In this issue of Blood, Vianna et al1 provide new evidence for the activity of B-cell maturation antigen (BCMA)-targeting bispecifics in light-chain (AL) amyloidosis, highlighting the activity of elranatamab in daratumumab-refractory patients.
The treatment paradigm for AL amyloidosis is based on anticlonal therapies aiming to rapidly eliminate the production of amyloidogenic light chains, a crucial factor for the improvement of patient outcomes. The current standard of care is the combination of daratumumab with VCD (bortezomib, cyclophosphamide, and dexamethasone); however, 40% to 50% of patients still fail to achieve a complete hematologic response and >50% fail to achieve an organ response.2 For the patients that require further treatment due to inadequate hematologic and/or organ response or because they relapsed after therapy, options include high-dose therapy with autologous stem cell transplantation for carefully selected patients, venetoclax for those with t(11;14) (but data are derived only from retrospective series, and the drug is not approved or widely available in many countries), and immunomodulatory drug-based combinations (but with relatively lower activity and more toxicity among patients with AL than in those with multiple myeloma) for patients without t(11;14) or those failing venetoclax.3 Targeting BCMA is a new approach to treat plasma cell malignancies. Two BCMA-targeting chimeric antigen receptor (CAR)–T products and 3 bispecific antibodies (BsAbs) are approved by the US Food and Drug Administration and European Medicines Agency, whereas combinations of Ab-drug conjugates targeting BCMA (belantamab mafodotin) will be approved for relapsed or refractory myeloma. For patients with AL amyloidosis, these therapies have emerged as new anticlonal therapy options. Teclistamab (the first approved anti-BCMA BsAb) has been found to rapidly induce extremely high response rates in retrospective case series of patients with relapsed or refractory AL (RRAL) that failed daratumumab-based therapy.4,5 Belantamab mafodotin monotherapy has been evaluated in a prospective phase 2 study in patients with RRAL6, with a small study evaluating an academic CAR-T in patients with heavily pretreated RRAL.7
In the current issue, Vianna et al report 9 patients with daratumumab-refractory RRAL treated with elranatamab (a BCMA-targeting BsAb) monotherapy. All patients achieved rapid (median, 9 days) hematologic responses, 89% at least hematologic VGPR, and 67% hematologic complete response (CR), with 78% achieving a dFLC of <10 mg/L. Bone marrow minimal residual disease (MRD) status was negative in 5 evaluated patients. All surviving patients remained in hematological remission after a median follow-up of 8 months. The median time from AL diagnosis was 8.4 months, suggesting that most patients were treated probably due to inadequate response to first- or second-line therapy. Organ responses were observed in 5 of 9 patients, with 5 and 1 evaluable for cardiac and renal responses, respectively. Cytokine release syndrome (CRS) was observed in 6 of 9 patients (including 1 with grade 3 CRS and 1 with the development of grade 2 immune effector cell-associated neurotoxicity syndrome), and cytopenias were mild, suggesting toxicity similar to that observed in myeloma. Three patients with advanced disease died despite hematologic response to elranatamab. Although this is a small case series with a short follow-up, it supports a signal of notable activity for anti-BCMA BsAbs in AL amyloidosis and raises significant questions.
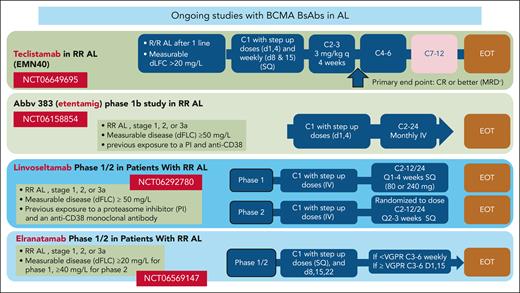
The management of RRAL is challenging and no approved therapies exist. Despite limited data, BCMA-targeting therapies are one of the most active treatments for RRAL reported to date. Nonetheless, before proposing BCMA-targeting as a primary treatment option for RRAL, more data from prospective studies are needed. It is also important to understand whether BCMA- or BCL2-targeting therapies may be preferable for patients with t(11;14), when both options are available. Ideally, anti-BCMA therapies could be approved for RRAL, a disease setting without approved therapies, based on well-designed phase 2 studies, as done in myeloma. The efficacy of belantamab combinations in relapsed or refractory myeloma and the experience of belantamab mafodotin monotherapy in RRAL6 justify further exploration in AL amyloidosis. Currently, 4 BCMA-targeting BsAbs are being evaluated in phase 1/2 studies in RRAL (NCT06158854, NCT06292780, NCT06569147, and NCT06649695; see figure), but the available efficacy data for these immunotherapies also point to new potential directions.
Ongoing clinical trials with BsAbs targeting BCMA in relapsed or refractory AL amyloidosis. Abbv-833, entetaming; d, day; dFLC, difference between involved and uninvolved serum free light chains; EOT, end of treatment; PI, proteasome inhibitor; q, every; SQ, subcutaneous administration; VGPR, very good partial response.
Ongoing clinical trials with BsAbs targeting BCMA in relapsed or refractory AL amyloidosis. Abbv-833, entetaming; d, day; dFLC, difference between involved and uninvolved serum free light chains; EOT, end of treatment; PI, proteasome inhibitor; q, every; SQ, subcutaneous administration; VGPR, very good partial response.
AL amyloidosis is a disease in which BsAb therapy may succeed for many reasons. Serum levels of BCMA are decreased in patients with AL amyloidosis, probably related to the relatively lower tumor burden. One of the proposed mechanisms of resistance to BCMA-targeting therapies may be the “sink effect” associated with circulating (soluble) BCMA, suggesting that their use in AL amyloidosis could be associated with a reduced probability of treatment failure and increased efficacy.8 In addition, the immune system of patients with AL amyloidosis may be less suppressed by the malignant clone or by the burden of previous chemotherapies. Whether due to the comparatively lower tumor burden, less aggressive clonal disease, “fitter” T cells, or other factors, BCMA-targeting BsAbs act rapidly while also allowing fixed-duration treatments, further improving their feasibility and safety.
At the next level, these regimens should be investigated in the primary treatment setting. However, AL has unique characteristics that require careful consideration. The safety of these therapies in the context of AL amyloidosis, especially in patients with more advanced disease, needs to be cautiously evaluated and optimized, including through the preemptive management of potential toxicities (CRS, ICANs, and infections). If these drugs were used for first-line treatment, a major question would be whether they should be evaluated as monotherapies or in combination with drugs that may further improve outcomes, without adding significant toxicity. Registration trials involving patients with newly diagnosed AL amyloidosis should aim to significantly improve hematologic CRs and subsequently organ responses over the current standard of care. Future trials should give paramount importance to considering the unique characteristics of patients with AL amyloidosis, especially in terms of safety. Before challenging current standards, it is critical to generate data from prospective trials, either for monotherapy or combination therapy. These clinical trials should include ancillary studies that will help us understand the role of the immune system and include response evaluations with sensitive assays such as mass spectrometry to detect residual light chains9 and bone marrow MRD (with next generation flow cytometry or next generation sequencing).
However, even with the most effective therapies, we fail to save the lives of several of our patients with advanced AL amyloidosis. Early diagnosis remains a major unmet need, and we should not solely rely on the highly effective anticlonal therapies but also invest heavily in innovative approaches for early diagnosis and the discovery of biomarkers that can identify patients at high risk to develop AL amyloidosis. Furthermore, strategies to improve affected organ recovery are needed.
Conflict-of-interest disclosure: E.K. received honoraria and research support from Johnson & Johnson, GlaxoSmithKline, and Pfizer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal