In this issue of Blood, Diorio et al1 present a series of 5 children (aged 2.5-14 years) with B-cell acute lymphoblastic leukemia who developed limb weakness following infusion of chimeric antigen receptor (CAR) T-cell constructs directed against CD19 or CD22. As toxicities involving the spinal cord after CAR T-cell treatment are exceedingly rare, to the best of our knowledge these are the first reports of pediatric patients where weakness was attributable to spinal cord injury (eg, myelitis or myelopathy) in 4 of the 5 cases. Collectively, this series highlights the heterogeneity of possible etiologies of acute limb weakness in CAR T-cell patients and provides a framework for diagnostic approaches and correlative investigations. This case series adds to emerging reports of paresis, with or without spinal cord injury, occurring as either a variant or an extension of immune effector cell-associated neurotoxicity syndrome (ICANS).
The American Society for Transplantation and Cellular Therapy’s definition of ICANS includes “deep focal motor weakness such as hemiparesis or paraparesis,” regardless of where in the nervous system the injury localizes.2 Recent reports of myelitis in adults with B-cell lymphoma receiving CD19 CAR T cells, who have inflammation and swelling of the brain stem extending into the spinal cord, demonstrate extension of ICANS beyond the brain.3-5 Accordingly, when myelitis arises with severe cytokine release syndrome (CRS) or ICANS, it is highly suspicious for relatedness to CAR T-cell treatment, especially if there are simultaneous imaging abnormalities in the brain or brain stem. In these cases, spinal cord injury likely represents the same underlying pathophysiology as “typical” ICANS. This exact pattern of injury was seen in 1 patient in this series, who after infusion of CD19 CAR T cells developed bilateral flaccid leg weakness with concurrent grade 4 CRS and ICANS with severely depressed mental status (see figure). As identifying paresis in critically ill patients can be challenging, it may not become apparent until the mental status improves. Thus, in severe CRS/ICANS, a heightened index of suspicion and early involvement from neurology for close monitoring is critical for quickly identifying and addressing progressive central nervous system (CNS) toxicity.
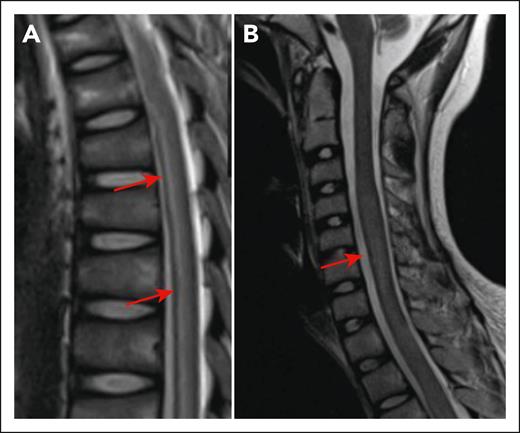
Myelitis following CD19 CAR T cells. (A) Longitudinally extensive T2 hyperintensity in the spinal cord of a 13-year-old presenting with bilateral leg weakness in the setting of CRS and ICANS (day 10 after CD19 CAR T-cell infusion). Arrows show areas of hyperintensity. (B) Increased expansion of the spinal cord T2 signal on day 21 after CAR T. The arrow shows increased expansion of the spinal cord. The figure has been adapted from Figure 1I,K in the article by Diorio et al that begins on page 1387.
Myelitis following CD19 CAR T cells. (A) Longitudinally extensive T2 hyperintensity in the spinal cord of a 13-year-old presenting with bilateral leg weakness in the setting of CRS and ICANS (day 10 after CD19 CAR T-cell infusion). Arrows show areas of hyperintensity. (B) Increased expansion of the spinal cord T2 signal on day 21 after CAR T. The arrow shows increased expansion of the spinal cord. The figure has been adapted from Figure 1I,K in the article by Diorio et al that begins on page 1387.
Distinguishing a potential CAR T-cell–associated neurotoxicity from alternative etiologies, however, can be especially challenging. Patients with relapsed or refractory leukemia are at high risk of neurologic sequelae from infection, chemotherapy or drug toxicity, and leukemic infiltration. Indeed, the remaining 4 cases presented by Diorio et al all have features that suggest contribution of other etiologies beyond CAR T-cell toxicity alone. Of the 3 other children who had acute spinal cord abnormalities on imaging, 2 had progressive leukemia, including 1 with extensive CNS involvement and 1 with likely cytomegalovirus encephalomyelitis. The final patient had quadriparesis but no evidence of spinal cord injury or residual leukemia. Here, diffuse white matter injury in the brain developed after recovery from both severe CRS and ICANS. The authors’ comprehensive description of these patients and outcomes (including pathology from autopsy findings, imaging results, cerebrospinal fluid [CSF] results, and serum and CSF cytokine profiling) highlights the type of approach needed as rare toxicities are first seen. As data accumulate, attribution of these cases may become more certain.
This series illustrates that spinal cord imaging should be performed in all patients who present with motor or sensory deficits after CAR T-cell infusion. Nonenhancing spinal cord T2 hyperintensities were common to all cases, but their patterns were not specific enough to secure a diagnosis. In this regard, systematic analysis of imaging findings in patients with ICANS or delayed neurotoxicity will be critical to understanding how to best interpret otherwise nonspecific findings in the context of multiple contributing factors. In addition to imaging, the authors also comprehensively measured biomarkers to provide insights into their clinical observations. Patients with paresis had a decreased inflammatory signature compared with unaffected patients, a finding that is hypothesis generating considering the limited sample size, clinical confounders, and potential impact of anti-inflammatory therapies. Nonetheless, the notion of a delayed, or noninflammatory, neurotoxicity after CAR T-cell therapy warrants further study, especially as patients are suboptimally responsive to current therapeutic approaches.
Endothelial activation6 or targeting of brain vascular mural cells7 with CD19 CAR T-cell constructs have been proposed as mechanisms of CD19 CAR T-cell toxicity. However, the question of possible on-target toxicity of CD19 CAR T cells remains unresolved. Although CD19 transcripts can be detected in fetal brain,7 there is no convincing evidence of its presence in the postnatal brain apart from the B cells that populate the healthy meninges. Notably, all patients reported by Diorio et al had a history of receiving CD19-targeted CAR T cells, including the 2 patients with limb weakness and disease progression following a CD22 CAR T-cell construct who had both developed severe ICANS with prior tisagenlecleucel therapy. Whether a history of severe ICANS predisposes to subsequent CAR T cell–associated or drug-associated neurotoxicity warrants further study.
As the CAR T-cell field continues to expand and more patients are treated, rare and novel forms or variants of established toxicities are to be expected. Similar to the recently described B-cell maturation antigen-associated movement disorders,8 astute clinical observations will need to be paired with appropriate imaging and comprehensive biomarker assessments to understand the underlying pathophysiology. Coexisting risk factors and diagnostic uncertainty remain a challenge in these rare toxicities, necessitating a broad differential. Although acute limb weakness that develops in the setting of severe neurotoxicity can be due to extension of the ICANS pathogenic process into the spinal cord, in all cases workup for leukemic CNS involvement and infection is warranted. This is especially critical if the presentation is delayed or atypical.
In conclusion, Diorio and colleagues report a first case series of paresis following CAR T cells in children, raising awareness of this complication. Despite being exceedingly rare, improved reporting and systematic assessment of paresis as an ICANS variant will help develop effective therapeutic and preventative interventions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal