In this issue of Blood, Gupta et al1 describe significant improvements in the recovery from renal failure and other, less frequent, methotrexate (MTX)-related toxicities associated with the use of glucarpidase in patients receiving high-dose MTX (HDMTX) therapy who develop acute kidney injury.
HDMTX has been widely used for over 50 years as a key treatment component in acute lymphoblastic leukemia, lymphoma, and osteosarcoma. It is one of very few antineoplastic therapies in which therapeutic drug monitoring is mandatory and essential for guiding leucovorin rescue that prevents otherwise potential lethal toxicities from the treatment. In the majority of patients, HDMTX is safely administered, but in a subset of patients, renal failure causes delayed drug elimination and enhances drug exposure, sometimes resulting in life-threatening MTX toxicities.2 In this subset of patients, augmented leucovorin doses are given, but these do not decrease MTX blood levels. In addition, there is growing evidence that higher leucovorin doses could diminish the antineoplastic effect of subsequent HDMTX treatment cycles.3,4 Extracorporeal drug elimination is not recommended due to poor efficacy and the invasiveness of the procedures. Also, extracorporeal elimination procedures remove leucovorin and potentially prolong the MTX toxicity time.5
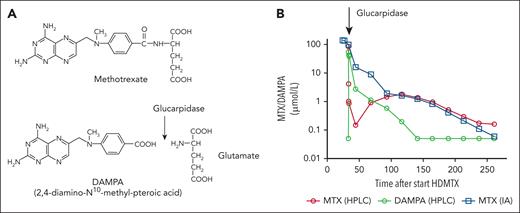
Glucarpidase, formerly termed carboxypeptidase G2, is a recombinant, bacterial-derived enzyme, which cleaves the glutamate residue from folate analogues. Early studies have demonstrated that administration of a nonrecombinant carboxypeptidase G1 could rapidly decrease MTX blood levels by cleavage of MTX into a nontoxic metabolite and glutamate.6,7 This pharmacological effect of the enzyme has been confirmed by subsequent studies with glucarpidase (see figure).8,9
Glucarpidase mode of action. (A) Glucarpidase cleaves the glutamate residue from MTX resulting in the nontoxic metabolite DAMPA. (B) Rapid decrease of the MTX blood concentration after glucarpidase administration in a patient with osteosarcoma and acute kidney injury after HDMTX therapy. Quantification by high-performance liquid chromatography (HPLC) demonstrates an increase of the metabolite DAMPA parallel to the decrease of MTX, whereas the MTX concentration is overestimated by immunoassay (IA) measurements due to interference with the DAMPA metabolite.
Glucarpidase mode of action. (A) Glucarpidase cleaves the glutamate residue from MTX resulting in the nontoxic metabolite DAMPA. (B) Rapid decrease of the MTX blood concentration after glucarpidase administration in a patient with osteosarcoma and acute kidney injury after HDMTX therapy. Quantification by high-performance liquid chromatography (HPLC) demonstrates an increase of the metabolite DAMPA parallel to the decrease of MTX, whereas the MTX concentration is overestimated by immunoassay (IA) measurements due to interference with the DAMPA metabolite.
In the present study, Gupta and colleagues retrospectively collected and analyzed data from a large number of patients who developed acute kidney injury after HDMTX therapy. The outcomes (ie, recovery from kidney injury) in patients who were treated with glucarpidase were compared with those who received no glucarpidase. Using a target trial emulation approach to diminish effects of confounders, rescue treatment with glucarpidase was found to be associated with a higher likelihood of and faster time to kidney recovery, despite the glucarpidase-treated patients having more morbidities (diabetes, hypertension) and higher steady-state MTX concentrations. In addition, toxicities such as neutropenia and transaminitis occurred less often in patients with glucarpidase therapy. Notably, patients with glucarpidase treatment had a higher average degree of acute kidney injury at baseline. Finally, glucarpidase-treated patients had longer hospital lengths of stay than non-glucarpidase-treated patients, and therefore may have had more time to recover.
This study, albeit providing clinically relevant data, which clearly support the use of glucarpidase in patients with HDMTX-induced acute kidney injury, has some limitations. First, the retrospective design, despite adjustments in data analysis, still carries a risk of a bias. However, a prospective trial comparing glucarpidase intervention vs nil in this medical emergency scenario would be unethical and is unlikely to be performed. Second, the optimal dosing of leucovorin postglucarpidase is unknown. In the present study, patients who received glucarpidase were treated with higher doses of leucovorin, and it remains unknown whether this might have skewed the results, at least to some extent with respect to hematological toxicity. Glucarpidase reliably and rapidly lowers MTX blood levels, but the effects on intracellular concentrations of MTX are unknown. In addition, leucovorin is also a substrate of glucarpidase, further obscuring the optimal leucovorin rescue after use of glucarpidase. However, MTX-immunoassay measurements, which are frequently used for therapeutic drug monitoring during HDMTX therapy, overestimate MTX levels due to interference with the nontoxic metabolite 2,4-diamino-N10-methyl-pteroic acid (DAMPA) (see figure),10 but these overestimations might approximate intracellular MTX concentrations, allowing a more appropriate leucovorin rescue. Nonetheless, administration of very high leucovorin doses carries the risk of hazardous hypercalcemia, if any of the widely available calcium-coupled leucovorin preparations are given. These important concerns require further studies to prevent additional toxicities and avoid “overrescue,” which might promote tumor cell growth and reduce the effect of further HDMTX treatments.3,4 Third, glucarpidase has variable effects on the concentrations of the natural, main metabolite 7-hydroxymethotrexate (7OH-MTX; 90% bound to albumin with a very slow renal elimination),8 which is considered partly cytotoxic, and its concentration likely modulates the required intensity of the leucovorin rescue. Finally, an optimal threshold for glucarpidase intervention defined by the MTX blood level, degree of renal failure, type of cancer, and MTX dose and regimen balancing the detrimental effects of a delayed MTX elimination against the potential negative effects of overrescue would be desirable. The further development of the freely available pharmacokinetic tool, which allows a prediction of the drug elimination kinetics after HDMTX, is not only beneficial for the management of individual patients, but also could help in the development of more refined criteria justifying the use of glucarpidase (MTXpk.org).
In conclusion, the present study clearly favors the use of glucarpidase in patients with delayed MTX elimination and acute renal failure after HDMTX therapy. However, the development of more precise threshold definitions for use of this pharmacologically effective enzyme and more data guiding an optimal leucovorin dosing postglucarpidase need additional studies.
Conflict-of-interest disclosure: S.S. has received a research grant from Protherics Medicines Development Ltd, consultancy fees from AMGEN, Pfizer, SERB SAS, speaker honoraria from Akademie für Infektionsmedizin e.V., AMGEN, CSi Hamburg GmbH, Pfizer, SERB SAS, and travel grants from AMGEN, Pfizer, SERB SAS. J.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal