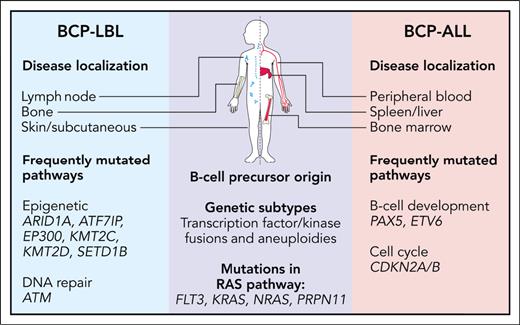
In this issue of Blood, Kroeze et al1 provide an in-depth analysis of the genomic and transcriptomic landscape of pediatric B-cell precursor lymphoblastic lymphoma (BCP-LBL). Pediatric B-cell precursor malignancies present in 2 distinct forms: BCP acute lymphoblastic leukemia (BCP-ALL), predominantly affecting the bone marrow and blood, and the less common BCP-LBL, characterized by its origin in extramedullary tissues and presentation as a solid tumor rather than widespread bone marrow involvement (see figure). Despite their morphologic and immunophenotypic similarities, most studies to date have focused on BCP-ALL, leaving BCP-LBL relatively understudied.
Overview of similarities and differences between pediatric BCP-LBL and BCP-ALL. Professional illustration by Patrick Lane, ScEYEnce Studios.
Overview of similarities and differences between pediatric BCP-LBL and BCP-ALL. Professional illustration by Patrick Lane, ScEYEnce Studios.
Pediatric BCP-ALL has been well characterized and has multiple recurrent somatic genetic alterations with biological as well as clinical significance.2 These genetic changes highlight the role of specific genes in the malignant transformation process, many of which are also crucial for normal B-cell development. However, because of the rarity of BCP-LBL and limited sample availability, cytogenetic and molecular genetic analyses are scarce, with previous studies hinting at a molecular genetic basis similar to BCP-ALL.3 Leveraging the comprehensive molecular classification of BCP-ALL,4 Kroeze et al conducted an extensive molecular characterization of the exomes and transcriptomes of 97 patients with BCP-LBL. Their findings offer valuable insights into the altered genes and their potential impact on biological pathways (see figure), helping to bridge the gap between these seemingly distinct conditions.5
The rarity of BCP-LBL, coupled with the challenges associated with analyzing predominantly formalin-fixed, paraffin-embedded (FFPE) tissue samples, has historically limited comprehensive genetic investigations of this disease entity. However, the successful integration of FFPE tissue samples in the study by Kroeze et al underscores the potential for applying these techniques and findings in routine clinical practice. The mutational landscape in BCP-LBL closely mirrors that observed in BCP-ALL, suggesting a substantial overlap in somatic mutations between the 2 conditions. Noteworthy differences include an overrepresentation of mutations in genes encoding epigenetic regulators, such as ARID1A, EP300, and KMT2D in BCP-LBL, and mutations in genes associated with B-cell development, such as PAX5 and ETV6 in BCP-ALL. This distinction holds potential clinical relevance, as epigenetic alterations may impact therapeutic strategy.6 However, a limitation of the study was the absence of matched normal controls, which restricted the mutational analysis to a predetermined set of known BCP-ALL genes. Future investigations are needed to confirm the somatic nature of these observations and evaluate the influence of other potentially mutated genes or pathways, further distinguishing these 2 diseases.
By applying gene expression profiling and genomic analyses, Kroeze et al demonstrate that most BCP-ALL molecular subtypes also exist in BCP-LBL. This pivotal observation emphasizes that BCP-LBL harbors genetic subtypes analogous to those observed in BCP-ALL, reinforcing the concept of 2 manifestations of the same entity.7 A particularly intriguing aspect of the study is the assignment of molecular genetic subtypes in BCP-LBL using RNA-sequencing data and a classifier developed for BCP-ALL.8 RNA-sequencing–based classification is an increasingly powerful tool for molecular subtype detection, and it is promising to see this study further validate its utility in this context.
A key clinical implication of this study is that the identification of molecular subtypes in BCP-LBL may lay the groundwork for risk-adapted treatment approaches. By applying BCP-ALL derived genetic risk categories to BCP-LBL, the authors identified high-risk genetics in 24% of BCP-LBL cases. Additionally, ∼7% of BCP-LBL cases exhibited tyrosine kinase or cytokine-receptor rearrangements, warranting further investigation given their potential clinical implications. Although relapses were more frequently observed in the high-risk group compared with the low-risk group, the cumulative incidence of relapse did not reach statistical significance, likely because of the overall low relapse rate in this BCP-LBL cohort (only 7 relapses). Larger studies will be imperative to determine the clinical utility of applying BCP-ALL risk stratification to BCP-LBL.
The reason why BCP-LBL manifests in the extramedullary tissues and not in the bone marrow is yet to be determined. Addressing this highly relevant question of tissue specificity will necessitate a combination of molecular and functional experimental approaches in the future. DNA methylation signatures imprint cell type and cell of origin, and are highly specific for BCP-ALL subtypes.9 These signatures could potentially elucidate questions regarding tissue-of-origin manifestation, cellular age, as well as proliferative history of the cells.10 Therefore, future studies focusing on DNA methylation or other epigenetic markers would be needed, particularly in light of the increased frequency of mutated epigenetic regulator genes observed in BCP-LBL.
In conclusion, this study represents the first comprehensive genomic analysis bridging the gap between BCP-ALL and BCP-LBL. Although questions still remain, it elucidates their shared genetic landscape and offers insights into the biology of BCP-LBL and its relationship to BCP-ALL, and may also serve as a basis for designing future diagnostic and risk stratification approaches.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal