In this issue of Blood, Oosterhoff et al1 revisit a widely cited hypothesis: that maternal alloantibodies directed preferentially against integrin αVβ3, rather than the platelet-restricted αIIbβ3, are key drivers of the most severe forms of fetal/neonatal alloimmune thrombocytopenia (FNAIT).
FNAIT is a nonmalignant hematologic bleeding disorder that arises when maternal antibodies specific for fetal platelet alloantigens cross the placenta and remove a baby’s platelets from circulation in the fetal and/or neonatal period. FNAIT complicates ∼1 in 350 pregnancies and leads to severe thrombocytopenia in ∼1 in 1000 live births; thus, among the ∼4 million babies born in the United States each year, FNAIT causes ∼11 500 cases of thrombocytopenia, 4000 of which are severe. Cases of mild thrombocytopenia often resolve without incident, but 10% to 20% of severely thrombocytopenic cases are unpredictably associated with major organ bleeds such as intracranial hemorrhage (ICH), which can cause irreversible brain damage and death.2 Despite attempts by scientists and clinicians to uncover the molecular and environmental features that might predispose an FNAIT pregnancy toward severity, there are currently few predictive parameters, outside of arguably maternal antibody titer,3 to guide antenatal management of this platelet alloimmune disorder.
More than 35 human platelet alloantigens (HPAs) have been mapped,4 yet a single polymorphism—the HPA-1a/1b diallelic system (formerly known as PlA1/PlA2)—accounts for most cases in populations of European descent. A Leu33→Pro substitution in the β3-integrin subunit generates the HPA-1b antigen and leaves HPA-1b–homozygous mothers susceptible to immunization by a paternally-inherited HPA-1a–positive fetus.5
Although named for its presence on platelets, the HPA-1a alloantigen is also expressed on other cells because the integrin β3 subunit on which the HPA-1a epitope resides can complex with either of 2 integrin α subunits: αIIb (GPIIb) or αV. αIIbβ3 is a platelet-specific receptor for fibrinogen and von Willebrand factor, whereas αVβ3 is expressed not only on platelets, but also on endothelial cells (ECs) and placental syncytiotrophoblasts (STs) where it functions as a receptor for vitronectin, fibronectin, and fibrinogen. β3 integrins have important functions on each of these cells, including formation and consolidation of platelet thrombi, maintenance of EC barrier function, and migration and invasion of STs into the uterus during early pregnancy. The upshot is that antibodies against HPA-1a have 2 potential cellular targets.
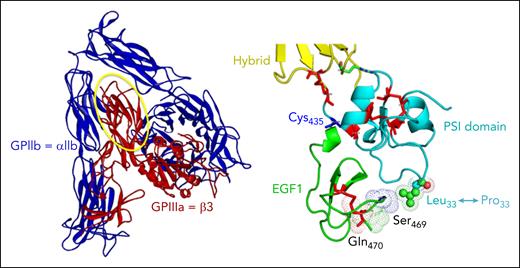
The immune response to the HPA-1a alloantigenic epitope is polyclonal and structurally heterogeneous in nature. Although all HPA-1a antibodies have an absolute requirement for leucine 33, some, referred to as type I antibodies, derive a meaningful proportion of their binding energy from surrounding amino acids solely within the PSI (plexins, semaphorins, integrins) domain, whereas others (type II) interact with residues in the linearly distant, but conformationally close, EGF1 (epidermal growth factor 1) domain6 (see figure). Still another level of complexity was proposed a number of years ago when Santoso et al7 (including one of us) described a third subtype that appeared to bind β3 preferentially when complexed with αV (type III), and was enriched in pregnancies complicated by ICH. That such antibodies might exacerbate FNAIT pathology, made mechanistic sense because they would have a propensity to damage cells expressing this integrin, namely ECs in the brain,8 and STs in the developing placenta,9 and raised hopes that a simple assay distinguishing αVβ3- vs αIIbβ3-reactivity might help stratify risk.
Schematic of the GPIIb-IIIa (αIIbβ3) complex (left), with the PSI and EGF1 domains magnified on the right to show the location of the Leu33Pro polymorphism that creates the HPA-1a epitope.
Schematic of the GPIIb-IIIa (αIIbβ3) complex (left), with the PSI and EGF1 domains magnified on the right to show the location of the Leu33Pro polymorphism that creates the HPA-1a epitope.
Oosterhoff et al now put that idea to a rigorous test. Using CRISPR-edited HEK293 cells expressing only αIIbβ3 or αVβ3, they absorbed 94 well-characterized maternal FNAIT sera (65 mild, 29 severe) and measured residual binding. Not a single serum displayed exclusive—or even dominant—affinity for αVβ3. Paradoxically, most bound αIIbβ3 slightly better, irrespective of clinical severity. Molecular modeling supported these empirical data, predicting greater exposure of the HPA-1a epitope in the resting αIIbβ3 conformation. The study therefore dispels the notion that αVβ3-restricted antibodies are a major determinant of ICH in FNAIT.
If antibody specificity is not the answer, what is? Several avenues merit exploration. First, affinity and avidity vary widely within the polyclonal response; high-affinity clones may drive pathology even at modest titers. Second, the orientation with which antibodies dock on β3 can stabilize or destabilize the integrin, altering outside-in signaling, platelet activation or endothelial integrity.10 Structure–function assays that capture these attributes, such as surface plasmon resonance, conformation-sensitive monoclonal antibody competition, or β3-reporter cell lines, could be developed in reference laboratories. Maternal Fc-glycosylation patterns, complement activation, and polymorphisms affecting Fcγ-receptor signaling also deserve systematic study. Environmental cofactors including intercurrent infection, may further modulate disease expression.
For clinicians, however, Oosterhoff et al reinforce a sobering reality: until better biomarkers emerge, management of high-titer HPA-1a–immunized pregnancies must continue to rely on established protocols—weekly intravenous immunoglobulin, with or without corticosteroids, and platelet transfusion of the neonate as needed. The quest for a reliable laboratory predictor of ICH goes on.
Scientific progress seldom proceeds in a straight line; it advances by successive approximation and often results in facts having a limited half-life. This is as it should be. To paraphrase Josh Billings (1874), “It ain’t so much the things we don’t know; it’s the things we know that just ain’t so.” By showing what αVβ3-selectivity is not, Oosterhoff et al help redirect the field toward what may yet be.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal