In this issue of Blood, Fürstenau et al report results from the German chronic lymphocytic leukemia (CLL) study group phase 2 trial with zanubrutinib, obinutuzumab, and venetoclax with optional bendamustine debulking (CLL2-BZAG) in patients with relapsed/refractory CLL. At the primary end point (6 cycles of the triple-drug combination), all 40 patients receiving at least 2 treatment cycles achieved a response, with an undetectable minimal residual disease (uMRD) rate of 52.5% for peripheral blood, based on flow cytometry. The uMRD rate increased over time, reaching a maximum of 85%, with no differences in response observed in patients previously exposed to Bruton tyrosine kinase inhibitors (BTKi) and/or venetoclax or in those carrying in TP53 aberrations. With a median follow-up of 21.5 months, the estimated 18-month progression-free survival (PFS) rate was 96%, with 2 patients showing disease progression according to criteria of the International Workshop on CLL.1
In an era in which most trials have focused on the frontline setting, the available trials on retreatment often investigated only a small number of patients, making the resultant data insufficient to determine an optimal strategy for proper sequencing of the available agents. In their study, Fürstenau et al analyzed the efficacy of fixed-duration therapy in a modern population of relapsed patients already exposed to targeted agents.
A time-limited approach can induce deep remissions, enabling treatment discontinuation while reducing toxicity, clonal evolution, resistance, and costs. Venetoclax-rituximab is the only fixed-duration combination treatment currently approved for previously treated CLL.2 However, the MURANO trial of venetoclax-rituximab did not reflect today’s patient population, as most patients are currently treated with targeted therapies only.
In the frontline setting, the GAIA study clearly answered the question of whether obinutuzumab is a better partner for venetoclax than rituximab, with higher rates of uMRD (87% vs 57%) and complete remissions (57% vs 49%) for the former. Similarly, triplets with covalent BTKi-venetoclax and obinutuzumab are actively being explored in treatment-naive patients.3 For previously treated patients, we currently have only limited real-world data for obunutuzumab-venetoclax, and limited evidence on triplets in small prospective cohorts.4-6
Importantly, the CLL2-BZAG trial demonstrated how it is possible to attain deep remission even in patients with very high risk (ie, TP53 aberrant), in whom continuous treatment is generally the preferred option. Furthermore, the combination proved effective, regardless of previous exposure to targeted agents. The trial also confirmed previous studies with triplets, where even relapsed/refractory patients were able to achieve high uMRD rates: a 50% uMRD after 14 cycles was achieved with an ibrutinib-based triplet,5 and a similarly impressive 76% uMRD was reported after 6 combination cycles using acalabrutinib as covalent BTKi.6
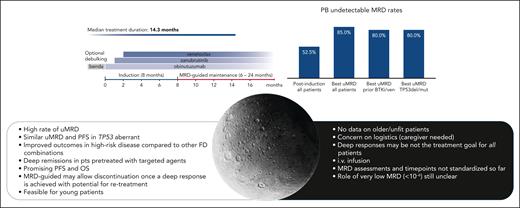
Longer term observation of the CLL2-BZAG trial showed a clearly improved uMRD over time, with best uMRD increasing by 33% compared with first assessment at 6 months (from 52.5% to 85%). Importantly, with a median treatment duration of 15 months, MRD-guided CLL2-BZAG is comparable to the fixed-duration regimens planned a priori for doublets and triplets in other studies.3,5
The authors argue that the first MRD assessment (primary end point) may have been captured too early. Considering that MRD is a promising surrogate for survival in CLL and has potential as a biomarker for guiding treatment duration, we believe that a full understanding of the MRD kinetics is essential in clinical trials with different combinations. This knowledge will lead to harmonization and interpretation of MRD time points. The same combination of agents in the treatment-naive setting has in fact demonstrated that early achievement of deep MRD is predictive of more rapid bone marrow clearance, allowing for therapy to be tailored accordingly.7
In the CLL2-BZAG trial, the authors reported similar PFS in those with TP53 aberrations compared with the whole population (18-month PFS: 100% vs 96%). Although promising, longer follow-up is needed for this specific category. Not all studies with triplets differentiated the outcome of patients with (del)17p/TP53 aberrations. Indeed, despite the rate of uMRD in patients with TP53 aberrations being comparable to the whole population with acalabrutinib, venetoclax, and obinutuzumab as frontline therapy, the 4-year PFS in this subgroup was lower.8
The CLL2-BZAG trial did demonstrate high concordance between circulating tumor DNA–based and flow cytometry–based MRD assessment. Although the clinical significance of very low MRD detection (<10−6) is still not fully clarified, such comparisons in prospective clinical trials are warranted to allow for standardization of MRD techniques.
Biology aside, the question remains whether a regimen like this is feasible for every patient. Triplet regimens likely add toxicity over doublet approaches. To our knowledge, AMPLIFY is the only study to date to directly compare a triplet with a BTKi plus B-cell 2 lymphoma inhibitor combination. Despite the enrollment of young, fit, treatment-naive patients, the safety data from this trial clearly show that adding obinutuzumab increased toxicity compared to the full-oral regimen.9
Although no age limits were set, the median age of patients enrolled in CLL2-BZAG was 64 years, in line with all the other triplet trials, where age was noticeably younger than in typical CLL-pretreated populations.
Although no unexpected toxicities were reported and only 16% of patients definitively discontinued treatment due to side effects, the feasibility of triplets in a population of unselected patients is still a matter of concern. Furthermore, the need for intravenous infusions with multiple initial doses, followed by a 5-week venetoclax ramp-up, emphasizes the importance of keeping consistent support for patients, many of whom are not entirely self-sufficient. There is still no consensus on the concept of fitness with targeted agents, so it is important to identify those patients who would benefit most from this type of combination if available in clinical practice.
Chemotherapy-based pretreatment seems unnecessary to prevent infusion-related reactions, but it may add to the risk of infection. In this trial, optional bendamustine debulking did not increase adverse events but it also did not reduce infusion reactions. Evidence suggests that use of BTKi as a lead-in can minimize infusion reactions and tumor lysis syndrome risk, making this type of agent a useful option for treatment initiation.
The favorable efficacy and tolerability data from the combination of zanubrutinib-venetoclax-obinutuzumab in the study by Fürstenau and colleagues represent another step forward toward understanding the potential of triplet regimens. However, many key questions remain, including the impact of comorbidities for individual patients and CLL characteristics that would benefit most/least from combination regimens, the optimal MRD monitoring method, timing to guide treatment duration, and how to incorporate novel agents (see figure).
The “bright and the dark side of the moon” in triplets. The top part of the figure shows the treatment schedule of the BZAG triplet combination and the rate of uMRD in peripheral blood, demonstrating the improved uMRD rate over time and the overlap of MRD rates between patients with TP53 aberrations and the entire population. The lower part of the figure shows the pros and cons of triplets in relapsed/refractory CLL. benda, bendamustine; del, deletion; FD, fixed duration; mut, mutation; pts, patients; ven, venetoclax.
The “bright and the dark side of the moon” in triplets. The top part of the figure shows the treatment schedule of the BZAG triplet combination and the rate of uMRD in peripheral blood, demonstrating the improved uMRD rate over time and the overlap of MRD rates between patients with TP53 aberrations and the entire population. The lower part of the figure shows the pros and cons of triplets in relapsed/refractory CLL. benda, bendamustine; del, deletion; FD, fixed duration; mut, mutation; pts, patients; ven, venetoclax.
Conflict-of-interest disclosure: A.M.F. has received honoraria for participation on advisory boards and has received sponsorships for congresses from Janssen, BeiGene, AbbVie, and AstraZeneca. A.T. has received honoraria for participation on advisory boards from Janssen, BeiGene, AbbVie, Lilly, and AstraZeneca and is a participant in speaker bureaus for BeiGene, AbbVie, and Johnson & Johnson.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal