In this issue of Blood, Alduaij and colleagues report on central nervous system (CNS) relapse rates in a retrospective cohort of patients with high-grade B-cell lymphoma (HGBCL) with MYC and BCL2 rearrangements (HGBCL-DH-BCL2).1 In addition, they identify high rates of CNS relapse in a molecularly defined group of germinal center B-cell (GCB) tumors expressing the dark-zone gene expression signature (DZsig+).
Outcomes for patients presenting with systemic relapses of diffuse large B-cell lymphoma (DLBCL) and HGBCL have improved significantly in recent years. This has been aided by the development of novel approaches such as anti-CD19 chimeric antigen receptor T cells, which are highly effective in inducing long-term remissions in the second- and third-line setting.2,3 Relapses involving the CNS are much rarer than systemic relapses and represent an unmet therapeutic challenge as they are associated with high rates of treatment refractoriness and short overall survival.4 There is therefore a great need to accurately identify patients at highest risk of CNS relapse; understand the clinical and molecular underpinnings of this risk; and develop biologically driven, therapeutic strategies to ameliorate this risk.
So, what tools do we currently have to assess CNS relapse risk? The CNS International Prognostic Index is a useful and well-validated index to identify high-risk clinical subsets of patients with DLBCL5; in addition, it is well established that certain aggressive B-cell subtypes, such as Burkitt lymphoma (BL) and HGBCL, relapse in the CNS with higher frequency than DLBCL, not otherwise specified (NOS).6 Despite these tools, we recognize that a large proportion of patients with aggressive BCL who develop CNS recurrence do not have high CNS International Prognostic Index scores or BL/HGBL biology and we therefore need additional and more precise predictors of CNS dissemination. To that point, recent genomic studies in LBCL suggesting potential associations of molecular subtypes and specific gene signatures with CNS recurrence are of interest.7
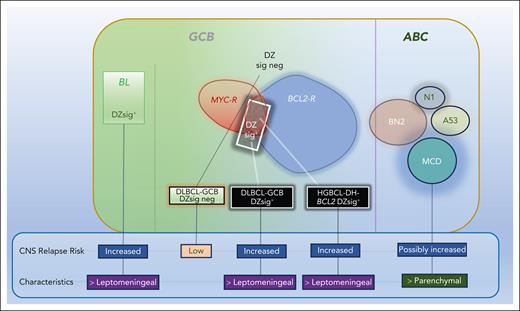
In their study, Alduaij et al report CNS relapse rates in HGBL-DH-BCL2 from a British Columbia Lymphoid Cancer database and, interestingly, identify a lower rate of CNS relapse (6.8% at 2 years) than many other reports. The underlying reasons for this are unclear, but the use of more intensive therapy approaches as well as varied use of CNS-directed therapy may have made some contribution. In addition, they stratified aggressive BCL tumors with GCB morphology into DZsig+ and DZsig− cases and looked at differential risk of CNS relapse between these groups---the DZsig, previously described by the authors, is a gene expression signature based on comparing the most significantly differentially expressed genes between HGBCL-DH-BCL2 and other GCB BCLs. DZsig was positive in most HGBCL cases as well as 8% of DLBCL-NOS cases. In the report, DZsig+ DLBCL-NOS cases (as well as DZsig+ HGBCL-DH-BCL2) were associated with a much higher rate of CNS relapse compared with DZsig− GCB-DLBCL cases.
The authors suggest that adverse biology in GCB-derived tumors extends beyond fluorescence in situ hybridization–defined HGBCL-DH-BCL2 and that one molecularly defined adverse subset expressing DZsig+ has similar CNS progression rates to HGBCLs. Although DZsig+ cases represent a small proportion of GCB-DLBCL tumors, given their association with CNS relapse, being able to identify them “a priori” could have potential therapeutic implications. Other studies that looked at associations between specific genetic signatures in DLBCL/HGBL include a study involving patients with isolated CNS or systemic recurrence after immunochemotherapy, which demonstrated that the MCD (characterized by MYD88L265P, CD79B mutations, and immune evasion) subgroup of ABC-DLBCL was associated with a higher prevalence of CNS recurrence compared with other subgroups.7
Another interesting finding by Alduaij and colleagues relates to CNS relapse characteristics and their association with biological subtypes (see figure). Despite the small number of patients, the authors found that DZsig+ DLBCL and HGBCL-DH-BCL2 relapses were mainly leptomeningeal and early, resembling those typically seen in BL. In contrast, ABC-DLBCL relapses are frequently parenchymal and further out from the time of diagnosis. This suggests that specific tumor biological factors, as well as being associated with a higher risk of CNS dissemination, may also align with distinct CNS relapse patterns (leptomeningeal vs parenchymal). If so, CNS prophylaxis approaches in LBCL may need to be tailored toward a specific CNS relapse pattern, depending on tumor biological factors. Many recent studies have shown that our current CNS prophylaxis standards are largely inadequate and need to be optimized.8,9 Developing effective prophylactic strategies will likely stem from more precise identification of high-risk patients and using biologically based approaches that are informed by a patient’s tumor biology. We currently have a spate of ongoing studies in patients newly diagnosed with aggressive B-cell lymphomas that incorporate CNS-penetrating agents, such as Bruton tyrosine inhibitors and anti-CD19 chimeric antigen receptor T cells. As the results of those studies become available, it will be of interest to identify whether the addition of any of these agents upfront ameliorates CNS relapse risk.10
Impact of DZsig+ on CNS relapse in aggressive B-cell lymphoma. BL and HGBCL-DH-BCL2 are typically DZsig+ and associated with increased CNS relapse compared with DZsig− GCB-DLBCL. DZsig+ is associated with leptomeningeal relapses compared with subtypes of ABC-DLBCL.
Impact of DZsig+ on CNS relapse in aggressive B-cell lymphoma. BL and HGBCL-DH-BCL2 are typically DZsig+ and associated with increased CNS relapse compared with DZsig− GCB-DLBCL. DZsig+ is associated with leptomeningeal relapses compared with subtypes of ABC-DLBCL.
Conflict-of-interest disclosure: K.D. has performed consulting work for AstraZeneca, AbbVie, BeiGene, Amgen, ADC Therapeutics, Genentech, Pharmacyclics, Incyte, and ONO Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal