In this issue of Blood, Su et al report that patients with sickle cell disease (SCD) have elevated quantities of a subset of B cells (B-1 cells), which significantly correlates with red blood cell (RBC) autoantibody levels. They also show that antiviral cytokines, type 1 interferons (IFN-Is), contribute to alterations in B-1 cell subsets, T-cell–independent antibody responses, and RBC autoantibody production in SCD mice (see figure).1 Although it has been shown that patients with SCD have an elevated frequency of RBC autoantibodies,2 mechanisms contributing to this frequency are lacking and represent one of the preeminent questions in the field.
Su et al address a perplexing issue: on one hand, many patients with SCD have functional asplenia rendering them susceptible to multiple infectious diseases, yet the frequency of anti-RBC allo- and autoantibody production is significantly elevated compared to immunocompetent populations.2 To address this, authors initially used multiple immunization strategies and found that responses to antigens that require T-cell help to B cells are minimally altered in SCD mice, despite disorganized splenic architecture in naive SCD mice. However, immunoglobulin M (IgM) and immunoglobulin G (IgG) responses to a T-cell–independent antigen are actually enhanced. These type 2 T-cell–independent (TI-2) antibody responses provide protection from encapsulated bacteria and viruses,3 and thus have implications for vaccine development.
The authors also investigated 2 B-cell subsets known to promote T-independent responses, marginal zone B cells and B-1 cells.3 After ruling out a significant role for marginal zone B cells, they observed an increase in B-1 cells following immunization. B-1 cells, in contrast to B-2 cells involved in germinal center reactions, represent a small fraction of spleen B cells and largely persist in peritoneal and pleural spaces. In addition to their role in T-independent antibody responses, B-1 cells can contribute to autoimmune hemolytic anemia in preclinical models.4 Accordingly, Su et al observed elevated RBC-bound autoantibodies in SCD mice that correlated with an increase in a B-1 cell subset (B-1b). To further evaluate the associations between B-1b cells, TI-2 responses, and RBC autoantibodies, the authors performed the relatively unique experiment of lavaging water into the peritoneum. Although this led to the lysis and depletion of B-1a cells, it surprisingly increased the levels of peritoneal B-1b cells, which correlated with increased TI-2 antibody responses and RBC autoantibodies.
Although the findings supported a pivotal role for B-1b cells, it is notable that the data indicating associations between B-1 cell levels, TI-2 responses, and RBC autoantibodies are correlative. It is unclear whether depletion of B-1b cells would alter antibody responses or autoantibody production. Additionally, it is notable that the alterations in B-1b cells and RBC autoantibodies in SCD mice are relatively modest. For example, the authors show that RBC autoantibodies in SCD mice are present on approximately 2% of RBCs, compared with 1% in controls.
The authors also investigated molecular mechanisms that may promote B-1 cell skewing in SCD. Multiple groups have demonstrated that patients and mice with SCD express type I interferons (IFN-Is) and IFN-stimulated genes in multiple innate immune cell types.5 Also, a recent study demonstrated a role for B-cell IFN-I signaling in TI-2 responses.6 Su et al found that a lack of IFN-I signaling resulted in reductions in B1-b cells, TI-2 responses, and RBC autoantibodies. Finally, Su et al observed an increase in B-1-like cells in patients with SCD, compared with healthy donors, and a striking correlation between B-1-like cell concentrations and RBC autoantibody levels in patients with SCD. It is yet to be seen whether B-1–cell levels influence T-dependent or TI-2 antibody responses and whether IFN-Is contribute to RBC autoantibody production in patients with SCD. Nevertheless, although IFN-Is have been implicated in production of RBC alloantibodies in transfusion models,7 this is the first report of a contributory role for IFN-Is in RBC autoantibody production in SCD.
The study has multiple implications. It suggests that IFN-I-targeted therapy may alleviate autoimmune hemolytic anemia in patients with SCD. However, it is unclear whether the identified RBC autoantibodies cause hemolysis. Moreover, a role for IFN-Is in patients with SCD has not been established and represents a significant gap in the field. In the present study, although IFN-Is significantly contributed to the observed autoantibody response, they are not required for B-1 cell alterations, RBC autoantibody production, or TI-2 responses. Thus, IFN-I signaling is one of multiple contributing factors that are likely to emerge. In addition to antibody production, it is plausible that IFN-Is may contribute to other sequelae of SCD, as IFN-Is can induce endothelial damage in other contexts.8 Studies of a potential role in vaso-occlusion are eagerly awaited.
In addition to SCD, the role for B-1 cells and IFN-Is in RBC autoantibody production has implications for patients with autoimmunity. IFN-Is play a pathogenic role in multiple autoimmune diseases, including lupus,9 in which up to 10% of patients experience autoimmune hemolytic anemia.10 Finally, RBC autoantibodies, elevated in SCD, can make it difficult to find compatible blood in the acute setting and can reduce the survival of RBCs in patients who require chronic transfusion therapy, leading to anemia-associated morbidity. In addition, RBC autoantibody production is associated with an increased frequency of RBC alloimmunization.2 Thus, targeted therapies that suppress autoantibody production would improve transfusion safety.
In closing, these findings provide important insight into the mechanisms to T-independent antibody responses and RBC autoantibody production in SCD. In doing so, this study improves our understanding of the elevated prevalence of RBC autoantibodies in patients with SCD and could pave the way toward therapeutic strategies to suppress their detrimental effects.
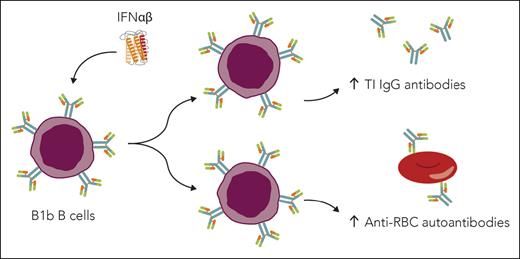
Type 1 interferons (IFNαβ) promote B1b cell skewing, T-independent (TI) antibody responses, and RBC autoantibody production in SCD. Elevated B1b cells correlate with increased anti-RBC antibodies in patients with SCD. IFNαβ promote production of B1b B cells, which contribute to TI IgG antibody responses and anti-RBC antibody production in mice with SCD. Professional illustration by Somersault18:24.
Type 1 interferons (IFNαβ) promote B1b cell skewing, T-independent (TI) antibody responses, and RBC autoantibody production in SCD. Elevated B1b cells correlate with increased anti-RBC antibodies in patients with SCD. IFNαβ promote production of B1b B cells, which contribute to TI IgG antibody responses and anti-RBC antibody production in mice with SCD. Professional illustration by Somersault18:24.
Conflict-of-interest disclosure: The authors report no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal