.
Time-dependent adhesion of PMNs to fibrin and the effect of fibrinogen. Isolated human neutrophils (2 × 106 cells/mL) were perfused over Fb-flow at a shear stress of 80 mPa for 15 minutes. The cells were diluted in HEPES buffer (▪) or in HEPES buffer containing soluble fibrinogen (5 g/L, ○). The mean ± SE of four experiments is shown. A significant time-dependent adhesion of PMNs to Fb-flow and a significant effect of soluble fibrinogen was observed (repeated-measures ANOVA, P < .01).
Time-dependent adhesion of PMNs to fibrin and the effect of fibrinogen. Isolated human neutrophils (2 × 106 cells/mL) were perfused over Fb-flow at a shear stress of 80 mPa for 15 minutes. The cells were diluted in HEPES buffer (▪) or in HEPES buffer containing soluble fibrinogen (5 g/L, ○). The mean ± SE of four experiments is shown. A significant time-dependent adhesion of PMNs to Fb-flow and a significant effect of soluble fibrinogen was observed (repeated-measures ANOVA, P < .01).
DISCUSSION
Deposition of mixed thrombi in vivo consisting of platelets and fibrin is found at sites of injured vessel wall. The interaction of leukocytes with these thrombi will influence vascular repair and is likely to be of great importance in the pathophysiology of thrombosis, atherosclerosis, and vasculitis. Until now, all leukocyte-fibrin(ogen) adhesion studies have been performed using purified proteins, such as fibrinogen and thrombin, or fibrin formed under static conditions.26 27 However, the formation of a fibrin network is a complex process influenced by many different factors such as the presence of (1) platelets, (2) plasma proteins, and (3) flow. In this report, we describe the cell-protein interaction under flow conditions between PMNs and fibrin and the role of platelets in this interaction. Different types of fibrin and fibrinogen deposition were used to determine which of the factors mentioned influence PMN adhesion to fibrin under flow conditions.
Platelets
Since surface-bound platelets are known to be potent in inducing PMN adhesion under flow conditions, we examined the effect of platelets in a fibrin network on PMN adhesion. Most of the experiments were performed using Fb-flow surfaces in which a few single platelets are present. This surface proved to be potent for adhesion of unstimulated PMNs under flow conditions. Using blocking MoAbs against P-selectin, no role for platelets in PMN adhesion to Fb-flow could be established (Table 4). In addition, by making fibrin from filtered plasma [Fb-flow (no platelets)], it was shown that the absence of residual platelets did not affect PMN adhesion to fibrin (Table 2). On the other hand, substantial deposition of platelets on top of the fibrin network (in Fb + platelets) caused an increase of neutrophil adhesion. This effect was more pronounced at higher shear stresses (Fig 2A), demonstrating the important role of platelets in leukocyte adhesion at high shear conditions. In earlier studies, we showed that P-selectin is expressed on surfacebound platelets and mediates PMN rolling on platelets at high shear stresses.22 Here, we show that platelets alone support up to 50% of rolling adhesion, whereas a fibrin network induces mainly static PMN adhesion (<10% rolling cells). Platelets and fibrin together on a surface form a potent substrate for firm, shear-resistant PMN adhesion (Fig 2B). In this mixed-thrombus model, P-selectin/ligand interactions induce rolling, while MAC-1 binding to fibrin induces static adhesion. In this manner, PMNs are able to adhere at high physiologic shear stresses.
Presence of Plasma Protein in Fibrin Network
Pronounced differences are described for fibrin networks developed in plasma and those developed in pure fibrinogen solution. Networks formed in plasma have thicker fibers, are more permeable, and have lower tensile strength.28 Evidently, fibrinogen and thrombin concentrations are important factors in fibrin formation, but calcium ions, albumin, factor XIII, and thrombospondin are also reported to affect fiber thickness and density.28 From Table 2, it can be concluded that the presence of plasma proteins during fibrin formation does not have an effect on PMN adhesion (compare Fb-static with Fb-pure and fibrinogen-pure). Accordingly, no direct effect of plasma proteins, present during fibrin formation, on the adhesive properties of fibrin for blood cells was described until now. Also, in a protocol similar to ours, platelet adhesion to fibrin formed from plasma was not different from adhesion to purified fibrin.25
Flow
Figure 1 shows that fibrin fibers formed under flow conditions on a prothrombin-activating surface are thick and oriented in the direction of the flow, whereas static-formed fibrin consists of thinner and more randomly distributed fibers. These typical structural features were also described in more detail by others.29,30 In vivo and in a nonanticoagulated whole-blood perfusion system, thick and flow-oriented fibers were also observed.5,31 Only recently, it was suggested that differences in network structure and fibrin orientation affect platelet adhesion and activation.25 Platelets adhering to a Fb-flow surface did form large aggregates, whereas fibrin formed under static conditions only induced adhesion of spread platelets with no aggregates, indicating a less activated phenotype. In our experiments with PMNs, the alignment of flow-directed fibrin fibers strongly promoted adhesion, particularly at higher shear stresses. This was illustrated by the gradual increase in PMN adhesion to more aligned fibrin (by its formation at increasing shear) (Fig 3). Whether additional mechanisms, like differential shear-induced exposition of adhesive sites, play a role remains to be elucidated. However, it seems unlikely that the difference in adhesion observed with Fb-flow versus Fb-static was a result of a changed contribution of neutrophil receptors for fibrinogen; blocking of MAC-1 consistently induced more than 80% inhibition (Table 4).
Receptors and Recognition Sites
Neutrophil receptors involved in the adhesion of PMNs to the fibrin network under flow conditions were characterized. Clearly, adhesion was critically dependent on β2-integrins. In particular, the potent ability of CD11b and CD18 MoAbs to inhibit PMN adhesion to fibrin under flow indicates the important role of MAC-1 (CR-3) in this process. Adhesion of PMNs to fibrinogen and fibrin without additional stimulation was also described in other systems. In most cases, this spontaneous binding occurred at a low affinity, which was increased after stimulation.14 In this respect, the resting status of the isolated neutrophils was confirmed by (1) no spontaneous homotypic aggregation and (2) a low FMLP-induced respiratory burst that could be measured. This indicates that no appreciable activation or priming occurred during isolation (results not shown). Consistently, fibrinogen bound with a low affinity to resting neutrophils. This binding of fibrinogen in (plasma) suspension was enough to inhibit PMN adhesion to fibrin-containing matrices, whereas albumin had no effect (Fig 4). Physiologic concentrations of soluble fibrinogen (2 to 4 g/L) only partially inhibited adhesion to fibrin. This suggests that in vivo under whole-blood conditions with a continuing flow, a fibrin network could allow a low but constant number of neutrophils to adhere per minute. In the case of a platelet-containing fibrin surface, adhesion of neutrophils is possible at higher shear stresses and is not inhibited by soluble fibrinogen (Figs 2 and 4). Our findings indicate that activated platelets present in a thrombus might be capable of promoting PMN adhesion under in vivo conditions.
A second mechanism promoting PMN adhesion to fibrin could be neutrophil activation, resulting in integrin activation. Under static conditions, neutrophil activation leads to increased adhesion to immobilized fibrinogen.14 Interestingly, preactivation with FMLP or the phorbol ester PMA inhibited adhesion to matrix-bound fibrin(ogen) in our perfusion assay.32 These findings were consistent with previous studies in which (rolling) adhesion of neutrophils to platelet-covered matrices was decreased after FMLP treatment. Using a fibrin surface, a role for selectins in PMN adhesion was not anticipated. However, it turned out that optimal adhesion of neutrophils to fibrin-coated surfaces under flow conditions required an L-selectin–mediated cooperative interaction between adherent and freely flowing neutrophils.32
In our adhesion assay, it was clearly seen that PMNs, once adhered to the fibrin network, exhibit strong shear resistance indicating high-affinity receptor/ligand interactions. This was illustrated by the observation that once PMNs adhered to fibrin, they did not detach from the surface by plasma or fibrinogen solutions (5 g/L) even at a high shear up to 640 mPa, which normally prevents PMN binding (results not shown). Several investigators have described activation of neutrophils by binding to different surfaces (ie, fibrinogen or CD18-antibodies) due to β2-integrin–mediated signaling.33 34 A fast upregulation of ligand-receptor affinity upon PMN adherence and activation may explain the observed firm attachment, and is under current investigation.
In conclusion, we show that unstimulated PMNs are able to adhere to a fibrin network under flow conditions in an integrin-dependent manner. The most important interaction is mediated by MAC-1 (CD11b/CD18). The structural features of the fibrin fibers, especially their orientation, affect the extent of neutrophil adhesion. However, the presence of plasma proteins within the network did not influence adhesion. Platelet deposition together with fibrin provides an optimal surface for both selectin-mediated rolling at high shear and integrin-mediated firm attachment. Neutrophil adhesion to such a mixed thrombus is hardly influenced by physiologic fibrinogen levels. This study provides more insight into the regulating role of fibrin with or without platelets in leukocyte interactions with the hemostatic response to damaged vessel wall.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr J.A.M. van der Linden for assistance in quantification of the data.
Supported by Dutch Heart Foundation Grant No. 92.106 and by Glaxo Wellcome, The Netherlands.
Address reprint requests to J.J. Zwaginga, MD, Department of Haematology, Room G.03.647, University Hospital Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal