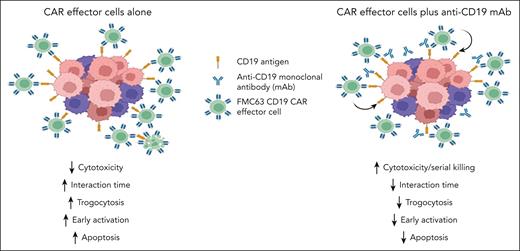
In this issue of Blood, Koh et al1 investigate how concurrent treatment with an anti-CD19 monoclonal antibody (mAb) can boost the antitumor efficacy of chimeric antigen receptor (CAR)–modified immune effector cells, specifically FMC63 CD19-targeted CAR natural killer and CAR T cells. This combined approach enhances cytotoxicity and antitumor activity through competitive CD19 binding and modulation of cell-cell interaction dependent on the anti-CD19 antibody affinity. Combined therapy leads to delayed but sustained CAR effector functions, reduced trogocytosis and apoptosis, and improved serial killing due to a faster detachment rate of CD19 CAR effector cells from target cells (see figure).
Schematic representation of the effects of concurrent treatment with an anti-CD19 mAb and FMC63 CD19 CAR effector cells in preclinical models.
Schematic representation of the effects of concurrent treatment with an anti-CD19 mAb and FMC63 CD19 CAR effector cells in preclinical models.
CAR T-cell activity relies on optimal antigen density thresholds.2 Recent findings revealed that sequential treatment with the CD19-targeting mAb, tafasitamab, followed by CD19 CAR T cells resulted in improved efficacy.3 Transient high-affinity CD19 occupancy reduced CD19 antigen availability, leading to decreased early CAR T-cell activation and apoptosis, and robust delayed CAR T-cell expansion. Building on this, Koh et al reveal that concurrent treatment with tafasitamab does not enhance CAR T-cell cytotoxicity and has a linear relationship between CD19 antigen-binding interference and CAR effectiveness. Furthermore, although concurrent exposure to tafasitamab, which has a high binding affinity for CD19, does not increase cytotoxicity, concomitant treatment with the lower affinity 4G7 or HIB19 mAb clones does augment the cytotoxicity of FMC63 CD19 CAR T cells. This suggests that concurrent treatment with lower affinity anti-CD19 antibody enhances CAR effector function by limiting CAR to CD19 binding, but not imposing excessive antigen competition.
Through single-cell live imaging, the authors observed that concurrent treatment with an anti-CD19 mAb significantly reduced the interaction time between CAR effector cells and target cells, leading to quicker cell killing, superior killing efficiency, and enhanced serial killing of target cells. Trogocytosis is an active process in which the target antigen is transferred to T cells, resulting in fratricide T-cell killing and T-cell exhaustion.4 Increasing anti-CD19 antibody doses correlated with a faster detachment rate, decreased trogocytosis, and apoptosis, suggesting reduced fratricide and/or reduced activation-induced cell death (AICD).5 Consistent with these findings, a CD19 CAR T-cell product targeting the membrane-proximal domain of CD19 with faster on- and off-rates compared with FMC63 CD19 CAR T cells had enhanced efficacy, reduced AICD, and better expansion.6
Under repetitive tumor challenge conditions simulating high tumor burden scenarios, anti-CD19 mAb concurrent treatment consistently resulted in reduced trogocytosis and maintained higher CAR expression levels. Cotreatment with anti-CD19 mAb appeared to result in lower early activation, reflected by reduced degranulation, interferon gamma production, and apoptosis, but subsequently enhanced effector function. In an immunodeficient murine leukemia (NALM6) model, concurrent treatment with CD19 CAR T cells and a single dose, but not multiple doses of anti-CD19 mAb improved survival compared with CAR T cells alone.
This study adds to the growing evidence that competition for CD19 binding can modulate CAR T-cell activation and enhance functionality. However, the lack of improved survival in mice treated with repeated anti-CD19 mAb doses, compared with CAR T cells alone, raises caution for clinical application. B-cell malignancies often exhibit heterogeneous CD19 expression levels,2 and lower pretreatment CD19 expression has been associated with worse outcomes after CAR T-cell therapy.7,8 It remains to be determined whether reducing trogocytosis, and thus preserving target density on tumor cells, might expand the therapeutic window of the combination of an anti-CD19 mAb and CD19-targeted CAR T cells. Controlled clinical trials are needed to investigate optimized antibody clones and dosing strategies.
Conflict-of-interest disclosure: A.V.H. received research funding from Juno Therapeutics (a Bristol Myers Squibb company) and Nektar Therapeutics and received honoraria from Bristol Myers Squibb. M.B. is an inventor on a patent describing HA-1 T-cell receptor therapy that is licensed to Promicell Therapeutics; served on the scientific advisory board of Orca Bio; has given sponsored talks for Miltenyi Biotec; and leads a clinical trial that receives some research funding from Miltenyi Biotec.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal