In this issue of Blood, Cole et al,1 using a novel cellular “biosensor” model, uncover that polyreactive immunoglobulin M (IgM)-initiated classical complement pathway activation could be the missing trigger initiating the disease in patients with complement-mediated or atypical hemolytic uremic syndrome (CM-HUS/aHUS). The classical complement proteins were once considered innocent bystanders in the pathological process of CM-HUS. However, the study by Cole et al reveals that they are really much like Dr. Jekyll, the celebrated transforming character from Robert Louis Stevenson.
CM-HUS is a kidney-predominant thrombotic microangiopathy that can lead to end-stage renal disease if left without specific treatment.2 It is characterized by the prevalence of, in up to 60% of CM-HUS cases, rare genetic variants in alternative complement pathway components and, in ∼10% of patients, autoantibodies against the key alternative pathway regulator, factor H (FH). Thus, it has long been considered a paradigm of disease with alternative pathway overactivation. Treatment was revolutionized by the anti–C5-blocking antibody, eculizumab; however, this drug targets the terminal pathway activated by any of the following complement pathways: classical, lectin, or alternative. Consequently, the excellent therapeutic efficacy highlights the importance of the complement, but not which pathway drives the condition.
Despite some suggesting that further exploration of CM-HUS was unnecessary due to a well-established pathophysiology and available treatment, several mysteries remained: Why does the disease predominantly affect the kidneys when complement dysregulation can affect the entire body? Why does it occur in the absence of identifiable genetic predisposing factors in at least 30% of patients? And why is the penetrance low among pathogenic variant carriers within the same family?
The study by Cole et al sheds light on some of these mysteries. Using a newly developed “biosensor,” a bioluminescent reporter cell line, HEK293, deficient in complement regulators CD46 and/or CD55/CD59 (knockout for PIGA, gene coding for an enzyme, generating the glycosylphosphatidylinositol anchor of these proteins), they discovered that complement activation on their surface, induced by sera from patients with CM-HUS, occurs through the classical pathway. This activation was inhibited by classical and terminal pathway inhibitors (C1s and C5), but strikingly not by factor B (FB) or even by C3 inhibition. The authors observed that using sera from most patients, both in the acute phase and in remission, resulted in a deposition of IgM on the cell surface in this model system, which triggered the classical pathway, bypassing the alternative pathway amplification loop as previously suggested for strong classical pathway activators.3,4
This new biosensor is based on a kidney embryonic cell line (HEK293), which seems particularly sensitive to the classical pathway, thus enabling this discovery. However, the disease affects specifically the glomerular endothelial cells. Previous studies on glomerular endothelial cells and human umbilical vein endothelial cells revealed that introducing a recombinant gain-of-function–mutant FB to normal human serum, immune depleted of normal FB, induced C3 overactivation on the cell surface, akin to levels achieved with serum from patients carrying C3 or FH genetic variants.5,6 These findings indicate that on a disease-relevant cell surface, the alternative pathway plays a key role in complement overactivation. Although IgM may serve as an initial trigger, it will be amplified on patient glomerular endothelium due to underlying alternative pathway dysregulation. Importantly, because the complement is activated on the ex vivo endothelial models from aHUS sera even in the absence of identifiable alternative pathway dysregulation, measuring IgM and C4 deposition should be incorporated in this assay to assess the presence of classical pathway triggers. Indeed, Cole et al found such C4 positivity in all 3 tested patients.
In healthy humans, circulating IgM, which represents a major part of the natural autoantibodies’ repertoire, manifests autoreactivity and ability to bind to multiple unrelated antigens, that is, polyreactivity. Physiologically, they participate in the clearance of apoptotic cells and damaged macromolecules and provide first line of defense against pathogens. However, binding of natural IgM to neoepitopes displayed on the surface of endothelial cells after ischemia-reperfusion triggers complement activation with ensuing tissue damage.7 The origin of the polyreactive IgM found in the patients with CM-HUS studied by Cole et al remains to be elucidated, as they were not detectable in healthy donors as a naturally occurring IgM and were not generated as a consequence of T-dependent immune responses, because they do not undergo class switch recombination and persist for a long time even during remission. Notably, polyreactive IgG does not seem to be implicated in complement activation within this model system. IgG is rarely polyreactive by itself but can become so in hemolytic conditions such as CM-HUS, where heme is released. Indeed, previous studies have revealed that heme binds to a fraction of IgGs in normal human repertoires, rendering them polyreactive,8,9 and that heme activates the alternative pathway.5 It remains to be determined whether, in this situation, the heme-sensitive IgG may also have pathogenic role in patients with CM-HUS.
The roles of these polyreactive immunoglobulins need further investigation using endothelial cell models. Extensive complement workups that colocalize IgM, IgG, C1q, C4, C3, and C5b-9 have to be performed on kidney biopsy specimens to determine the extent of classical pathway activation. A recent study using a proximity ligation assay in kidney biopsy specimens indicated the absence of classical pathway activation and the presence of an alternative pathway C3 convertase.10 Finally, results from the ongoing phase 3 clinical trial APPELHUS (NCT04889430), which tests alternative pathway inhibition with the FB inhibitor iptacopan in CM-HUS, are eagerly awaited and will clarify the balance between classical pathway and alternative pathway in this disease.
In conclusion, the work of Cole et al provides solid evidence of polyreactive IgM in patients with CM-HUS, which can activate the classical pathway in a novel in vitro biosensor model (see figure). This paper is sure to spark considerable debate within the complement community and renew interest in this disease. Just as Dr. Jekyll transforms into the monstrous Mr. Hyde, the polyreactive IgM and the classical pathway may emerge as a mysterious culprit in CM-HUS, and its identification could reveal new pathophysiological principles.
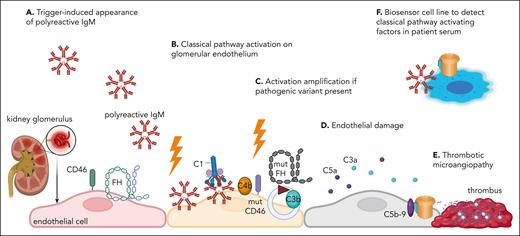
Hypothesis for the pathophysiology of CM-HUS (aHUS), based on the study of Cole et al. (A) A triggering event (infection, pregnancy, inflammation, etc) may result in the appearance of polyreactive IgM antibodies. (B) If directed to glomerular endothelium, they will activate the classical pathway. If this activation is sufficiently strong, it alone may be sufficient to cause endothelial damage and thrombotic microangiopathy. This may occur in patients without genetic risk or anti-FH autoantibodies. (C) In the presence of an at-risk genetic variant (dysfunctional mut FH or CD46 presented in the figure, or factor I or gain-of-function C3 or FB, not illustrated for simplicity) or anti-FH autoantibodies, this classical pathway activation may be amplified to a level, overcoming the tolerable endothelial cell stress, causing (D) endothelial damage, leading eventually to (E) thrombotic microangiopathy. (F) Cole et al propose a new biosensor cell line, sensitive to the presence of classical pathway activators (such as polyreactive IgM), which could be used to detect complement triggers and potentially, after validation, to distinguish CMHUS from other forms of thrombotic microangiopathy. mut, mutated. Figure generated with BioRender.com.
Hypothesis for the pathophysiology of CM-HUS (aHUS), based on the study of Cole et al. (A) A triggering event (infection, pregnancy, inflammation, etc) may result in the appearance of polyreactive IgM antibodies. (B) If directed to glomerular endothelium, they will activate the classical pathway. If this activation is sufficiently strong, it alone may be sufficient to cause endothelial damage and thrombotic microangiopathy. This may occur in patients without genetic risk or anti-FH autoantibodies. (C) In the presence of an at-risk genetic variant (dysfunctional mut FH or CD46 presented in the figure, or factor I or gain-of-function C3 or FB, not illustrated for simplicity) or anti-FH autoantibodies, this classical pathway activation may be amplified to a level, overcoming the tolerable endothelial cell stress, causing (D) endothelial damage, leading eventually to (E) thrombotic microangiopathy. (F) Cole et al propose a new biosensor cell line, sensitive to the presence of classical pathway activators (such as polyreactive IgM), which could be used to detect complement triggers and potentially, after validation, to distinguish CMHUS from other forms of thrombotic microangiopathy. mut, mutated. Figure generated with BioRender.com.
Conflict-of-interest disclosure: L.T.R. received consultancy fees from Alexion and research grants from Novartis, CSL Behring, Roche, and Commit Biologics. J.D.D. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal