Key Points
After a transient decrease after the STOP trial, the rates of ischemic stroke are increasing in children with SCD in California.
Stroke in adults with SCD linked to modifiable risk factors like hypertension and hyperlipidemia warrants additional prevention strategies.
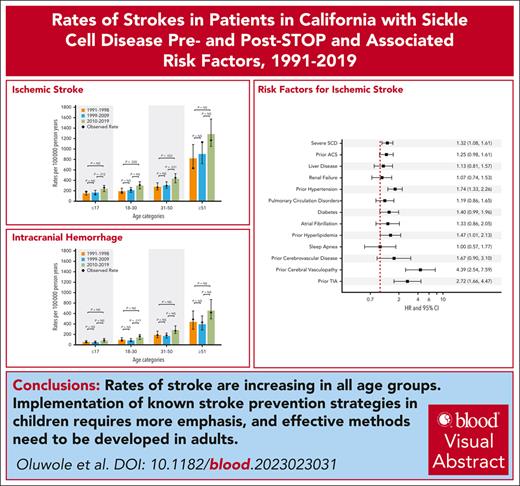
Visual Abstract
Neurovascular complications, including strokes and transient ischemic attacks (TIAs), are common and cause significant morbidity in individuals with sickle cell disease (SCD). The Stroke Prevention Trial in Sickle Cell Anemia (STOP) (1998) established chronic transfusions as the standard of care for children with SCD at high risk for stroke. Using statewide data from the California Department of Health Care Access and Innovation (1991-2019), we determined the cumulative incidence (CMI) and rates of primary and recurrent strokes/TIAs in people with SCD pre- and post-STOP trial. For the 7636 patients included in our SCD cohort, the cumulative incidence of the first ischemic stroke was 2.1% by the age of 20 years and 13.5% by the age of 60 years. The CMI of the first intracranial hemorrhage (ICH) was 0.5% and 6.8% by the age of 20 and 60 years, respectively. Ischemic stroke rates increased in children (age <18 years; 234.9 vs 165.1 per 100 000 patient years [PY]; P = .012) and adults (age 31-50 years; 431.1 vs 303.2 per 100 000 PY; P = .031) in 2010 to 2019 when compared with the preceding decade. There was an increase in the rates of ICH in those aged 18 to 30 years and TIA in children <18 years from 2010 to 2019 when compared with the previous decade. Risk factors for strokes included increasing age, hypertension, and hyperlipidemia. These findings underscore the need for stroke prevention in adults with SCD, suggesting an emphasis on management of modifiable cerebrovascular risk factors that have been proven to be effective in the general population.
Introduction
Strokes are a frequent and potentially catastrophic complication in people living with sickle cell disease (SCD). Individuals with SCD are susceptible to both ischemic and hemorrhagic strokes and transient ischemic attacks (TIAs) with or without subsequent stroke. The Cooperative Study of Sickle Cell Disease (CSSCD), which collected longitudinal data on 4082 patients between 1978 and 1988, is the largest prospective cohort of children and adults with SCD to date.1,2 Within the CSSCD cohort, without any preventive measures, the cumulative incidence of cerebrovascular accidents (which included TIA or stroke) in patients with sickle cell anemia (SCA; homozygous S [HbSS]) was 11% by the age of 20 years and 24% by the age of 45 years.3 The overall incidence rate of stroke in SCD was 0.46 per 100 patient years (PY) with the highest rate in those with SCA (0.61/100 PY).3 In addition, the study revealed a bimodal distribution of ischemic strokes in individuals <20 years and >30 years, although hemorrhagic strokes were more common in the 20- to 29-year age group.3
The Stroke Prevention Trial in Sickle Cell Anemia (STOP) demonstrated that, in children with abnormal transcranial Doppler (TCD) velocities, chronic transfusion therapy led to a 90% reduction in the risk for stroke when compared with the standard of care alone.4 The follow-up study, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2), showed that, in individuals who had normalized their TCD velocities, discontinuation of transfusion therapy after 30 months led to a return of aberrant velocities and an increased risk for stroke.5 After the publication of results from the initial STOP trial, several studies have demonstrated a decline in the incidence of strokes.6-11 STOP was conducted on children, and the majority of studies have focused on the outcomes in children. Only a few studies have examined stroke incidence in adults. Only 2 studies6,8 have used population-based data to describe the trends of strokes in children and adults with SCD, the most recent of which was published in 2009. Therefore, we conducted a real-world, population-based study to describe the incidence of primary and subsequent strokes and TIAs in a diverse and more contemporary cohort and to determine risk factors associated with strokes in patients with SCD in California.
Methods
Database
We used the Health Care Access and Innovation (HCAI) database, which included the Emergency Department Utilization (EDU; 2005-2019) database and the Patient Discharge Data (PDD; 1991-2019) hospitalization database. People with SCD were identified using a previously described method.12-16 Since 1990, the state of California has mandated that nonfederal hospitals have to report up to 25 diagnoses and up to 21 procedures performed during each patient hospitalization. These diagnoses were classified using the 9th (ICD-9-CM) and 10th (ICD-10-CM) revisions of the International Classification of Diseases, Clinical Modification. Although there is a potential for systematic error with the change from ICD-9 to ICD-10 codes in 2015, the diagnosis codes for strokes have shown similar positive predictive values (90% [95% confidence interval (CI), 86-93] vs 92% [95% CI, 88-95]).17 All emergency department encounters have been reported since 2005. Both databases contained additional information, such as sociodemographic and insurance information. A unique record linkage number, which is an encrypted form of a social security number, was assigned to each individual and enabled longitudinal tracking of encounters across all reporting institutions. Of note, the HCAI did not include information on laboratory results or medications prescribed or received. Patient mortality data were available in the HCAI through linkage to death products18 using 1 of the following 2 methods: (1) in 1990 to 2013, the unique best match of a single death record to a patient’s last identifiable record in the PDD, regardless of the type of care, or (2) in 2014 to 2017, the annual PDD and ED discharges were linked to the same year of the California Comprehensive Death File. The methodology changes in 2014 likely led to underreporting of death rates in people without PDD/EDU encounters in the calendar year preceding their death. Discharge data from 1991 to 2019 and mortality data through 2017 were used for this study.
This study used data obtained through chart reviews of existing medical records with deidentified information and did not involve direct interaction with participants. Informed consent was not obtained for this study as it was exempt under category 4, as determined by the University of California Davis Institutional Review Board; therefore, informed consent was not required.
Cohort
Using ICD-9 and ICD-10-CM codes (supplemental Table 1, available on the Blood website),12-16,19,20 we defined our SCD study cohort using the following criteria: 2 admissions with SCD as the principal diagnosis or 1 admission with SCD as the principal diagnosis and 2 additional admissions with SCD as a secondary diagnosis. This study was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects and the University of California, Davis Institutional Review Boards. It was performed in accordance with the Declaration of Helsinki.
Outcomes
The primary outcome was the incidence of a cerebrovascular event (CVE), which included strokes or TIAs. ICD-9 and ICD-10-CM codes, which both have been validated for acute stroke,21 were used to identify PDD and/or EDU visits for ischemic stroke, hemorrhagic stroke, and TIAs (supplemental Table 2). A first CVE was defined as an event of interest (ischemic or hemorrhagic stroke or TIA) if recorded in any diagnosis position. To increase confidence that a relevant code in subsequent encounters represented a new event, patients with a subsequent CVE had to meet 1 of the following 3 more restrictive criteria: (1) the subsequent CVE had to be in the principal diagnosis position; (2) if SCD was in the principal position, the stroke code had to be in the first other diagnosis position; or (3) the stroke code could be in any position if it was not present on admission and therefore deemed a hospital-acquired event. In addition, the subsequent encounter date had to be >14 days after the first CVE discharge date or be documented as a different CVE type (if ≤14 days after first discharge date).
Covariates
The first encounter recorded in either the PDD or EDU database was used to determine gender and race/ethnicity. As done in our previous studies, the frequency of hospitalization was defined by using a patient’s average number of visits per year for any cause; those with ≥3 PDD/EDU encounters per year were defined as frequent hospitalizations, because we have previously shown that this frequency of acute care visits is associated with an increase in SCD morbidity and mortality12-15 SCD-related complications, such as acute chest syndrome (ACS), moyamoya disease, and pulmonary hypertension, were included as covariates. Liver disease, renal failure, diabetes, and hypertension from the Elixhauser comorbidity index22 were included, and atrial fibrillation, hyperlipidemia, sleep apnea, arteriovenous malformation, nonruptured cerebral aneurysm, cerebrovascular disorders, posterior reversible encephalopathy syndrome/hypertensive encephalopathy, and occlusion without infarction were also identified as covariates of interest. A diagnosis of vessel occlusion without infarction was considered to be evidence of cerebral or extracerebral (ie, carotid artery) vasculopathy. Supplemental Table 3 includes a detailed list of the ICD-9 and ICD-10 codes that were used.
Statistical analysis
Descriptive frequencies were used to describe the overall SCD cohort and those with each CVE outcome. The median potential follow-up time was calculated using the reverse Kaplan Meier method from each patient’s first admission encounter to the last known discharge encounter.23 The cumulative incidence functions of each first CVE were calculated on the age scale and took into account the competing risk of death using the Fine and Gray methodology.24,25 Similarly, the cumulative incidence functions of a subsequent CVE were calculated from the time of the first CVE discharge date to the admission date for a subsequent CVE, the date of death, or the last known discharge date in HCAI.
Predicted age-specific stroke incidence rates per 100 000 person-years were calculated for each time interval (1991-1998, 1999-2009, 2010-2019) and CVE using Poisson regression. Rates were compared between time periods by age categories. Person-years were calculated from either the start of the HCAI database (1 January 1991) or the estimated birth date (which ever occurred later) to the CVE of interest, the date of death, or the last known discharge date in HCAI database. Person-years were stratified by age groups (0-17, 18-30, 31-50, and ≥51) and time interval, as previously described, using SAS macro %stratify.26 To compare these data with those of previous reports that showed declining stroke rates in California children (<20 years of age) in the 2 years after publication of the STOP results, age-specific incidence rates for the years 1999 to 2000 were also calculated. To further explore the location of SCD inpatient or emergency department care among the pediatric population (age 0-17 years), we categorized all non-federal hospitals and emergency rooms in California as high-volume SCD facilities (≥100 unique patients seen over the 29-year study period) or low-volume SCD facilities (<100 unique patients). We then classified patients with SCD who received care during this period as receiving mostly high-volume (≥80% admissions at a high-volume SCD facility), mostly low-volume (≤20% admissions at a high-volume SCD facility) or mixed-volume care. Ischemic strokes rates per 100 000 person-years using Poisson regression were then calculated among 0- to 17-year olds by time intervals and SCD facility volume. Unadjusted P values are presented; no Bonferroni multiple-comparison correction was implemented.
Multivariable Cox proportional hazards regression models, using the methods of Fine and Gray24 to account for competing risk of death, were used to identify risk factors associated with ischemic stroke and ICH separately. Time was calculated from the first known admission date recorded in the PDD or EDU databases to the CVE date of interest, death date, or last known discharge date. Each risk factor was included as a time-dependent covariate at the first time it was coded for each patient.
Results
Patient characteristics
Our study cohort comprised 7636 patients with SCD (n = 4070 females) identified between 1991 and 2019. Their baseline characteristics are summarized in Table 1. Overall, 733 patients (9.6%) had at least 1 CVE; 451 (5.9%) had an ischemic event, 227 (3%) had an ICH, and 205 (2.7%) had a TIA. CVEs were more common in women (ischemic, 56.3%; ICH, 52.4%; TIA, 64.9%) and in frequently hospitalized patients (≥3 hospitalizations per year) (ischemic, 59.0%; ICH, 59.0%; TIA, 68.8%). Preexisting hyperlipidemia was more common among those with ischemic stroke and TIA (11.5% and 13.7%, respectively) when compared with the entire cohort (9.7%). For those with ICH, when compared with the entire cohort, a history of ACS (61.7% vs 58.7%), renal failure (22% vs 12.3%), and liver disease (17.2% vs 12.5%) were more common. A history of hypertension was common among those who presented with any of the CVEs (38.8% ischemic stroke, 42.7% ICH, and 35.6% TIA) when compared with an incidence of 33.1% in the entire cohort.
Characteristics among California patients with SCD with and without CVEs, 1991-2019
| Variables . | All . | First ischemic . | First ICH . | First TIA . |
|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | |
| All | 7636 (100%) | 451 (5.9%) | 227 (3%) | 205 (2.7%) |
| Sex | ||||
| Male | 3566 (46.7%) | 197 (43.7%) | 108 (47.6%) | 72 (35.1%) |
| Female | 4070 (53.3%) | 254 (56.3%) | 119 (52.4%) | 133 (64.9%) |
| Race/ethnicity | ||||
| Non-Hispanic Black | 6787 (88.9%) | 413 (91.6%) | 215 (94.7%) | 182 (88.8%) |
| Non-Black | 849 (11.1%) | 38 (8.4%) | 12 (5.3%) | 23 (11.2%) |
| Hospitalization frequency∗ | ||||
| Less frequent | 4162 (54.5%) | 185 (41%) | 93 (41%) | 64 (31.2%) |
| Frequent | 3474 (45.5%) | 266 (59%) | 134 (59%) | 141 (68.8%) |
| Comorbidities present before each first event type | ||||
| ACS | 4482 (58.7%) | 249 (55.2%) | 140 (61.7%) | 115 (56.1%) |
| Renal failure | 937 (12.3%) | 63 (14%) | 50 (22%) | 26 (12.7%) |
| Liver disease | 953 (12.5%) | 56 (12.4%) | 39 (17.2%) | 23 (11.2%) |
| AFIB | 481 (6.3%) | 41 (9.1%) | 17 (7.5%) | 12 (5.9%) |
| Pulmonary circulatory disorder | 1262 (16.5%) | 88 (19.5%) | — | 35 (17.1%) |
| Hypertension | 2531 (33.1%) | 175 (38.8%) | 97 (42.7%) | 73 (35.6%) |
| Cerebral or extracerebral vasculopathy | 143 (1.9%) | 22 (4.9%) | 14 (6.2%) | 8 (3.9%) |
| Ischemic stroke | 451 (5.9%) | — | 28 (12.3%) | — |
| Moyamoya | 84 (1.1%) | — | 6 (2.6%) | — |
| AVM | 19 (0.2%) | — | 1 (0.4%) | — |
| Nonruptured cerebral aneurysm | 75 (1%) | — | 2 (0.9%) | — |
| Cerebrovascular disease | 191 (2.5%) | 18 (4%) | — | 8 (3.9%) |
| PRES/hypertensive encephalopathy | 124 (1.6%) | 15 (3.3%) | 89 (1.3%) | 4 (2.0%) |
| Sleep apnea | 501 (6.6%) | 14 (3.1%) | — | 14 (6.8%) |
| Hyperlipidemia | 740 (9.7%) | 52 (11.5%) | — | 28 (13.7%) |
| Coagulopathy | 189 (2.5%) | — | 6 (2.6%) | — |
| Thrombocytopenia | 1473 (19.3%) | — | 55 (24.2%) | — |
| Variables . | All . | First ischemic . | First ICH . | First TIA . |
|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | |
| All | 7636 (100%) | 451 (5.9%) | 227 (3%) | 205 (2.7%) |
| Sex | ||||
| Male | 3566 (46.7%) | 197 (43.7%) | 108 (47.6%) | 72 (35.1%) |
| Female | 4070 (53.3%) | 254 (56.3%) | 119 (52.4%) | 133 (64.9%) |
| Race/ethnicity | ||||
| Non-Hispanic Black | 6787 (88.9%) | 413 (91.6%) | 215 (94.7%) | 182 (88.8%) |
| Non-Black | 849 (11.1%) | 38 (8.4%) | 12 (5.3%) | 23 (11.2%) |
| Hospitalization frequency∗ | ||||
| Less frequent | 4162 (54.5%) | 185 (41%) | 93 (41%) | 64 (31.2%) |
| Frequent | 3474 (45.5%) | 266 (59%) | 134 (59%) | 141 (68.8%) |
| Comorbidities present before each first event type | ||||
| ACS | 4482 (58.7%) | 249 (55.2%) | 140 (61.7%) | 115 (56.1%) |
| Renal failure | 937 (12.3%) | 63 (14%) | 50 (22%) | 26 (12.7%) |
| Liver disease | 953 (12.5%) | 56 (12.4%) | 39 (17.2%) | 23 (11.2%) |
| AFIB | 481 (6.3%) | 41 (9.1%) | 17 (7.5%) | 12 (5.9%) |
| Pulmonary circulatory disorder | 1262 (16.5%) | 88 (19.5%) | — | 35 (17.1%) |
| Hypertension | 2531 (33.1%) | 175 (38.8%) | 97 (42.7%) | 73 (35.6%) |
| Cerebral or extracerebral vasculopathy | 143 (1.9%) | 22 (4.9%) | 14 (6.2%) | 8 (3.9%) |
| Ischemic stroke | 451 (5.9%) | — | 28 (12.3%) | — |
| Moyamoya | 84 (1.1%) | — | 6 (2.6%) | — |
| AVM | 19 (0.2%) | — | 1 (0.4%) | — |
| Nonruptured cerebral aneurysm | 75 (1%) | — | 2 (0.9%) | — |
| Cerebrovascular disease | 191 (2.5%) | 18 (4%) | — | 8 (3.9%) |
| PRES/hypertensive encephalopathy | 124 (1.6%) | 15 (3.3%) | 89 (1.3%) | 4 (2.0%) |
| Sleep apnea | 501 (6.6%) | 14 (3.1%) | — | 14 (6.8%) |
| Hyperlipidemia | 740 (9.7%) | 52 (11.5%) | — | 28 (13.7%) |
| Coagulopathy | 189 (2.5%) | — | 6 (2.6%) | — |
| Thrombocytopenia | 1473 (19.3%) | — | 55 (24.2%) | — |
AFIB, atrial fibrillation; AVM, arteriovenous malformation; PRES, posterior reversible encephalopathy syndrome.
Hospitalization frequency: patients with SCD with an average of ≥3 visits per year (inpatient or ED) were defined as frequent; patients with an average of <3 visits per year were defined as less frequent.
Incidence of first CVEs
The cumulative incidence of the initial CVE is shown in Figure 1. The cumulative incidence of the first ischemic stroke was 2.1% (95% CI, 1.8-2.4) by the age of 20 years and 13.5% (95% CI, 12.3-14.7) by the age of 60 years. The cumulative incidence of ICH was 0.5% (95% CI, 0.4-0.7) by the age of 20 years and 6.8% (95% CI, 5.9-7.7) by age 60 years. Lastly, the cumulative incidence of the first TIA was 0.7% (95% CI, 0.5-0.9) by the age of 20 years and 5.9% (95% CI, 5.1-6.8) by the age of 60 years.
Cumulative incidence of first CVE type, accounting for the competing risk of death, among California patients with SCD, 1991-2019. (A) Ischemic stroke. (B) Intracranial hemorrhage ICH. (C) TIA.
Cumulative incidence of first CVE type, accounting for the competing risk of death, among California patients with SCD, 1991-2019. (A) Ischemic stroke. (B) Intracranial hemorrhage ICH. (C) TIA.
Rates of strokes and TIAs
Stroke rates stratified by age groups during the previous 3 decades are presented in Figure 2. The rate of ischemic stroke increased with age and by time period with the highest rates occurring in the most recent decade (2010-2019) across all age groups (0-17 years, 234.9; 18-30 years, 298.8; 31-50 years, 431.1; ≥51 years, 1285.9/100 000 PY). ICH occurred at similar rates in the 1990s and early 2000s across all age categories with a trend toward increased rates in the 2010s. Similarly, the rate of the first TIA increased with age and by time period with the 2010s having the highest rate across all age categories. When compared with the previous decade, there was an increase in ischemic stroke (P = .012) and TIAs (P = .027) in patients aged ≤17 years in 2010 to 2019. The SCD genotype was inaccurately coded in our administrative data set, and we were unable to risk stratify by genotype.
Predicted rates of first CVE type per 100 000 PY by age category and time period among California patients with SCD between 1991 and 2019. Shown are the rates of ischemic strokes (A), ICH (B), and TIAs (C). Rates were compared between each time period by age categories. Unadjusted P values are presented; no Bonferroni multiple-comparison correction was implemented.
Predicted rates of first CVE type per 100 000 PY by age category and time period among California patients with SCD between 1991 and 2019. Shown are the rates of ischemic strokes (A), ICH (B), and TIAs (C). Rates were compared between each time period by age categories. Unadjusted P values are presented; no Bonferroni multiple-comparison correction was implemented.
Rates of ischemic stroke in pediatric patients by hospital volume
Stroke rates in children ≤17 years of age are shown in supplemental Figure 1. Among patients followed at high-volume SCD facilities, there was a decline in the ischemic stroke rates from the 1991 to 1998 period (pre-STOP) to the 1999 to 2009 period (359.0 vs 202.6 per 100 000 PY; P = .01). There was a trend toward increasing rates of ischemic strokes in 2010 to 2019 when compared with 1999 to 2009 regardless of hospital SCD volume.
Risk factors of ischemic and hemorrhagic strokes
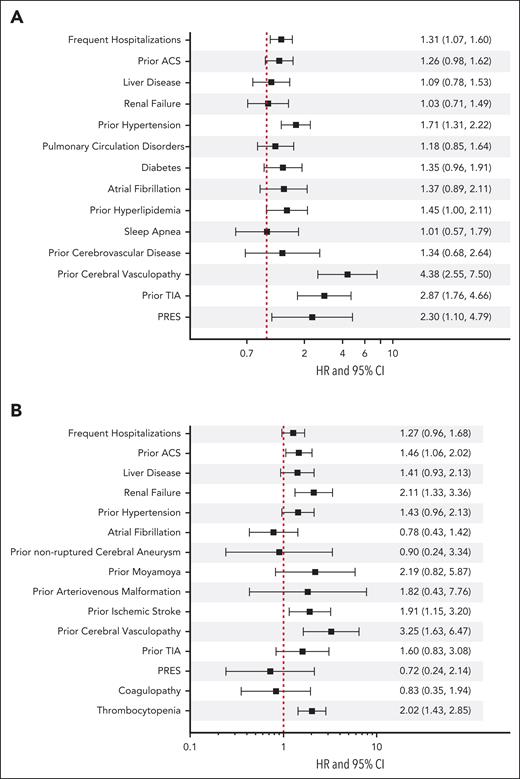
We identified various risk factors for both ischemic stroke and ICH (Figure 3). Frequent hospitalization (hazard ratio [HR], 1.31; 95% CI, 1.07-1.60), hypertension (HR, 1.71; 95% CI, 1.31-2.22), hyperlipidemia (HR, 1.45; 95% CI, 1.00-2.11), previous cerebral or extracerebal vasculopathy (HR, 4.38; 95% CI, 2.55-7.50), previous TIA (HR, 2.87; 95% CI, 1.76-4.66), and posterior reversible encephalopathy syndrome (HR, 2.30; 95% CI, 1.10-4.79) were significantly associated with ischemic strokes. ICH, however, was associated with a history of ACS (HR, 1.46; 95% CI, 1.06-2.02), renal failure (HR, 2.11; 95% CI, 1.33-3.36), previous ischemic stroke (HR, 1.91; 95% CI, 1.15-3.20), previous cerebral or extracerebral vasculopathy (HR, 3.25; 95% CI, 1.63-6.47), and thrombocytopenia (HR, 2.02; 95% CI, 1.43-2.85).
Risk factors for ischemic stroke (A) and intracerebral hemorrhage (B) among California patients with SCD from 1991 to 2019. This figure illustrates the risk factors associated with ischemic stroke and intracranial hemorrhage (ICH), accounting for the competing risk of death among California patients with SCD between 1991 and 2019. Cox regression models with correction for the competing risk of death was adjusted for sex, age at entry into the cohort, year of entry into the cohort, and Black race. All variables, except frequent hospitalizations, were included as time-dependent covariates. Frequent hospitalizations was defined as an average of ≥3 admissions per year. Previous cerebrovascular disorder includes cerebral atherosclerosis, hypertensive encephalopathy, and other ill-defined or unspecified cerebrovascular disease.
Risk factors for ischemic stroke (A) and intracerebral hemorrhage (B) among California patients with SCD from 1991 to 2019. This figure illustrates the risk factors associated with ischemic stroke and intracranial hemorrhage (ICH), accounting for the competing risk of death among California patients with SCD between 1991 and 2019. Cox regression models with correction for the competing risk of death was adjusted for sex, age at entry into the cohort, year of entry into the cohort, and Black race. All variables, except frequent hospitalizations, were included as time-dependent covariates. Frequent hospitalizations was defined as an average of ≥3 admissions per year. Previous cerebrovascular disorder includes cerebral atherosclerosis, hypertensive encephalopathy, and other ill-defined or unspecified cerebrovascular disease.
Incidence of subsequent stroke
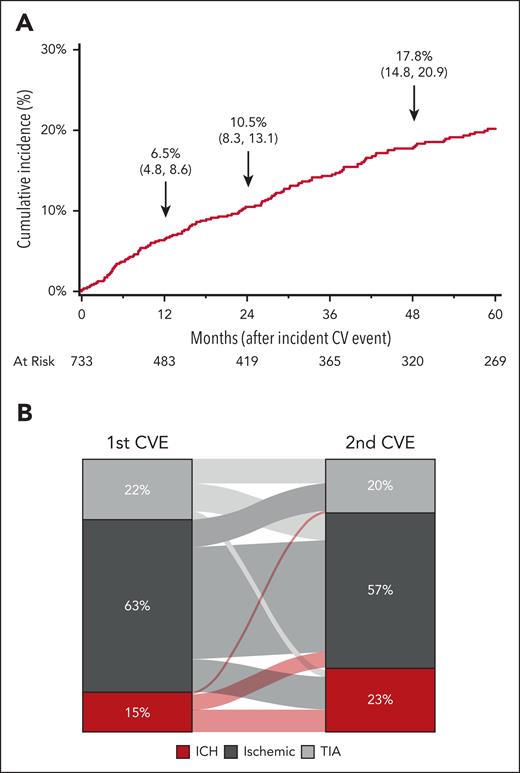
Of the 733 patients with SCD who had their first CVE, 158 patients (21%) had a subsequent stroke or TIA during the study period. The cumulative incidence of a subsequent CVE is shown in Figure 4A. The cumulative incidence increased from 6.5% at 12 months to 10.5% at 24 months and 17.8% at 48 months after the initial CVE. The distribution of the primary and secondary CVE types is shown in Figure 4B. The majority (63%) of the initial events were ischemic strokes, and subsequent events were a second ischemic stroke (65%), ICH (19%), or TIA (16%). Moreover, ischemic strokes were the most common (57%) subsequent CVE type, followed by ICH (23%) and TIA (20%).
Subsequent CVE in 733 California patients with SCD with a first event in 1991 to 2019. (A) Cumulative incidence of a subsequent event. (B) Distribution of the first CVE to second CVE type among 158 California patients with SCD with both a first and second event during 1991 to 2019.
Subsequent CVE in 733 California patients with SCD with a first event in 1991 to 2019. (A) Cumulative incidence of a subsequent event. (B) Distribution of the first CVE to second CVE type among 158 California patients with SCD with both a first and second event during 1991 to 2019.
Discussion
In this report, we provide an update on the incidence of CVEs in a large population-based cohort of people living with SCD in California. We found that 9.6% of patients had an incident CVE. The cumulative incidence of ischemic stroke more than doubled for every 20-year increase in age; the rates of ischemic strokes also increased with age and by decade despite a transient decline following publication of the STOP results.4 The incidence of ICH increased about 13-fold from 20 to 60 years of age. Cerebral/extracerebral vasculopathy and antecedent TIA were associated with the highest risk for ischemic strokes, whereas renal failure and cerebral/extracerebral vasculopathy were associated with a higher risk for ICH.27
STOP primarily showed that chronic red blood cell (RBC) transfusions led to a 90% decrease in incident strokes in high-risk children with SCA and elevated TCD velocities.4 This finding, in part, led to the National Institutes of Health/National Heart, Lung, and Blood Institute and the American Academy of Pediatrics to recommend TCD screening for people with SCD aged 2 to 16 years.28,29 Using data from the California PDD, Fullerton et al found an 80.6% decrease in the incident stroke rates (both ischemic and hemorrhagic) among children with SCD in the 2 years following the publication of the STOP guidelines (1999-2000).6 We found a 66% reduction in the observed rate of ischemic strokes (176.92/100 000 PY in 1991-1998 [before STOP] to 60.05/100 000 PY in 1999-2000; supplemental Figure 2); however, we found no decrease in the predicted rates using Poisson regression. Following an initial decrease in the stroke rates in the 2 years after STOP, we found that the ischemic stroke rates increased from 165.8 per 100 000 PY (1999-2009) to 235.0 per 100 000 PY (2010-2019; P = .017). The ischemic stroke rates increased in a similar pattern in pediatric patients followed at facilities with high or low SCD patient volume. Plausible reasons for this discouraging trend of increased strokes in children with SCD living in California, include (1) decreasing adherence to the TCD screening guidelines recommended by the National Institutes of Health/National Heart, Lung, and Blood Institute or the American Academy of Pediatrics, (2) low rates of transfusion therapy and hydroxyurea use, and (3) improved sensitivity of neuroimaging that may lead to the diagnosis of ischemic strokes.
Other investigators have shown underuse of TCD screening with yearly rates ranging from 22% to 75%.30-32 A study using Medicaid claims data from 2005 to 2010 showed that each year of increasing age was associated with a 3% lower odds of TCD screening.32 Further data from the post-STOP study (a retrospective observational study of patients who were enrolled in the STOP and STOP II clinical trials and who were followed for a mean of 9.1 ± 3.4 years) showed that only 57% of patients who were eligible for screening (range, 18%-91% based on site) underwent TCD rescreening,33 and 63% of strokes occurred in patients who did not receive guideline concordant care.7 Although chronic RBC transfusion remains the mainstay of treatment for primary and secondary stroke prevention, seminal clinical trials such as the Transcranial Doppler with Transfusions Changing to Hydroxyurea trial,34 the Stroke Prevention in Nigeria trial,35 the Hydroxyurea for Primary Stroke Prevention in Children with Sickle Cell Anemia in Nigeria trial,36 and a long-term French cohort study37 all demonstrated that hydroxyurea is an alternative to RBC transfusions for primary stroke prevention in patients without significant CNS vasculopathy. However, the clinical response to hydroxyurea in patients with SCD is variable and several studies have demonstrated low rates of hydroxyurea adherence.38-40 Thus, decreased adherence rates to TCD screening guidelines that lead to underuse of appropriate hydroxyurea transfusion therapy are potential contributing factors to the increasing stroke rates since STOP.
Brain magnetic resonance imaging (MRI) is more sensitive for detecting ischemic strokes and small lacunar infarcts than head computed tomography imaging (initially the primary imaging modality for ruling out strokes). A study showed that MRI has 83% sensitivity for any acute ischemic stroke diagnosis as opposed to 26% for computed tomography; the sensitivity was found to be similar for hemorrhagic stroke.41,42 The increasing use of MRI in the late 1990s and early 2000s (during the post-STOP period) likely contributed to the rising stroke rates observed during the study period.43 A study in the Northern Kentucky/Cincinnati area showed a trend toward higher stroke rates (excluding TIAs) in young adults from 1993/1994 to 2005, along with an increase in MRI use from 27% to 58% during that period.44
Stroke recurrence is high in people with SCD with recurrence rates as high as 67%, and it was found to be the highest in the first 2 to 3 years after the initial event.3,45,46 Within the CSSCD cohort, the rate of recurrence was 14% among those who survived the first CVE with a mean time to recurrence after a TIA, hemorrhagic, and ischemic stroke of 3, 7.2, and 22 months, respectively. Our study showed a 10.5% incidence of a subsequent CVE within 2 years and a 17.8% incidence within 4 years. In addition, the subsequent CVE followed a similar distribution as the primary event. Without intervention, stroke recurs in 2 of 3 patients with SCA.47 Chronic RBC transfusions remain the mainstay of treatment for secondary stroke prevention. Although the Stroke with Transfusions Changing to Hydroxyurea trial48 showed that hydroxyurea and phlebotomy treatment following an initial 18 months of chronic transfusion therapy was within the noninferiority boundary when compared with continuation of chronic transfusion and iron chelation, the study was stopped early because of futility for the composite end point of liver iron loading.48
Given the logistical challenges and time constraints of chronic RBC transfusions, the development of other primary and secondary stroke prevention strategies is needed. Many studies of neurologic outcomes following curative strategies for SCD, such as hematopoietic stem cell transplant (HSCT), have demonstrated either improved or stable neurovascular abnormalities on imaging post-HSCT.49-51 Conversely, the development of new or worsened vascular abnormalities after HSCT have also been reported.50-52 There are no studies that directly compare HSCT with chronic RBC transfusions for secondary stroke prevention.
In the post-STOP cohort, the investigators found an overall ICH incident rate of 63 per 100 000 PY (50/100 000 PY in children and 134/100 000 PY in adults).9 About half of these patients had some underlying structural cerebrovascular abnormalities, and no cause was identified in 22.8% of patients. Strouse et al also used data from California and estimated that the ICH incidence rate was 32 per 100 000 PY in children and 330 per 100 000 PY in adults.8 Similarly, we found a much higher rate of ICH in adults aged >51 years (617/100 000 PY) than in children (89/100 000 PY). There are currently no evidence-based guidelines for the prevention of ICH in SCD cases because most previous studies focused on ischemic strokes.53 Investigators from the post-STOP study group recommend adding a brain magnetic resonance angiography to the screening brain MRI recommended by the American Society of Hematology guidelines to be obtained at least once in children and adults with SCD.9,53
In this study, stroke rates increased with age and there was a trend toward even higher rates in more recent years of follow-up. Although TCDs are feasible in adults with SCD,54,55 the TCD velocity cutoffs used for children have been unreliable in adults and adult TCD velocity patterns have yet to be determined; consequently, there are no current screening guidelines for strokes in adults. Despite a lack of prospective studies, young adults on chronic RBC transfusions since childhood for secondary stroke prevention are continued into adulthood; this may be based on a retrospective cohort study in which 8 of 22 who did not continue regular transfusions after transition to an adult sickle cell program died within 5 years.56 Because patients with SCD are living well into the age of higher stroke risk, prospective studies aimed at screening and primary and secondary stroke prevention strategies are needed. Clinical trials of new SCD disease-modifying therapies should have CNS events as outcome measures.
Consistent with previous studies8 we found that hypertension and hyperlipidemia were associated with the development of ischemic strokes. Lower systemic blood pressures and relative hypertension have been well described in patients with SCD.57-59 Studies showed that higher blood pressures, albeit normal by conventional standards, are associated with CVEs in SCD.58,59 Individuals with SCA exhibit a distinct lipid profile of elevated triglycerides and decreased total cholesterol when compared with the general population.60,61 However, the implications of this distinct lipid profile on cardiovascular outcomes in SCD remain unclear. Evidence abounds that blood pressure and lipid control lowers stroke risk in the general population.62 Because patients with SCD live longer owing to the advent of newer disease-modifying therapies, SCD-related risk factors of strokes will ideally be minimized and the proportion of strokes attributable to traditional risk factors may increase. In this study, we found that the median age of the first hyperlipidemia code was 45 years old (interquartile range, 33-55). Given that these are inpatient codes, the age of diagnosis is unclear. The United States Preventive Services Task Force F recommends screening for men aged 20 to 35 years depending on the family history and cardiovascular risk factors, and the optimal screening time has not been established. During 2015 to 2018, the prevalence of hyperlipidemia in the general population was 11.4% in adults ≥20 years with the greatest prevalence in adults aged 40 to 59 years (15.7%).63 When extrapolating data from the general population into the routine care of adults with SCD, we recommend screening patients with SCD similar to the general population. We recommend that hypertension and hyperlipidemia should be aggressively treated in this patient population that is most vulnerable to adverse CVEs. In addition, pharmacotherapies, such as statins, may provide additional benefits for pain management in SCD.64 Further studies on the safety and efficacy of lipid-lowering agents in this population are needed. Prospective studies on the prevalence and management of these risk factors are also needed.
This study has several limitations. Our database lacks reliable documentation of the SCD genotype and tobacco use, which modify the risk for stroke.65-67 Imaging reports are not available; therefore, we cannot radiographically confirm a diagnosis of stroke. Furthermore, we lack data on silent cerebral infarction, a frequent and underdiagnosed neurovascular event that is associated with an increased risk for overt stroke and neurocognitive impairments,68,69 because no ICD 9/10 codes exist for silent cerebral infarction. CVEs may be underestimated if Californians had strokes outside of the state, patients died of stroke before hospital presentation, or TIA symptoms were managed at home. There have been evolving definitions of TIA vs ischemic stroke and increasing sensitivity of neuroimaging may partially contribute to the observed increase in stroke rates although a concomitant decrease in TIA because of reclassification based on MRI was not observed. Subsequent stroke events may have been underreported because of the stringent coding criteria employed. Although risk factors documented before the CVE were included, some factors like hypertension and hyperlipidemia may not have been diagnosed or documented before the CVE encounter. In addition, data on the use of disease-modifying therapies and stroke screening protocols is lacking. Most of the limitations stem from the use of administrative data. There is abundant literature on data provenance regarding administrative discharge data and limitations,70,71 however, it is reassuring that our previous findings have yielded similar results as other independent research groups, including our work on pregnancy outcomes in SCD.19,72,73 The lack of a comparable population-based data source for a cohort study design restricts our ability to replicate the findings of this study. Nevertheless, this limitation opens opportunities for future population-based studies. Conversely, a major strength of our study was our ability to create a large population-based cohort to calculate incidence rates, which is in contrast with other studies that estimated the underlying SCD population. We could also determine risk factors for different types of CVEs from longitudinal follow-up of SCD-related complications and comorbidities in this large SCD cohort.
In summary, ischemic stroke rates in children and adults have increased in California after publication of the STOP results. In addition, our work confirms previous findings that the incidence of strokes in patients with SCD markedly increased with age. Because people with SCD are living longer, there remains an unmet need to develop guidelines for primary and secondary stroke prevention in adults at highest risk for neurologic debility from adverse CVEs.
Acknowledgments
The authors acknowledge Daniel Tancredi’s biostatistical consultation.
This work was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute T32 Hematology training grant (5T32HL007093-47; O.O.).
Authorship
Contribution: O.O., O.O.A., K.Y.F., and T.W. developed the study concept; O.O., O.O.A., K.Y.F., A.M.B., and T.W. designed the study; A.M.B. and T.W. acquired the data and A.M.B., T.W., and O.O. analyzed the data; O.O., A.M.B., and T.W. drafted the manuscript; and all authors made revisions and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olubusola Oluwole, Division of Classical Hematology, University of Pittsburgh, 200 Lothrop St, Pittsburgh, PA 15213; email: oluwoleob2@upmc.edu.
References
Author notes
The data that support the findings of this study are available from the California Cancer Registry and/or the California Department of Health Care Access and Information. Access to the data is granted through an application process by the management or data custodians (DataAndReports@hcai.ca.gov).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal