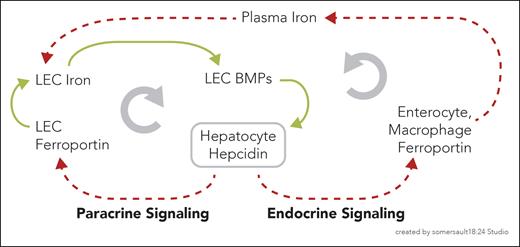
In this issue of Blood, Fisher and colleagues demonstrate that the iron exporter ferroportin contributes to the iron-sensing properties of liver endothelial cells (LECs) in the regulation of systemic homeostasis.1 Their observations support a model in which LECs and hepatocytes crosscommunicate in a paracrine feed-forward loop, to amplify the relationship between LEC iron content and hepatocellular hepcidin production (see figure).
Model of positive (paracrine) and negative (endocrine) feedback between hepatocellular hepcidin and LEC BMPs. The downstream consequences of hepcidin on LEC BMP production are presented as opposing paracrine (clockwise) and endocrine (counterclockwise) regulatory loops. In the paracrine loop, hepcidin blocks or degrades LEC ferroportin and increases LEC iron. Increased LEC iron increases production of BMPs, which upregulate hepatocellular hepcidin. In the endocrine loop hepcidin blocks or degrades ferroportin on duodenal enterocytes and reticuloendothelial macrophages. Iron retention in these cells decreases plasma iron, and thus LEC iron uptake, to attenuate LEC BMP production and hepatocyte hepcidin expression. Green = promoting; red and dashed = attenuating.
Model of positive (paracrine) and negative (endocrine) feedback between hepatocellular hepcidin and LEC BMPs. The downstream consequences of hepcidin on LEC BMP production are presented as opposing paracrine (clockwise) and endocrine (counterclockwise) regulatory loops. In the paracrine loop, hepcidin blocks or degrades LEC ferroportin and increases LEC iron. Increased LEC iron increases production of BMPs, which upregulate hepatocellular hepcidin. In the endocrine loop hepcidin blocks or degrades ferroportin on duodenal enterocytes and reticuloendothelial macrophages. Iron retention in these cells decreases plasma iron, and thus LEC iron uptake, to attenuate LEC BMP production and hepatocyte hepcidin expression. Green = promoting; red and dashed = attenuating.
LECs play a central role in the regulation of iron homeostasis. They do so by sensing yet to be completely defined indicators of both circulating and stored iron, to change production of specific BMPs (primarily BMP6 and BMP2),2,3 which interact with receptor complexes on hepatocytes to increase hepcidin transcription. Hepcidin binds to and degrades ferroportin, the only known cellular iron exporter, and has an endocrine function in regulating iron release from enterocytes and reticuloendothelial macrophages. More recent attention has been directed to autocrine and paracrine functions of hepcidin in cell types with little iron turnover, and thus little consequence on systemic iron status. In this current work, LEC ferroportin is identified as a hepcidin paracrine target with consequences on systemic iron homeostasis. This observation was made during investigations on the mechanisms by which systemic iron signals regulate LEC BMP production.
Published data by this group and others indicate that changes in LEC iron content influence BMP transcription, possibly via the redox-sensitive transcription factor Nrf2.4 These observations bring attention to the source of the iron participating in such regulation. Several candidate iron uptake mechanisms have been considered, including transferrin receptor 1 (TFR1), ZIP14 (encoded by Slc39a14), and ZIP8 (encoded by Slc39a8). Mice with global knockout of Slc39a14 appear to maintain iron-mediated regulation of BMPs.5 Mice with LEC-specific knockout of Tfr1,6,7Slc39a8,8 or combined Tfr1 and Slc39a88 have only modestly attenuated liver BMP and hepcidin expression. None of these models, however, has approximated the profound hemochromatosis phenotype seen with knockout of LEC Bmp6.2
Alternatively, LEC iron content and thus BMPs might be regulated by modulation of iron export rather than import. This possibility had been examined previously by other investigators who targeted LEC ferroportin in mice expressing Cre from the Tie2 promoter.9 The resulting phenotype was complex, including a significant anemia, leading those investigators to speculate that signals unrelated to LEC iron might be at play. Their findings may be plausibly related to off-target knockout of ferroportin in hematopoietic or other cell types in which the Tie2 promoter is now known to be active. In this current study, the investigators used mice expressing Cre from the Stab2 promoter, which has much greater specificity for LECs. They found that loss of endothelial ferroportin was associated with increases in LEC iron and Bmp6 messenger RNA (mRNA) expression. Hepcidin expression was inappropriately high, at least relative to liver or serum iron concentrations. There was no apparent hematologic phenotype.
The investigators’ observations suggest a feed-forward series of events resulting in a positive feedback loop: increased LEC iron, LEC BMP production, hepatocellular hepcidin expression, LEC ferroportin degradation, and LEC iron. What might be the purpose of such a vicious cycle? It could plausibly serve to amplify the signals relating iron status to hepcidin expression. Small initial changes in LEC iron content could feed-forward to produce a more substantial BMP signal than would otherwise occur. Indefinite persistence of such a cycle would of course not be physiologic. Reasonably, the endocrine effects of hepcidin would over time lead to a decrease in serum iron and thus LEC iron uptake, that is, a negative feedback loop (see figure). The investigators tested this possibility by administering supplemental iron to the LEC ferroportin knockout mice sufficient to equalize the serum iron concentrations. These iron-supplemented mice had higher BMP and hepcidin expression than did the control mice, reasonably attributable to overcoming hepcidin endocrine-mediated effects on serum iron.
The iron-responsive protein system in LECs is another negative feedback mechanism to potentially offset the paracrine feed-forward loop. By this mechanism, increases in LEC iron would decrease transferrin receptor 1 mRNA stability, and increase translation of ferroportin transcripts with a 5′ iron-responsive element. The consequent decreased uptake and increased release of iron would serve to normalize LEC iron content. Regardless of these potential feedback events, the net effect of loss of LEC ferroportin is relatively increased LEC BMP production and inappropriately high hepatocellular hepcidin.
Although this study clearly supports a role for LEC ferroportin in iron homeostasis, the consequences of its knockout are relatively mild. As such, regulation of LEC ferroportin appears to be an important modifier, but not the primary mediator, of iron-responsive BMP production. Perhaps other signals (eg, iron-containing ferritin10) may have a more profound influence on LEC BMP expression than forms of iron trafficked through the transporters studied to date. Alternatively, aggregate small changes in LEC iron uptake from multiple transport mechanisms, along with small changes in ferroportin-mediated iron release, may lead to substantial changes in LEC BMP production.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal