In this issue of Blood, Van Laer et al have expressed and extensively characterized 8 thrombomodulin (TM) variants of uncertain significance identified in patients with venous thromboembolism (VTE) or bleeding, demonstrating defective anticoagulant activity in 2 of them (L433P and C175S).1 Their findings, reported in this issue of Blood, support a contributory role of these variants in the pathogenesis of VTE, strengthening the link between TM defects and VTE risk.
TM, encoded by the THBD gene, is a transmembrane glycoprotein mainly expressed on the surface of endothelial cells, where it acts as a thrombin receptor. Its structure comprises a lectinlike domain, 6 epidermal growth factor (EGF)-like modules, a Ser/Thr-rich region, a transmembrane domain, and a short cytoplasmic tail (see figure). Soluble forms of TM (sTM), which lack the transmembrane and cytoplasmic domains but retain the ability to bind thrombin via EGF-like domains 5 and 6, also circulate at low levels in plasma. TM regulates coagulation, fibrinolysis, complement activity, and inflammation by multiple mechanisms.2 By binding thrombin, TM inhibits the activity of thrombin on its procoagulant substrates (eg, fibrinogen), while greatly (>1000-fold) enhancing the activation of protein C, a major anticoagulant enzyme, and of thrombin activatable fibrinolysis inhibitor (TAFI). Activated protein C (APC), assisted by its cofactor protein S, inactivates the essential coagulation cofactors Va and VIIIa, effectively shutting down thrombin formation.
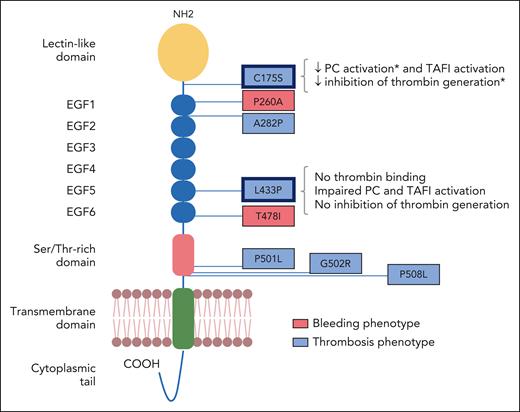
TM domain structure and positions of the investigated variants. The individual protein domains are indicated. The 8 missense variants identified in patients with bleeding (red) or venous thrombosis (blue) are annotated using the single-letter amino acid code. The detected functional alterations of the C175S and L433P variants are summarized on the right-hand side. ∗Effect only significant for the soluble mutant. Adapted from Figure 1 in the article by Van Laer et al that begins on page 1929.
TM domain structure and positions of the investigated variants. The individual protein domains are indicated. The 8 missense variants identified in patients with bleeding (red) or venous thrombosis (blue) are annotated using the single-letter amino acid code. The detected functional alterations of the C175S and L433P variants are summarized on the right-hand side. ∗Effect only significant for the soluble mutant. Adapted from Figure 1 in the article by Van Laer et al that begins on page 1929.
The pivotal anticoagulant functions of TM suggest that quantitative and qualitative alterations of TM may contribute to both bleeding and thrombotic tendencies. Accordingly, 2 rare THBD variants (ie, C537∗, identified in several unrelated families, and P496Rfs∗10) have been recently shown to cause a bleeding disorder known as TM-associated coagulopathy.3 These mutations truncate TM within or just before the transmembrane domain, favoring its release in soluble form, as elegantly elucidated for C537∗.4 This results in massively elevated sTM levels, leading to excessive protein C activation and posttraumatic bleeding. Conversely, the role of TM defects in VTE occurrence has been mainly supported by sporadic case reports in which potentially deleterious THBD variants were identified by targeted resequencing of this gene. However, few of these variants have been functionally characterized to demonstrate their mechanistic implication.5,6 More recently, new evidence for THBD as a candidate gene for VTE has come from a genome-wide association study in African Americans7 and from a large prospective study in which carriers of rare loss-of-function or nonbenign missense THBD variants proved to be 3 times more likely to develop VTE than noncarriers.8 Yet, the contribution of TM defects to VTE risk is still less well established than for other components of the protein C pathway, such as protein C and protein S.8
The article by Van Laer et al provides functional evidence for the involvement of 2 novel THBD variants in the pathogenesis of VTE.1 Using next-generation sequencing to screen patients with hemostatic disorders for mutations in candidate genes, the authors identified 8 THBD missense variants of uncertain significance in patients with VTE (n = 6) or bleeding (n = 2) (see figure). Following recombinant expression in membrane-bound and soluble form, all TM mutants were probed for thrombin-cofactor activity in protein C and TAFI activation assays and for inhibition of thrombin generation in plasma. Two variants, identified in the heterozygous state in 2 different patients with severe thrombotic phenotypes, demonstrated defective anticoagulant activity (see figure). The first variant (L433P), which maps to the thrombin interaction site within the EGF5 domain, completely abolished the ability of TM to bind thrombin, stimulate protein C and TAFI activation, and inhibit thrombin generation. This functional profile resembles that of the nearby G430D variant (TM-Nagasaki), which was reported in the homozygous state in an infant with a past cerebral infarction who later presented with a bleeding phenotype in the presence of markedly elevated coagulation activation markers and excessive fibrinolysis (all attributable to the loss of thrombin-dependent TM functions).9 The second variant (C175S), located between the lectin and EGF1 domains (and hence far away from the thrombin-binding site), showed a mild reduction of protein C and TAFI activation and of thrombin generation inhibition. This was probably the result of conformational changes, as suggested by an in silico structural analysis and by failure of some antibodies to recognize the mutant protein. In fact, another variant in the lectin domain of TM (D126Y), previously identified in a 10-year-old Chinese boy with recurrent VTE and arterial thrombosis, was also shown to interfere with several thrombin-dependent functions (PC and TAFI activation as well as HMGB1 cleavage), despite normal thrombin binding.6 All other TM mutants investigated by Van Laer et al, including the ones associated with bleeding, did not significantly differ from wild-type TM for expression levels or any of the tested functional properties. However, it cannot be excluded that some of these variants affect the (thrombin-independent) anti-inflammatory and complement regulatory functions of TM. For example, the A282P variant, which was identified in a toddler with portal vein thrombosis, but was also present in his brother with atypical hemolytic-uremic syndrome, might alter the ability of TM to downregulate complement activity.10
Interestingly, 4 of the 6 patients with VTE in whom a THBD variant was identified also carried factor V Leiden or the prothrombin 20210G>A mutation. This observation suggests that coinheritance of these common thrombophilic defects may increase the penetrance of mildly prothrombotic THBD variants through synergistic interactions. The authors provide experimental support for this hypothesis by showing that supplementation of the test plasma with extra prothrombin (to mimic the effect of the prothrombin 20210G>A mutation) increases the difference between wild-type and mutant TM (L433P and C175S) in the thrombin generation assay.
In summary, the findings by Van Laer et al provide additional evidence that deleterious THBD variants may contribute to the development of VTE. Moreover, as more THBD variants are likely to be discovered through the increasing application of exome sequencing for diagnostic purposes, the article highlights the importance of accurate functional characterization to distinguish pathogenic from benign variants.
Conflict-of-interest disclosure: E.C. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal