Abstract
We have identified a gene that has a high level of mRNA expression in undifferentiated, multipotential hematopoietic cells (FDCP-Mix) and that downregulates both transcript and protein, as these cells are induced to differentiate into mature myeloid cells. Sequence analysis of this gene has identified it as a serine protease inhibitor EB22/3 (serpin 2A). Constitutive expression of serpin 2A in FDCP-Mix cells was associated with an increase in the clonogenic potential of the cells and with a delay in the appearance of fully mature cells in cultures undergoing granulocyte macrophage differentiation when compared with control cells. Serpin 2A was also found to be expressed in bone marrow-derived bipotent granulocyte macrophage progenitor cells (GM-colony forming cell [CFC]), but not in erythrocyte progenitor cells from day 15 fetal liver. Expression of serpin 2A also showed a marked up regulation during the activation of cytotoxic suppressor CD8+ T cells, with a clear lag between the appearance of transcript and detection of protein.
THE MATURE CELLS of the regenerating tissues are continually being replenished from more primitive stem cells.1 These stem cells, although few in number, possess two features that distinguish them from the mature cells: they are multipotent and can undergo self-renewal.2 How the balance between self-renewal and differentiation is maintained remains an enigma. We have previously described the use of multipotent hematopoietic cell lines (FDCP-Mix cells) to investigate the molecular genetic changes that accompany (and perhaps regulate) the process of stem cell self-renewal versus differentiation.3 These cell lines are karyotypically normal, nonleukemic, and require the continual presence of appropriate growth factors such as interleukin-3 (IL-3) for the maintenance of a primitive self-renewing blast phenotype.4 Also like normal cells, if FDCP-Mix are cultured on bone marrow stromal cells in the absence of added growth factors, they undergo multilineage differentiation into mature myeloid cells.5 Furthermore, they can also be induced to undergo multilineage differentiation in the absence of stromal cells by culturing in the presence of an appropriate range and concentration of growth factors.6 This property has allowed large numbers of both primitive and mature cells to be obtained to perform detailed molecular studies and isolation of genes that are downregulated during differentiation.3
Using the method of differential cDNA library screening, we now report the cloning of the serine protease inhibitor EB22/3 or serpin 2A7 from undifferentiated FDCP-Mix cells together with its pattern of expression at various stages of differentiation in FDCP-Mix cells, in normal tissue, freshly isolated hematopoietic progenitor cells, and quiescent and activated T cells. We also report the results of constitutively expressing the gene in FDCP-Mix cells.
MATERIALS AND METHODS
Cell culture.The origin and characterization of the FDCP-Mix cell line (clone A4) have been previously described.4,8 The cells were maintained in a self-renewing primitive phenotype by culturing in 2% (vol:vol) X63 AG81653 cell-conditioned medium as a source of IL-39, 20% (vol:vol) horse serum (various sources: Medical Veterinary Supplies, Fred Baker Science, Runcorn, Cheshire, UK; GIBCO, Paisley, UK, and Flow Labs, Rickmansworth, Herts, UK) and Iscoves medium GIBCO. The development of FDCP-Mix cells into granulocytes and macrophages was promoted by changing the culture conditions to 20% (vol:vol) fetal calf serum (Sera Lab, Crowley Down, Sussex, UK; NBL, Cramlington, Northumberland, UK; or GIBCO) 1.5 U/mL IL-3, 50 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF ), and 1,000 U/mL G-CSF in Iscoves medium.6 All cells were grown at 37°C in 5% CO2 (vol:vol) in air.
Clonogenic assays of FDCP-Mix were performed as previously described.6 Each assay was performed in triplicate and incubated at 37°C, 5% CO2 for 7 to 12 days, and the number of colonies greater than 50 cells were counted.
Retroviral gene transfer to FDCP-Mix cells.This has been extensively described elsewhere.10 Briefly, the ecotropic packaging cell line GP+E-8611 was lipofected with 30 μg of M5 vector12 containing the serpin 2A open reading frame. This was selected with 500 μg/mL of G-418 (neomycin analogue, GIBCO-BRL) and used to infect the amphotropic producer cell line GP+envAm12.13 G-418 resistant colonies were isolated, expanded, and assayed for the ability to produce virus by infection of 3T3 cell.10 Producer cell clones were then assayed for production of 2A transcript and protein by Northern and Western blotting. Confluent monolayers of producer cells shown to express the 2A gene were irradiated and used to infect FDCP-Mix cells by cocultivation. G-418 resistant FDCP-Mix clones were selected by cloning in soft agar, expanded, and assayed for production of serpin 2A transcript and protein.
Differentiation assay.Differentiation of the retrovirally transfected FDCP-Mix clones was performed as described previously. Three identical T25 cm2 flasks were seeded with each clone from cells cultured under conditions that promote self-renewal to a density of 1 × 105 cells/mL in granulocyte/differentiation medium with the remaining undifferentiated cells being used for RNA and protein extraction. These cultures were incubated for 8 days at 37°C, 5% CO2 whereupon cytospin preparations were made, and the remainder of the cells harvested for RNA and protein.
Hematopoietic growth factors.The growth factors used were as follows: GM-CSF (recombinant murine, 1.25 × 107 U/mg, Biogen, Geneva, Switzerland); G-CSF (recombinant human, 108 U/mg, Amgen, Thousand Oaks, CA); IL-3 (recombinant murine, 2.1 × 107 U/mg, Biogen).
Isolation of granulocyte macrophage colony-forming cells (GM-CFC) from bone marrow.These were isolated by the procedure of Cook et al,14 which was adapted from that of Williams et al.15 Briefly, 20 mice were intraperitoneally injected with 200 mg/kg of cyclophosphamide. On day 4, the femora were removed and the marrow cells washed with Fischer's medium. These were then suspended in low density (1.06 g/mL) metrizamide, which was layered onto 1.08 g/mL of metrizamide and centrifuged 800g for 15 minutes at 4°C. The low density interface cells were collected, washed with Fischer's medium, and pumped into the elutriator JE6B rotor (Beckman Instruments, Irvine, CA) spinning at 2,000 rpm with Fischer's plus 5% horse serum (FHS) at a flow rate of 10 mL/min. A total of 100 mL of eluent was collected and the flow switched to bypass the loading chamber. A further 100 mL of eluent was collected for each FHS flow rate of 14, 16.5, and 19.5 mL/min, which was discarded. The flow rate was sequentially increased to 22, 25.5, 29, and 32 mL/min of FHS collecting 100 mL of eluent per flow rate. These fractions contained the GM-CFC that were used for RNA extraction and in vitro differentiation.
GM-CFC were stimulated to undergo development into mature granulocytes or macrophages by culturing in Iscove's medium supplemented with 20% vol:vol fetal calf serum and either 5,000 U/mL G-CSF or 2 U/mL M-CSF6 for a period of 4 days.
Erythropoietic fetal liver cells.Fresh fetal livers were surgically removed from 15-day gestation BDF1 fetuses and sheared through a 23-gauge hypodermic needle in ice cold phosphate-buffered saline (PBS) to give a unicellular suspension. Cytospin preparations were made from this and the remainder gently pelleted (1,200g ) for RNA extraction.
Activated T-cell cultures.Primary splenocytes were cultured at a density of 1 × 106/mL in HEPES buffered RPMI containing 10% fetal bovine serum (FBS), and 2 mercaptoethanol (ME) maintained at 37°C, 5%CO2 . Cultures were stimulated with either 10 μg/mL of Con A or a 1:300 dilution of anti-CD3 plus 60 U/mL of IL-2. On day 3 of activation, the cultures were split 1:1 with fresh activation medium except that Con A and anti-CD3 was left out. The same procedure was performed when taking the day 4 sample.
RNA isolation and cDNA library construction.RNA was purified by the guanidinium isothiocyanate/cesium chloride method.16 This was poly A+ selected by the use of oligo dT magnetic beads (Dynal, Wirral, Merseyside, UK) according to the manufacturer's instructions. cDNA library construction was adapted from the method of Watson and Jackson,17 whereby approximately 100 ng of double-stranded R1 linkered cDNA was ligated into 1 μg of Eco-R1-cut λgt10 (Stratagene, La Jolla, CA). This was packaged (Gigapack Gold, Stratagene), plated and found to have a primary titer of 1.3 × 107 clones.
cDNA probe synthesis.The differential screening procedure has been described in detail elsewhere.3
Random primed probes of isolated cDNA inserts were made by boiling 50 ng of DNA for 2 minutes, followed by incubation with 50 μCi of α32[P]dCTP using the same conditions.
cDNA library screening.Plaque lifts of 10,000 primary clones were prepared on 22 × 22 cm nylon filters (Hybond-N; Amersham, Little Chalfont, UK) and these were subsequently probed with the undifferentiated followed by the differentiated FDCP-Mix cDNA probes, as previously described,3 and exposed to Kodak XAR5 film (Eastman Kodak, Rochester, NY).
Polymerase chain reaction amplification (PCR) of λgt10 cDNA inserts.Individual plaque picks were eluted at 4°C overnight into 500 μL of SM18 + 20 μL of chloroform. A total of 4 μL of this extract was added to a standard 100 μL PCR reaction using λgt10 screening amplimers (Clontech Labs, Palo Alto, CA) and 2.5 U of Taq DNA polymerase (Boehringer Mannheim, Lewes, UK) according to the suppliers instructions. A 227-bp specific serpin 2A probe was PCR amplified from the isolated cDNA using the oligonucleotide primers: 5′ CTCTTTATGGCAAAA 3′ and 5′ TCAGGAACCTGATT 3′ with the following cycle parameters: 10 × (94°C for 1 minute, 52°C for 45 seconds, 72°C for 30 seconds). The entire 1,320-bp serpin open reading frame was PCR amplified using BamHI site mismatch oligonucleotides and ligated into the retroviral expression vector M5 Neo.12
Analysis of cDNAs.This has been described in detail elsewhere.3 Briefly, the PCR amplified products from each plaque pick were separated by agarose gel electrophoresis, capillary transferred to Hybond nylon filters, and sequentially reprobed with cDNA probes made from undifferentiated and differentiated FDCP-Mix RNAs. Filters were again exposed to Kodak XAR5 film.
Isolation of cDNA inserts and subcloning.Inserts identified as positive with the undifferentiated and negative with the differentiated probe were isolated by preparative agarose gel electrophoresis. These were then digested with EcoRI (Lambda gt10 cloning site), ligated into EcoRI digested, phosphatased Puc13, and transformed into competent Escherichia coli HB101.
DNA sequencing.Double-stranded cDNA clones were sequenced by the dideoxy method of Sanger et al19 using a Sequenase II kit (US Biochemicals, Cleveland, OH).
Northern analysis.This was based on the method of Lehrach et al20 and has been described previously.3 Hybridization was performed essentially as for DNA blots using conditions of 7% sodium dodecyl sulfate (SDS), 1% bovine serum albumin (BSA), 0.25 mol/L NaCl, and 0.25 mol/L Na2HPO4.21
Nuclear run off transcription assays.The preparation of FDCP-Mix nuclei and the run off transcription reactions were performed according to the method of Marzaluff and Huang.22 Cells were lysed in the absence of detergent, and the nuclei centrifuged through 2 mol/L sucrose. A 200-μL transcription reaction containing 1 × 107 nuclei with 100 μCi α32[P]UTP (Amersham) made up to a concentration of 0.4 μmol/L UTP, was performed. RNA was extracted using the guanidinium isothiocyanate acid phenol procedure.23 Slot blots of 10 μg of linearized target plasmid per slot were prepared on Hybond-N nylon membrane and the hybridization performed using the conditions of Church and Gilbert21 with the addition of 10 μg/mL of polyA (Boehringer Mannheim). The membrane was washed to a stringency of 20 mmol/L sodium phosphate pH 7.2 at 68°C with the inclusion of a ribonuclease digest according to the method of Becker and Schutz.24 Hybridization signals were quantified by the use of a Molecular Dynamics Phosphorimager.
Antiserum production.Initially, it proved difficult to clone the serpin 2A ORF into various bacterial expression vectors, hence three potentially antigenic peptides were selected from the protein sequence (WKNPFDPNDTFKSEV, ILPDGGRMQQVEASL, and MAKVTNPERCMNFPN) using the Wisconsin GCG antigenicity plot. These were synthesized (ALTA Bioscience, Birmingham, UK), conjugated to BSA by the use of glutaraldehyde, and the resultant hapten complex used to immunize a rabbit. Following boosting, the serum was assayed by Western blot for reactivity against proteins isolated from GP+envAm12 cells13 transfected with a serpin 2A expression vector (M5 neo12 ). Subsequent to this, the 2A protein was successfully expressed using the pet3a vector in the p-lys S bacterial host (Novogen25 ). This product was used to test the reactivity of the 2A peptide antiserum.
Recombinant granzyme B protein was prepared by cloning the open reading frame (ORF ) into the glutathione fusion vector pGEX 2T and expressing this in E coli.26 The insoluble protein product was purified by preparative SDS polyacrylamide gel electrophoresis27 and used to immunize a rabbit. After boosting, the serum was assayed by Western blot for reactivity against recombinant granzyme B (manuscript in preparation).
Western blot analysis.Cells were pelleted, washed briefly in phosphate-buffered saline (PBS) and dissolved in 2× Laemmli buffer (0.06 25 mol/L Tris/HCl pH 6.8, 2.5% SDS, 10% glycerol). The equivalent of 2 × 105 cells per track of total cell protein was heated to 100°C for 2 minutes and separated by electrophoresis through a 12% polyacrylamide gel according to the method of Laemmli.27
The separated proteins were transferred to Hybond-C extra membranes (Amersham) which were preblocked for 2 hours with blocking buffer (3% wt:vol BSA, 0.1% vol:vol Tween 20 in PBS) at room temperature with shaking. This was then incubated for 1 hour with the rabbit antiserpin 2A antisera at a concentration of 1:300 in blocking buffer followed by extensive washing with 0.1% (vol:vol) Tween 20 in PBS. The membrane was finally incubated with peroxidase conjugated swine antirabbit secondary antibody (Dako, High Wycombe, UK) at a concentration of 1:4,000 in blocking buffer for 30 minutes again followed by washing with 0.1% (vol:vol) Tween 20 in PBS. Detection of bound antibody was performed by the use of an enhanced chemiluminescence (ECL) fluorescence kit (Amersham) with exposure times of 1 to 5 minutes to Hyperfilm (Amersham) being typical.
RESULTS
Cloning and analysis of serpin 2A expression pattern in self-renewing and differentiating FDCP-Mix cells.Several clones were isolated by the differential cloning procedure described, some of which were identified as the previously characterized protease CCP1 (Granzyme B).3 Sequence analysis of additional clones identified a previously cloned but incomplete serine protease inhibitor mRNA, serpin 2A7 (EMBL Accession No. M64085), as being present in undifferentiated, but absent in differentiated FDCP-Mix cells. Figure 1 clearly demonstrates that the transcript is highly expressed in the primitive self-renewing day 0 population and undergoes extensive downregulation as cells are induced to differentiate along the G/M lineage for 8 days. When corrected for different exposure times, the level of expression of this gene in the day 0 cells approaches that of α-actin,28 which is approximately 0.5% of the mRNA population (L. Hampson, unpublished observation, October 1993) and drops to virtually undetectable levels by day 6. This pattern of expression can be correlated with the decline of clonogenic cells present in the differentiating culture at each time point, as previously reported.3 It does not correlate with the proliferative activity of the cultures, which are actively expanding up to day 7. The additional smaller transcript observed on the Northern blot was shown to be present irrespective of whether a complete full coding sequence probe or various fragments of this were used as probes. This implies an alternative 5′ UTR splicing event, which is the subject of continued investigation.
Northern blot of RNA isolated from an FDCP-Mix granulocyte macrophage differentiation time course probed with labeled serpin 2A cDNA followed by α-actin. The lanes represent undifferentiated day 0 to fully differentiated day 8 of the time course. Signals were visualized by phosphorimaging.
Northern blot of RNA isolated from an FDCP-Mix granulocyte macrophage differentiation time course probed with labeled serpin 2A cDNA followed by α-actin. The lanes represent undifferentiated day 0 to fully differentiated day 8 of the time course. Signals were visualized by phosphorimaging.
Nuclear run off transcription analysis.The results shown in Fig 2 indicate that a rapid >250-fold decrease in the rate of serpin 2A mRNA transcription occurs concomitant with G/M development in FDCP-Mix cells. This is in contrast to the myeloid-specific gene lysozyme M,29 which increases throughout the 6-day differentiation time course, and glyceraldehyde phosphate dehydrogenase (GAPDH), which remains constant. These data clearly demonstrate that the difference in 2A transcript levels between undifferentiated and mature cells results from transcriptional regulation and not simply from an alteration of serpin 2A mRNA stability. Granzyme B undergoes a less rapid, sixfold transcriptional downregulation, which is entirely consistent with previously published data.3
Nuclear run off transcription assays using probes made from nuclei isolated from day 0 undifferentiated and day 6 G/M differentiated FDCP-Mix cells. Signals were detected by phosphorimaging.
Nuclear run off transcription assays using probes made from nuclei isolated from day 0 undifferentiated and day 6 G/M differentiated FDCP-Mix cells. Signals were detected by phosphorimaging.
Sequence analysis.The longest FDCP-Mix-derived serpin 2A clone was 2.3 kb in length and differed from the teratocarcinoma-derived clone of Inglis et al7 in that it possessed a functional exon 2 (Fig 3). Because there is a high degree of homology between serpin 2A and other serpins, in particular α-1–antichymotrypsin (ACT 59% AA identity), it was necessary to derive a specific DNA probe. This was done by sequence comparison with other serpins found in the EMBL database, using the Wisconsin GCC gap program, and the 227-bp fragment, shown in Fig 3, was subsequently PCR amplified and cloned. This product was used as a probe in all subsequent Northern blots.
cDNA sequence of FDCP-Mix Serpin 2A. The FDCP-Mix–derived sequence is complete unlike the previously reported teratocarcinoma derived cDNA,7 which lacked exon 2. The splice junction between exons 2 and 3 is marked with a #. We have also shown that the FDCP-Mix serpin 2A cDNA also has an untranslated exon 1 (data not shown). The Cys-Cys P1 and P1′ active site residues are marked and double underlined. The oligonucleotide primers sites indicated by underlining at positions 1255-1266 bp and position 1469-1482 bp were used to PCR amplify a 227-bp serpin 2A specific probe, which was used for all the Northern blots shown.
cDNA sequence of FDCP-Mix Serpin 2A. The FDCP-Mix–derived sequence is complete unlike the previously reported teratocarcinoma derived cDNA,7 which lacked exon 2. The splice junction between exons 2 and 3 is marked with a #. We have also shown that the FDCP-Mix serpin 2A cDNA also has an untranslated exon 1 (data not shown). The Cys-Cys P1 and P1′ active site residues are marked and double underlined. The oligonucleotide primers sites indicated by underlining at positions 1255-1266 bp and position 1469-1482 bp were used to PCR amplify a 227-bp serpin 2A specific probe, which was used for all the Northern blots shown.
Influence of growth factors on serpin 2A transcript expression.Readdition of high concentration IL-3 (150 U/mL used to maintain FDCP-Mix cells in a proliferative self-renewal mode) to differentiated day 9 cells, resulted in a slight increase in expression of serpin transcripts (Fig 4A). This was only a fraction of the expression observed in undifferentiated cells and probably reflects the response of low numbers of residual progenitor cells present in the culture, similar to that previously reported for Granzyme B.3 The addition of GM-CSF, M-CSF, G-CSF, transforming growth factor-β (TGF-β), IL-6, IL-4, or IL-1 produced no increase in serpin 2A mRNA levels (results not shown). We thus conclude that high level expression of serpin 2A is not a characteristic of mature myeloid cells even when treated with a variety of growth factors known to activate mature cell function.
Northern blots of RNAs isolated from (A) day 9 G/M differentiated FDCP-Mix cells before (lane 1), 30 minutes after (lane 2), and 4 hours after (lane 3) addition of 150 U/mL of IL-3, probed with labeled serpin 2A cDNA and α-actin. (B) Undifferentiated GM-CFC (lane 1), day 4 in vitro macrophage differentiated GM-CFC (lane 2), and day 4 in vitro granulocyte differentiated GM-CFC (lane 3) probed with labeled serpin 2A cDNA and α-actin. (C) Undifferentiated FDCP-Mix cells (lane 1), 15-day gestation fetal liver (lane 2), and adult liver (lane 3) probed with labelled serpin 2A cDNA and α-actin.
Northern blots of RNAs isolated from (A) day 9 G/M differentiated FDCP-Mix cells before (lane 1), 30 minutes after (lane 2), and 4 hours after (lane 3) addition of 150 U/mL of IL-3, probed with labeled serpin 2A cDNA and α-actin. (B) Undifferentiated GM-CFC (lane 1), day 4 in vitro macrophage differentiated GM-CFC (lane 2), and day 4 in vitro granulocyte differentiated GM-CFC (lane 3) probed with labeled serpin 2A cDNA and α-actin. (C) Undifferentiated FDCP-Mix cells (lane 1), 15-day gestation fetal liver (lane 2), and adult liver (lane 3) probed with labelled serpin 2A cDNA and α-actin.
Expression of serpin 2A in bipotent GM-CFC and their maturing progeny.High level expression of serpin 2A in undifferentiated multipotential FDCP-Mix cells encouraged us to examine expression of the gene in more developmentally restricted myeloid progenitor cells. To do this, we used a freshly isolated, highly enriched population of bipotent neutrophil/macrophage progenitor cells (40% soft agar granulocyte macrophage colony forming efficiency) from the bone marrow. Figure 4B demonstrates that serpin 2A is also expressed in the bipotent cells but is downregulated as a consequence of differentiation and development into mature macrophages (differential score 98% macrophages, 2% granulocytes) and neutrophils (differrential score 9% macrophages, 83% mature granulocytes, 9% early granulocytes). To exclude the possibility that the serpin 2A signal observed in undifferentiated GM-CFC was due to contaminating activated T cells, we reprobed the blot with granzyme B, which proved totally negative.
Expression of serpin 2A in erythropoietic fetal liver cells.Fetal liver is the main site of hematopoiesis in the neonate, and on day 15 of gestation, this is confined mainly to erythroid cells that comprise about 97% of the total cellularity. The results, shown in Fig 4C, clearly demonstrate that the 2A mRNA is not expressed in these cells even with prolonged exposure times.
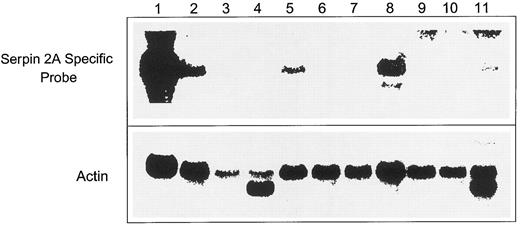
Tissue distribution of serpin 2A.Having established that the gene is expressed in multipotent cells and in granulocyte macrophage progenitor cells, we next examined the tissue distribution of serpin 2A mRNA expression. Figure 5 shows a Northern blot of various murine organs probed with the 227-bp specific serpin 2A fragment and α-actin. The adult liver track confirmed that this was negative for 2A expression in agreement with Fig 4C, whereas thymus, spleen, and testes were all positive. A weak signal was also detected in bone marrow, which presumably reflects the low numbers of progenitor cells present. While the significance of this distribution is not yet known, it is interesting to note that the spleen is not only an organ that can exhibit extramedullary hematopoiesis, but together with thymus and testes, represents a major site of cells undergoing apoptosis. The thymus is involved in T-cell maturation and the testes in spermatogenesis, both of which are processes that involve differentiation, proliferation, and apoptosis.
Northern blot of RNAs isolated from adult murine organs. Lane 1, undifferentiated FDCP-Mix cells; lane 2, lung; lane 3, liver; lane 4, heart; lane 5, thymus; lane 6, small intestine; lane 7, bone marrow; lane 8, spleen; lane 9, kidney; lane 10, brain; and lane 11, testis probed with labeled 3′ specific serpin 2A cDNA fragment.
Northern blot of RNAs isolated from adult murine organs. Lane 1, undifferentiated FDCP-Mix cells; lane 2, lung; lane 3, liver; lane 4, heart; lane 5, thymus; lane 6, small intestine; lane 7, bone marrow; lane 8, spleen; lane 9, kidney; lane 10, brain; and lane 11, testis probed with labeled 3′ specific serpin 2A cDNA fragment.
Expression of serpin 2A by activated T cells.The expression of serpin 2A in thymus and spleen, together with our previous finding of granzyme B expression in hematopoietic progenitors, as well as in activated T cells, prompted us to look more closely at expression in populations of T cells. Resting nonactivated T cells do not express the gene (Fig 6), however, stimulation with mitogenic activators, such as Concanavalin A plus IL-2, caused an upregulation of expression of serpin 2A mRNA. This increase is found within 1 day and occurs 24 hours earlier than the previously reported30 upregulation of Granzyme B transcripts, as shown in Fig 6. In further work, we have demonstrated that this upregulation of serpin 2A expression is mainly confined to fluorescence-activated cell sorting (FACS) sorted CD8+ cytotoxic and suppressor cells with a much reduced signal in activated helper CD4+ cells (data not shown).
Northern blot of RNA isolated from a 7-day T-cell (splenocyte) Con A-induced activation time course probed with labeled serpin 2A and granzyme B cDNAs.
Northern blot of RNA isolated from a 7-day T-cell (splenocyte) Con A-induced activation time course probed with labeled serpin 2A and granzyme B cDNAs.
Western blot analysis.To determine the level of serpin 2A protein production in FDCP-Mix and activated T cells, we raised a rabbit polyclonal antiserum against antigenic peptides, selected from the 2A protein, and coupled to BSA. This antisera showed reactivity with the serpin 2A protein produced by both the pet3a bacterial expression system (Fig 7A and in a mammalian cell expression system following retroviral-mediated gene transfer of the 2A open reading frame (+ve track, Fig 7B). No 50-kD reactive band was detected in a control expression system with the gene in reverse orientation (−ve track). The serpin 2A protein is clearly seen in undifferentiated FDCP-Mix cells, but undergoes rapid downregulation as these are induced to differentiate (Fig 7B). Analysis of proteins isolated from a T cell Con A/IL-2 activation time course showed no signal in quiescent T cells (Fig 7C, track 0) with the accumulation of several signals following activation (Fig 7C, tracks 2, 3, 4, and 5). Interestingly, although high levels of the 2A mRNA were transcribed 24 hours after activation (Fig 6), the protein was translated 24 hours later (Fig 7C). This pattern of mRNA/protein expression was in contrast to that observed for Granzyme B protein, which was detected 48 hours after activation (Fig 7C) concomitant with detection of Granzyme B mRNA (Fig 6). The observation of multiple serpin 2A protein signals is most likely explained by the mode of action of serpins. It is known that serpins can form SDS stable complexes with their target proteases,31 which is the most probable explanation for the 100-kD signal. Furthermore, these serpin/protease complexes undergo gradual proteolysis at the serpin active site thereby producing cleavage products that would explain the bands smaller than the 50-kD native serpin 2A signal. Investigation of these complexes and their products will be the subject of further study.
SDS polyacrylamide gel electrophoresis showing (A) Coomassie brilliant blue stained 12% gel of proteins isolated from lane 1, noninduced culture of BL21DE3 plys S transformed with serpin 2A ORF cloned into the Pet 3A T7 RNA polymerase expression vector; lane 2, the same culture after 2 hours of induction at 37°C with 0.2 mmol/L IPTG and a Western immunoblot of a duplicate gel probed with the serpin 2A peptide antiserum. Detection was by ECL and the exposure time for the blot shown was 3 seconds. (B) Western immunoblot of proteins isolated from an FDCP-Mix G/M differentiation time course. The +ve control lane was made up of proteins isolated from the amphotropic retrovirus packaging cell line GP + envAm1213 that had been transfected with the serpin 2A ORF cloned into the high level retroviral expression vector M5. The −ve control lane was made up of proteins isolated from the wild type packaging cell line. This was incubated with the serpin 2A peptide antiserum with detection by peroxidase conjugated antiimmunoglobulin and ECL. Exposure times were typically between 1 and 2 minutes. (C) Western immunoblot of proteins isolated from Con A + Il-2 activation time course of primary splenocyte T cells. Lanes 0 to 5 represent the period of the activation in days. These were incubated with the serpin 2A antiserum and detection was by ECL with an exposure time of 1 minute.
SDS polyacrylamide gel electrophoresis showing (A) Coomassie brilliant blue stained 12% gel of proteins isolated from lane 1, noninduced culture of BL21DE3 plys S transformed with serpin 2A ORF cloned into the Pet 3A T7 RNA polymerase expression vector; lane 2, the same culture after 2 hours of induction at 37°C with 0.2 mmol/L IPTG and a Western immunoblot of a duplicate gel probed with the serpin 2A peptide antiserum. Detection was by ECL and the exposure time for the blot shown was 3 seconds. (B) Western immunoblot of proteins isolated from an FDCP-Mix G/M differentiation time course. The +ve control lane was made up of proteins isolated from the amphotropic retrovirus packaging cell line GP + envAm1213 that had been transfected with the serpin 2A ORF cloned into the high level retroviral expression vector M5. The −ve control lane was made up of proteins isolated from the wild type packaging cell line. This was incubated with the serpin 2A peptide antiserum with detection by peroxidase conjugated antiimmunoglobulin and ECL. Exposure times were typically between 1 and 2 minutes. (C) Western immunoblot of proteins isolated from Con A + Il-2 activation time course of primary splenocyte T cells. Lanes 0 to 5 represent the period of the activation in days. These were incubated with the serpin 2A antiserum and detection was by ECL with an exposure time of 1 minute.
Effect of constitutive expression of serpin 2A in FDCP-Mix cells.Northern blot analysis of RNAs extracted from serpin 2A and control vector transfected FDCP-Mix clones confirmed that the appropriate constructs had been successfully transferred to the cells. Figure 8, track 1 shows the 2.3 kb endogenous serpin 2A transcript present in undifferentiated vector only control cells, whereas tracks 3, 5, and 7 show the additional 5.2-kb retrovirally encoded band that is present in the undifferentiated serpin 2A transfected clones. Interestingly, the levels of retrovirally encoded expression obtained, were considerably less than the endogenous 2A mRNA levels observed for each undifferentiated clone. In addition, variation in endogenous transcript levels in different clones was also observed.
Northern blot of RNAs isolated from undifferentiated and day 8 G/M differentiated serpin 2A transfected FDCP-Mix clones. Lane 1, undifferentiated; lane 2, differentiated Neomycin vector only control cells; lane 3, undifferentiated; lane 4, differentiated serpin 2A clone 4 cells; lane 5, undifferentiated; lane 6, differentiated serpin clone 5 cells; lane 7, undifferentiated; lane 8, differentiated serpin clone 6 cells. This was probed with serpin 2A and α-actin with signals being visualized by phosphorimaging (the RNAs were isolated from one of the experiments shown in Fig 9A).
Northern blot of RNAs isolated from undifferentiated and day 8 G/M differentiated serpin 2A transfected FDCP-Mix clones. Lane 1, undifferentiated; lane 2, differentiated Neomycin vector only control cells; lane 3, undifferentiated; lane 4, differentiated serpin 2A clone 4 cells; lane 5, undifferentiated; lane 6, differentiated serpin clone 5 cells; lane 7, undifferentiated; lane 8, differentiated serpin clone 6 cells. This was probed with serpin 2A and α-actin with signals being visualized by phosphorimaging (the RNAs were isolated from one of the experiments shown in Fig 9A).
When the transfected FDCP-Mix cells were induced to differentiate into granulocytes/macrophages, their was a decrease in expression of the endogenous transcript, whereas the retrovirally encoded transcript was maintained (Fig 8, tracks 4, 6, and 8). Western blots confirmed that the serpin transduced cells continued to produce the serpin 2A protein (albeit at lower levels), but the protein was not detectable in the differentiated control transfected cells. This expression of the transfected serpin 2A in FDCP-Mix cells was associated with a delay in maturation: a significantly higher proportion of immature cells was clearly seen at the end of the 8-day differentiation time course (Fig 9A). However, no significant effect was seen in the relative proportions of neutrophils/macrophages that were produced from the control or serpin transfected cells when sampled on a daily basis (three experiments, data not shown). This delay in maturation along with the lack of bias to either the neutophil or macrophage lineage indicates that expression of the transduced serpin 2A may be influencing the probability of differentiation of the clonogenic FDCP-Mix cells. In support of this, analysis of the ability of the retrovirally transfected clones to form colonies in soft agar6 reproducibly demonstrated that cells transfected with the 2A gene had a significant (P = .005) threefold to fivefold higher clonogenic potential than the corresponding control cell clones transfected with vector alone (Fig 9B).
(A) Represents the results of differential cell morphology scores performed on the serpin 2A and vector only control transfected FDCP-Mix cells after 8 days of culturing under conditions that promote granulocyte macrophage differentiation. The results represent the pooled data of three experiments for each clone, and the sample standard deviations are shown. Primitive cells comprise blasts and early granulocytes; mature cells represent metamyelocytes, mature granulocytes, and macrophages. The mean 8-day terminal counts were 5.0 × 105 cells/mL for vector only transfectants and 5.3 × 105cells/mL for serpin 2A transfectants with standard deviations not exceeding 10% of the mean (not shown). Statistical comparison of the Neo clone with the highest mean number of primitive cells (Neo 7) to each of the serpin clones using a two sample t-test gave P values of <.005 for serpin clones 4 and 5 judged as a highly significant difference and <.05 for serpin 6, which is judged as significant. (B) Represents the results of the soft agar clonogenic assays performed on serpin 2A and vector only transfected FDCP-Mix cells. Clones were plated at 5 × 103 cells/assay and the serpin 2A transfected clones were also scored at plating densities of 2 × 103 cells/assay. Each data point was the mean result of three separate plating assays and is representative of three repeat experiments with the standard deviations shown. Statistical comparison of the neo clone with the highest clonogenic potential (Neo1) with each of the serpin 2A transfected clones using a two sample t-test gave a P value of <.005 for all clones, which is judged as a highly significant difference.
(A) Represents the results of differential cell morphology scores performed on the serpin 2A and vector only control transfected FDCP-Mix cells after 8 days of culturing under conditions that promote granulocyte macrophage differentiation. The results represent the pooled data of three experiments for each clone, and the sample standard deviations are shown. Primitive cells comprise blasts and early granulocytes; mature cells represent metamyelocytes, mature granulocytes, and macrophages. The mean 8-day terminal counts were 5.0 × 105 cells/mL for vector only transfectants and 5.3 × 105cells/mL for serpin 2A transfectants with standard deviations not exceeding 10% of the mean (not shown). Statistical comparison of the Neo clone with the highest mean number of primitive cells (Neo 7) to each of the serpin clones using a two sample t-test gave P values of <.005 for serpin clones 4 and 5 judged as a highly significant difference and <.05 for serpin 6, which is judged as significant. (B) Represents the results of the soft agar clonogenic assays performed on serpin 2A and vector only transfected FDCP-Mix cells. Clones were plated at 5 × 103 cells/assay and the serpin 2A transfected clones were also scored at plating densities of 2 × 103 cells/assay. Each data point was the mean result of three separate plating assays and is representative of three repeat experiments with the standard deviations shown. Statistical comparison of the neo clone with the highest clonogenic potential (Neo1) with each of the serpin 2A transfected clones using a two sample t-test gave a P value of <.005 for all clones, which is judged as a highly significant difference.
DISCUSSION
Our primary objective in these studies was the identification of genes and their products that are associated with, and perhaps, influence self-renewal of stem cells and their differentiation into more lineage-restricted progeny. We are somewhat intrigued, therefore, by the nature of two genes isolated using differential hybridization: the previously reported serine protease Granzyme B3,30 and the presently documented serine protease inhibitor serpin 2A.7 Both of these are highly expressed in the undifferentiated self-renewing FDCP-Mix multipotent cells and both are downregulated during myeloid cell lineage commitment and development. Downregulation of Granzyme B, however, may be an early event in the differentiation of multipotential hematopoietic progenitor cells because transcripts were not detected in bipotent GM-CFC. Serpin 2A, on the other hand, was also shown to be highly expressed in more lineage restricted bipotent GM-CFC from bone marrow, but not in erythroid progenitor cells isolated from fetal liver. Mature myeloid cells did not express the gene even after treatment with a variety of growth factors. Clearly, these data do not support an association of serpin transcripts with cycling cells, as FDCP-Mix cells undergo rapid clonal expansion up to day 7 of a G/M differentiation time course (a point at which the 2A message is virtually undetectable) and a 2A signal is absent in fetal liver cells (which are also rapidly proliferating).
Granzyme B has been found primarily in activated T cells32 and curiously we have shown that serpin 2A expression is also stimulated in activated, but not in resting T cells. What then, could explain the presence of these products in both myeloid stem cells and activated T cells? It has been argued that multipotent hematopoietic cells express transcripts, but not necessarily the protein products, of a variety of genes that will ultimately characterize specific mature cells of the different cell lineages and that commitment of the multipotent cells into “inappropriate” cell lineages (where such products have no functional role to play) leads to downregulation of these genes. This does not appear to be the case for granzyme B, however, where both high levels of mRNA and of protein are produced in the undifferentiated cells and where the gene is under different regulatory transcriptional control elements than are found in activated T cells33 (B. Johnson, unpublished, May 1994). Similarly with serpin 2A, where levels of expression of mRNA are apparently equal to that of actin and where protein is clearly being produced. It is, therefore, reasonable to assume that the products of these genes do have a functional role to play in such cells. It could be argued, of course, that expression of granzyme B and serpin 2A is an aberrant activity of the FDCP-Mix cells and not truly representative of normal hematopoiesis. However, there are numerous examples29 34 of genes, characteristic of mature cells, that are apparently not expressed in the multipotent FDCP-Mix myeloid cells, but which are upregulated as a consequence of differentiation into cells with a mature phenotype. In addition, our observation that the 2A gene is expressed in a highly enriched population of freshly isolated, marrow derived, bipotent hematopoietic progenitor cells (GM-CFC), leads us to conclude that the expression of serpin 2A is indeed a characteristic of normal hematopoietic progenitor cells of the neutrophil/macrophage, but not of the erythroid lineage.
What could be the function of this serpin? Previous work has indicated that serpins are associated with (and perhaps influence) differentiation of; teratocarcinoma cells,35,36 neuronal cells,37 muscle cells,38 and the enteric villus formation.39 What is significant about these reports is that they all describe increased serpin synthesis associated with differentiation. Our data clearly demonstrate a marked downregulation of the 2A gene product associated with mature myeloid cell development, but an upregulation during T-cell activation. In other words, expression of serpin 2A during myelopoiesis is associated with maintenance of a primitive, rather than a mature, phenotype. Indeed, we have shown that FDCP-Mix cells transfected with the 2A gene show a delay (rather than block) in the appearance of mature cells compared with the vector only controls. In addition, the 2A transfected clones also exhibited a higher clonogenic potential than control cells. It should be noted, however, that observed levels of the transduced 2A transcript and protein were substantially lower than endogenous levels and that the effects we have observed on self- renewal and differentiation may well be further enhanced if higher expression of the transfected gene could be obtained.
In terms of expression in T cells, we note that the serpin 2A gene has several interesting structural features. For example, we have preliminary evidence (L. Hampson, unpublished observation, June 1995) that the 5′ end has an untranslated exon 1, as has been reported for the previously characterized J6 serpin gene.40 In addition, this untranslated exon 1 has been shown to possess inverted repeat structures, which produce a high degree of secondary structure that may be involved in translational regulation. Our results would support a translational control mechanism by virtue of the delay observed between transcript and protein production in activated T cells.
In conclusion, we have identified a serine protease inhibitor, serpin 2A, which is expressed in proliferating multipotent, in bipotent myeloid cells, and in activated T cells, but is not expressed by activated CD4+ helper T cells, mature myeloid cells, erythroid progenitors, or resting T cells. We have also shown that constitutive expression of this inhibitor in myeloid progenitors increases their clonogenic potential and delays their differentiation. These results, along with the observed tissue distribution, indicate that serpin 2A probably has a role to play in myeloid cell development and in the processes associated with T-cell activation.
ACKNOWLEDGMENT
The authors thank Stella Pearson and Janice McCormack for technical assistance.
Supported by Cancer Research Campaign, Manchester, UK and the National Cancer Institute of Canada. T.M.D. is a Gibb Fellow of the Cancer Research Campaign. R.C.B. is an Alberta Heritage Foundation for Medical Research (AHFMR) Scientist and M.P. is an AHFMR student. I.N.H. and L.H. contributed equally to this work.
Address reprint requests to I.N. Hampson, CRC Department of Experimental Haematology, Patterson Institute of Cancer Research, Christie Hospital, Wilmslow Rd, Manchester M20 9BX, UK.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal