In this issue of Blood, Farrokhi et al1 have identified a crucial role for early postnatal viral infection in preventing childhood B-cell leukemia (B-ALL) by depleting preleukemic cells (PLCs), thus offering new insights into potential preventive strategies. B-ALL is a clonal blood disorder whose earliest stages take place during embryonic life, when different types of germ line or somatic alterations occur prior to birth that alter the behavior of hematopoietic progenitors, giving rise to the so-called PLCs. At least 5% of all newborns have PLCs. Fortunately, the presence of PLCs is compatible with normal hematopoietic development, and, in the majority of the cases, B-ALL will never develop. However, in 1% of predisposed children, PLCs can transform over time through the acquisition of additional somatic genetic alterations, thereby leading to full-blown childhood B-ALL.
A century ago, infections were hypothesized to be a significant factor contributing to the progression from the preleukemic state to leukemia,2,3 and early-age infections have been proposed to be either protective of or to trigger the progression to B-ALL. However, given the low incidence of the disease, testing these hypotheses was not possible using only clinical or epidemiological data. Recently, the advent of genetically engineered mouse models capable of recapitulating the human B-ALL predisposition and evolution to leukemia has demonstrated the existence of infection-triggered mechanisms leading to B-ALL development.4,5 These models have also shown that there are several types of stress affecting the immune system (ie, not only infections, but also, for example, antibiotic treatment) that can promote the malignant evolution of PLCs.6 Importantly, immune stress does not selectively favor the preleukemic clone already possessing the second mutation; instead, the infection promotes the acquisition of the second mutation, directly contributing to the progression to full-blown B-ALL.7 Together, these observations support the hypothesis that eliminating PLCs could potentially prevent childhood B-ALL.2 However, specific strategies to effectively target PLCs for the prevention of B-ALL remain unclear.
Farrokhi et al present the “other side of the coin” (ie, the role of infections in controlling PLC-to-B-ALL progression) by elegantly demonstrating that neonatal but not adult viral infection with murine cytomegalovirus (MCMV) leads to depletion of PLCs in a mouse model of hyperploid B-ALL. Using the Eμ-ret transgenic mouse model, where B-ALL arises from an aneuploid B-cell precursor population that is present at birth, they show that infection with MCMV in the right time window (the first week of life) led to specific depletion of the precursor B-cell population containing the PLCs, thus significantly delaying the onset of B-ALL in neonatally infected mice. Subsequently, they employed mouse genetics and gene expression analyses to identify the cell types and signaling pathways responsible for this outcome, to determine that this effect is mediated by activation of neonatal innate immunity and the production of interferon-γ (IFN-γ). Indeed, antibody-mediated neutralization of IFN-γ completely prevented the elimination of PLCs in MCMV-infected mice and, conversely, injection of IFN-γ in uninfected animals resulted in a significant decrease of PLCs in neonatal mice. This result demonstrates a primary role for IFN-γ in the process and confirms that it is the host’s response to the virus, rather than the virus itself, that leads to PLC depletion.
This is the first mechanistic study that clearly demonstrates the role of an early postnatal infection provoking an immune response that protects the host from development of hyperploid B-ALL. In combination with the results from other B-ALL-predisposed models like Pax5+/− or ETV6RUNX1+ mice (where the immune stress caused by infection triggers leukemic development in predisposed animals), the remarkable findings from Farrokhi et al reinforce the “infectious” hypothesis that posits that exposure to infection represents a key event in the evolution of the disease. However, the data suggest that the final outcome of these genetic predisposition and infection interactions is influenced by multiple factors (see figure); indeed, depending on the specific mutations, the age of exposure to the infection, and the pathogen involved, among other variables, the interactions may either create an oncogenic environment that promotes leukemia development or result in the selective death of PLCs, thereby preventing the disease. These data raise the possibility that boosting innate immunity might represent a viable strategy to reduce the risk of leukemic progression in predisposed children. Recent results have shown that the elimination of the PLCs by transient treatment either with a JAK inhibitor or by boosting innate immunity prevents leukemic development in genetically predisposed Pax5+/− mice.8-10 However, in this type of predisposition, early exposure to infection does not prevent B-ALL progression,8-10 thus reinforcing the idea that the role of immune deregulation in the evolution toward leukemia is heterogeneous and varies among the different genetic subtypes of childhood B-ALL.6,10
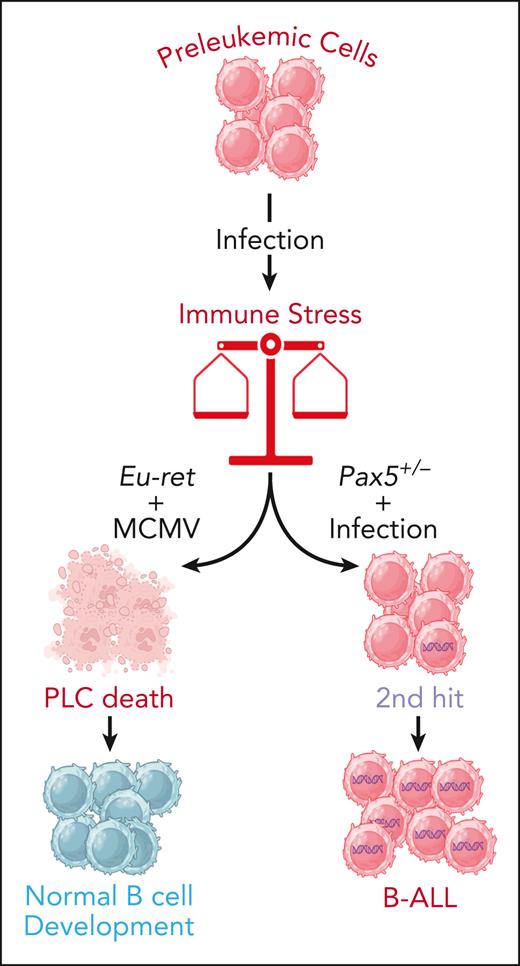
The double-edged sword of immune stress in leukemic progression. Different types of mutations can cause genetic predisposition to B-ALL in at least 5% of newborns, as they lead to the appearance of PLCs from the earliest stages of embryonic life. In the majority of cases, these PLCs will not evolve to full-blown B-ALL, but exposure to environmental stresses such as infections can alter this benign path. The interplay between the nature of the genetic predisposition, the infectious agent, and the age at the time of exposure can produce opposite results. Indeed, in some cases the immune stress triggered by the infection can cause the destruction of the preleukemic clone and prevent leukemia progression, while in some others, it can trigger the appearance of secondary mutations (2nd hit) leading to BALL development. Figure created with BioRender.com.
The double-edged sword of immune stress in leukemic progression. Different types of mutations can cause genetic predisposition to B-ALL in at least 5% of newborns, as they lead to the appearance of PLCs from the earliest stages of embryonic life. In the majority of cases, these PLCs will not evolve to full-blown B-ALL, but exposure to environmental stresses such as infections can alter this benign path. The interplay between the nature of the genetic predisposition, the infectious agent, and the age at the time of exposure can produce opposite results. Indeed, in some cases the immune stress triggered by the infection can cause the destruction of the preleukemic clone and prevent leukemia progression, while in some others, it can trigger the appearance of secondary mutations (2nd hit) leading to BALL development. Figure created with BioRender.com.
The findings of this study have significant implications for our understanding of the disease, and for epidemiology and public health, from the moment when we might soon have the potential of targeting the preleukemic condition in carrying children. However, certain aspects remain unresolved. A very relevant question in this context is why most genetically predisposed children (and many of the genetically predisposed animals) never develop leukemia. Furthermore, what are the mechanisms through which environmental factors like infection facilitate the acquisition of secondary mutations, thus driving the malignant progression of PLCs? Addressing these and other key questions is imperative for gaining a comprehensive understanding and preventing B-ALL in newborns with PLCs. However, it is essential to validate the relevance of this study systematically in a human setting first. This would most likely imply prospective screening of newborns to identify those at risk of leukemia, with all the difficult ethical implications that such a measure entails. Looking forward, there is a real potential for innate immunity to become a modifiable therapeutic target that can helps us prevent childhood leukemia. In the end, this broader perspective will reshape our approach to childhood leukemia, ultimately prioritizing prevention over diagnosis and treatment.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal