In this issue of Blood, Xie et al1 provide an elegant, integrative analysis that identified the RNA-binding protein Rbm38 as a lineage-specific posttranscriptional regulator of ferrochelatase (Fech) and erythroid heme biosynthesis.
Previous work on Rbm38 in erythropoiesis, largely based on in vitro cell culture and knockdown approaches,2-4 suggested that it had a role in late erythroid differentiation and hemoglobin production. However, its role in vivo and the underlying mechanisms remained unclear. By combining whole-body and hematopoietic-specific knockout models, multiomics profiling, and functional rescue experiments, Xie et al identified the link between Rbm38 deficiency, impaired Fech expression, and erythropoietic protoporphyria (EPP)-like syndrome (see figure panel A). Across distinct genetic contexts, Rbm38 loss consistently led to microcytic hypochromic anemia and fragile, oxidant-sensitive erythrocytes, along with impaired erythropoiesis. Transplantation experiments confirmed the cell-intrinsic nature of these defects. Notably, the authors detected the accumulation of free protoporphyrin IX (PPIX) in erythrocytes and feces, along with skin photosensitivity. The finding that oral 5-aminolevulinic acid (5-ALA) exacerbated the photosensitivity recapitulates clinical reports in which heme precursors precipitate porphyric crises.5 Thus, an Rbm38 deficiency provides an EPP model without a Fech mutation, thereby expanding the mechanistic spectrum beyond classic FECH lesions. They further uncovered a human single-nucleotide variant of RBM38 that modulates red blood cell protoporphyrin levels. These findings have great translational relevance for patients with unexplained microcytic anemia, photosensitivity, or atypical porphyria.
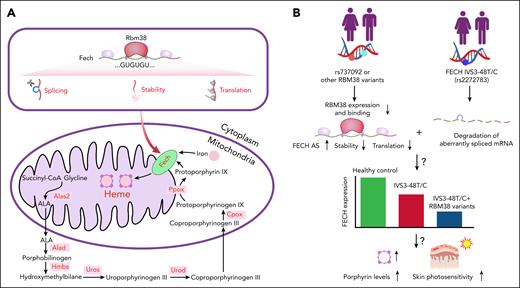
The Rbm38-Fech axis in heme synthesis and skin photosensitivity. (A) Schematic illustration of the Rbm38-Fech axis in erythroid heme biosynthesis. Rbm38 governs the pre-mRNA splicing, stability, and translational efficiency of Fech mRNA, thereby ensuring Fech expression to chelate ferrous iron into protoporphyrin for heme synthesis. (B) The proposed model for how single-nucleotide polymorphisms in RBM38 and FECH jointly modulate human porphyrin levels and skin photosensitivity. The RBM38 single-nucleotide polymorphism rs737092 (C/C) reduces RBM38 expression, thereby lowering FECH levels. When this genetic variant is combined with the FECH IVS3-48 C/C, FECH activity is further compromised, thereby leading to porphyrin accumulation and skin photosensitivity.
The Rbm38-Fech axis in heme synthesis and skin photosensitivity. (A) Schematic illustration of the Rbm38-Fech axis in erythroid heme biosynthesis. Rbm38 governs the pre-mRNA splicing, stability, and translational efficiency of Fech mRNA, thereby ensuring Fech expression to chelate ferrous iron into protoporphyrin for heme synthesis. (B) The proposed model for how single-nucleotide polymorphisms in RBM38 and FECH jointly modulate human porphyrin levels and skin photosensitivity. The RBM38 single-nucleotide polymorphism rs737092 (C/C) reduces RBM38 expression, thereby lowering FECH levels. When this genetic variant is combined with the FECH IVS3-48 C/C, FECH activity is further compromised, thereby leading to porphyrin accumulation and skin photosensitivity.
Mechanistically, the study integrated RNA sequencing, RNA-immunoprecipitation sequencing, and proteomics to identify convergent targets, and Fech emerged as the bona fide downstream effector. Fech catalyzes the terminal step of heme biosynthesis by inserting Fe2+ into PPIX. The authors demonstrated that Rbm38 binds to GU-rich motifs in Fech pre-messenger RNA (mRNA), prevents exon skipping, stabilizes the transcript, and promotes ribosome loading. A loss of Rbm38 led to compromised Fech expression, reduced mitochondrial heme biosynthesis, the accumulation of free PPIX in erythrocytes and feces, and photosensitivity, closely paralleling human EPP despite an intact Fech coding sequence.6 Importantly, enforced Fech expression in Rbm38-deficient hematopoietic progenitors restored hemoglobin production, normalized erythroid maturation, and reduced porphyrin accumulation in vivo, thereby providing direct evidence of the link between defective Fech regulation and the hematologic and metabolic phenotypes caused by an Rbm38 deficiency.1
The translational relevance of these findings is amplified by human genetic studies. The common intronic variant rs737092 located within an erythroid-specific enhancer of RBM38 was previously associated with lower gene expression and altered red blood cell traits.7,8 Xie et al found that individuals who were homozygous for the C allele, exhibited higher erythrocyte protoporphyrin concentrations.1 Interestingly, a minority of symptomatic patients with EPP lacked FECH mutations,5 and individuals with clinical EPP usually harbor a rare null or mutant FECH allele and a common hypomorphic variant, IVS3-48T/C (rs2272783).9 This intronic variation in FECH, which causes aberrant splicing and nonsense-mediated FECH mRNA decay, also commonly exists in healthy cohorts (ie, ∼40% prevalence in the Chinese Han and Japanese population10). The observation from this study therefore strongly suggests that rs737092, along with other RBM38 variants and the IVS3-48T/C allele of FECH, may act as modifier alleles in patients with unexplained porphyria or microcytic anemia even when routine genomic sequencing fails to identify pathogenic FECH mutations (see figure panel B). Prospective sequencing of FECH mutation–negative porphyria cohorts is now warranted to determine the clinical significance of RBM38 variants and their impact on disease outcomes.
This work also raises the possibility that RNA-directed therapeutics can be applied in EPP. Whether stabilization or enhanced translation of the residual FECH transcript, either through antisense oligonucleotides that block cryptic splice sites or small molecules that mimic Rbm38’s stabilizing interaction, might prove effective in humans and benefit patients remains to be further explored.
The specific molecular mechanisms through which Rbm38 coordinate RNA splicing, stability, and translational control remain unclear. Whether Rbm38 interacts with other protein factors to facilitate these processes remains to be determined in depth. It is also of interest to elucidate the RNA-binding landscapes governed by Rbm38 across other tissues and organs, especially in hematologic malignancy.
In conclusion, this study established a posttranscriptional safeguard for heme biosynthesis in erythroid cells. The Rbm38-Fech axis not only provides mechanistic insight into certain unexplained symptomatic EPP cases but also exemplifies a framework for dissecting RNA-mediated metabolic control in hematology.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal