In this issue of Blood, Xue et al provide compelling evidence that platinum-based chemotherapy triggers platelet thrombosis by caspase-3–gasdermin E axis–mediated pyroptosis.1 Their intriguing study places platelet gasdermin E at a crossroad between the immune and thrombosis functions of platelets.
Chemotherapy continues to be an essential armament in the war on cancer. Platinum-based derivatives such as cisplatin are commonly used first-line chemotherapeutic agents to treat carcinomas and sarcomas. Other chemotherapeutic drug cocktails include cisplatin for the management of metastatic disease as a second line of treatment.2 Platinum-based drugs crosslink DNA in the nucleus to form adducts, which impair the DNA repair process and induce caspase-mediated tumor cell–programmed death.2 Although these agents are highly effective at killing cancer cells, they can also exert off-target cytotoxicity by increasing the risk of thrombotic events in patients with cancer. Significantly high rates of thromboembolic complications have been reported in patients treated with cisplatin-based chemotherapy for esophageal, gastric, small cell lung, testicular, and germ cell cancers. One meta-analysis showed an association between cisplatin use and a significantly increased risk of venous thromboembolism in patients with advanced solid tumors, when compared with non–cisplatin-based chemotherapy.3 The prothrombotic feature associated with cisplatin treatment likely reflects activation of endothelial cells, coagulation pathways, and platelets. Another report showed that cisplatin potentiates agonist-induced platelet aggregation,4 but it remains unclear how exposure of anucleated platelets to cisplatin promotes occlusive thrombi.
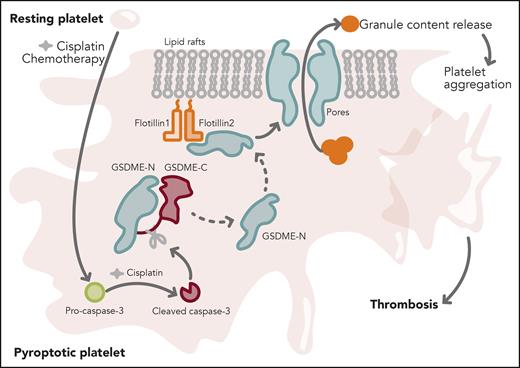
In their study, Xue et al provide basic science mechanistic insights into this significant clinical problem. They show that cisplatin can trigger activation of caspase-3 in the megakaryoblastic cell line Meg-01 and in human and mouse platelets. Activated caspase-3 cleaved full-length gasdermin E (GSDME) to generate the GSDME–N-terminal fragment. Flotillin-2, a scaffold protein, recruited the N-terminal GSDME fragments to the lipid raft fractions of the platelet membrane, wherein GSDME–N-terminal fragments assembled to form pores. These pores facilitated leakage of platelet α, dense and lysosomal granular contents. Increased secretion of platelet granular contents promoted aggregation and integrin αIIbβ3 inside-out and outside-in signaling responses, and supported occlusive clots in vivo and in vitro (see figure). Platelets from patients with hepatocholangiocarcinoma after cisplatin treatment displayed increased caspase-3 activation and GSDME–N-terminal cleavage as well as enhanced aggregation and adenosine triphosphate release, when compared with the same patients before cisplatin treatment. Finally, GSDME-null mice displayed reduced aggregation, secretion, outside-in signaling responses, and thrombosis. Treatment with cisplatin in GSDME-null mice resulted in reduced thrombi. Taken together, the study reveals a significant role for the caspase-3–GSDME pyroptosis pathway in promoting cisplatin-induced occlusive thrombi.
Chemotherapy (cisplatin) converts resting platelet into a pyroptotic state. Cisplatin triggers activation of caspase-3. Active caspase-3 cleaves full-length gasdermin E (GSDME) to generate GSDME–N-terminal and GSDME–C-terminal fragments. GSDME–N-terminal protein is recruited to the platelet lipid raft membrane via an interaction with flotillin-2. GSDME–N-terminal protein assembles into pores in the raft fraction and causes leakage of platelet granular contents. Increased platelet secretion triggers platelet aggregation and thrombosis. Professional illustration by Somersault18:24.
Chemotherapy (cisplatin) converts resting platelet into a pyroptotic state. Cisplatin triggers activation of caspase-3. Active caspase-3 cleaves full-length gasdermin E (GSDME) to generate GSDME–N-terminal and GSDME–C-terminal fragments. GSDME–N-terminal protein is recruited to the platelet lipid raft membrane via an interaction with flotillin-2. GSDME–N-terminal protein assembles into pores in the raft fraction and causes leakage of platelet granular contents. Increased platelet secretion triggers platelet aggregation and thrombosis. Professional illustration by Somersault18:24.
The word pyroptosis has a Greek origin: pyro (fire) and ptosis (falling). Pyroptosis is referred to as pro-inflammatory programmed cell death mediated by gasdermin (GSDM) A, B, C, D, or E superfamily proteins. Pyroptosis is well understood to be an immune functional response in myeloid cells responding to pathogen-associated molecule patterns and/or danger-associated molecular patterns. The distinctive features of pyroptosis include cell swelling and formation of pores on the cell membrane. Pore formation is mediated by oligomerization of the cleaved GSDM–N-terminal proteins, which facilitate the release of interleukin-1β and trigger systemic inflammation.5 Emerging studies suggest that pyroptosis can occur in platelets as well. During severe sepsis, upregulated gasdermin protein D triggers platelet pyroptosis and supports neutrophil extracellular trap (NET)-mediated inflammation.6 Although platelets express gasdermin A, B, C, D, and E proteins, the link between platelet pyroptosis and thrombosis remains unclear.
This interesting study has 2 important findings. First, the authors provide new mechanistic insight into serious thromboembolic complications associated with platinum-based chemotherapy, and the data suggest that the caspase-3–GSDME signaling axis in platelets promotes platelet hyperactivity. Surprisingly, cisplatin did not cleave gasdermin A, B, C, and D proteins in platelets. Although the bulk of the data is focused on cisplatin, limited evidence is presented that the GSDME–N-terminal cleavage product is increased in platelets exposed to etoposide (a topoisomerase II inhibitor). These observations raise additional questions: How does cisplatin activate specifically GSDME-mediated pyroptosis? Can caspase-3–GSDME axis–mediated platelet pyroptosis be a common feature with other chemotherapeutic agents? Second, the study illustrates a novel role for pyroptosis in platelet physiology and thrombosis, with an emphasis on gasdermin E protein. Interestingly, gasdermin D protein–mediated platelet pyroptosis was previously shown to promote formation of NETs.6 As NETs facilitate thrombosis,7 it remains to be investigated whether the newly described platelet thrombosis triggered by gasdermin E pyroptosis is independent or dependent on NETs.
Finally, Xue and colleagues provide compelling evidence of GSDME-induced pyroptosis in chemotherapy-induced platelet dysfunction. The challenge now is to translate these findings and develop gasdermin E as a potential target to reduce thrombotic events in patients on chemotherapy.
Conflict-of-interest disclosure: K.V.V. reports no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal