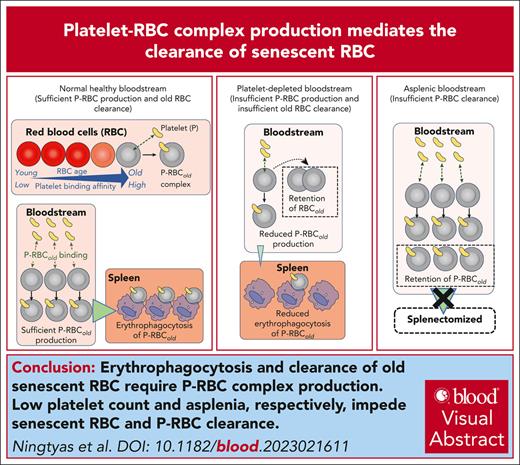
Senescent RBCs are bound to platelets, forming P-RBC complexes that are selectively consumed by erythrophagocytes.
A sufficient supply of platelets is required to maintain efficient complex-dependent clearance of senescent RBCs.
Visual Abstract
In humans, ∼0.1% to 0.3% of circulating red blood cells (RBCs) are present as platelet-RBC (P-RBC) complexes, and it is 1% to 2% in mice. Excessive P-RBC complexes are found in diseases that compromise RBC health (eg, sickle cell disease and malaria) and contribute to pathogenesis. However, the physiological role of P-RBC complexes in healthy blood is unknown. As a result of damage accumulated over their lifetime, RBCs nearing senescence exhibit physiological and molecular changes akin to those in platelet-binding RBCs in sickle cell disease and malaria. Therefore, we hypothesized that RBCs nearing senescence are targets for platelet binding and P-RBC formation. Confirming this hypothesis, pulse-chase labeling studies in mice revealed an approximately tenfold increase in P-RBC complexes in the most chronologically aged RBC population compared with younger cells. When reintroduced into mice, these complexes were selectively cleared from the bloodstream (in preference to platelet-free RBC) through the reticuloendothelial system and erythrophagocytes in the spleen. As a corollary, patients without a spleen had higher levels of complexes in their bloodstream. When the platelet supply was artificially reduced in mice, fewer RBC complexes were formed, fewer erythrophagocytes were generated, and more senescent RBCs remained in circulation. Similar imbalances in complex levels and senescent RBC burden were observed in humans with immune thrombocytopenia (ITP). These findings indicate that platelets are important for binding and clearing senescent RBCs, and disruptions in platelet count or complex formation and clearance may negatively affect RBC homeostasis and may contribute to the known risk of thrombosis in ITP and after splenectomy.
Introduction
Platelets are endowed with a multitude of cell adhesion molecules that support physical interactions with most other blood cell types, including red blood cells (RBCs). One well-known interaction is the binding of platelets to RBCs in venous thrombi, in which RBCs provide a scaffold for platelet aggregation and stabilize clot structure through the release of molecules such as adenosine diphosphate.1,2 Additionally, constant and seemingly harmless aggregates called platelet-RBC (P-RBC) complexes are present in the normal bloodstream,3-5 engaging 1% to 2% of mouse RBCs6,7 and 0.1% to 0.3% of human RBCs.8,9 The origin, purpose, and impact of P-RBC complexes remain unknown. However, in conditions that compromise RBC health, such as sickle cell disease (SCD)10,11 and hemodialysis,12 P-RBC complexes occur at 2 to 3 times greater frequency than in healthy individuals and play a role in pathogenesis and increase the risk of thrombosis. In malaria, platelet binding to Plasmodium-infected RBC is ∼10 times greater than uninfected cells and serves a protective function against infection.6-8 The characteristics that distinguish platelet-bound cells from unbound cells in these examples have not been investigated. Diseased RBCs are presumed to interact more with platelets by virtue of RBC membrane changes that activate platelets10,11 and/or express disease-specific ligands.13 Under in vitro conditions, additional ligand-receptor interactions are known to facilitate P-RBC binding,14-16 suggesting that molecular redundancy may be involved in P-RBC complex formation.
As RBC approach senescence, which occurs after ∼60 days in mice and 120 days in humans, they undergo membrane changes that promote recognition and removal by phagocytes of the reticuloendothelial system, a process known as erythrophagocytosis (EP).17,18 These EP-promoting changes include externalization of phosphatidylserine (PS) on the membrane,19,20 downregulation of the CD47 self-antigen molecule,21,22 and opsonization of neoantigens generated by oxidative damage.23 An added layer of surveillance involves spleen-based mechanical filtration of cells with age and damage-induced reductions in cell deformability.24 These mechanisms ensure that senescent RBCs do not accumulate in the circulation, because they possess increased procoagulant activity and raise the risk of thromboembolic complications.2
Diseased RBCs that generate excessive P-RBC complexes exhibit abnormal PS exposure and oxidative damage effects similar to aged and senescent cells.25-30 Hence, we investigated whether chronologically old but healthy RBCs also exhibited an increased propensity for P-RBC formations and explored how platelet binding influenced their clearance from the bloodstream. Mice were subjected to whole blood pulse-labeling, and blood samples were analyzed to quantify and characterize platelet binding to differently aged RBCs, and in other experiments, the effects of splenectomy and platelet depletion in mice and humans on senescent RBC clearance and EP were investigated. The results enabled us to deduce that senescent RBCs are the preferential target for platelet binding and that P-RBC generation and subsequent consumption in the reticuloendothelial system serves an important function in maintaining senescent RBC clearance.
Methods
Mice, antibodies, and other reagents
The mice, antibodies, and other reagents used in this study are described in supplemental Methods, available on the Blood website.
Murine P-RBC complex studies
Mice were injected IV (lateral tail vein) with N-hydroxysuccinimide ester (NHS)-biotin or carboxyfluorescein succinimidyl ester (CFSE) for RBC chronological age studies, as described previously,31,32 and platelet-specific anti-murine GPIbβ-DyLight649 (X649, emfret Analytics) for platelet chronological age analyses.33 For complex clearance studies, separately isolated mouse platelets and RBCs were labeled with CFSE and Atto633, respectively, coincubated (5:1::platelet:RBC) with gentle mixing (2 hours room temperature), and then transfused into recipients. Blood samples (collected from the tail) were stained and analyzed within 1 to 2 hours of collection. Imaging flow cytometry was performed on an ImageStreamX Mk II instrument (Amnis Corporation, Seattle, WA) equipped with 488 nm (180 mV) and 785 nm (0.57 mV) lasers and INSPIRE 200.1.620.0 acquisition software. Further details of these methods are described in supplemental Methods.
Platelet and phagocyte depletion and splenectomy
Mice were platelet-depleted using anti-mouse glycoprotein 1b α chain (GPIbα) or αIIbβ3 antibodies according to manufacturer and published protocols34-36 (emfret Analytics), and counting beads were used to enumerate platelets and RBCs in some experiments. Mice were administered clodronate liposomes (Liposoma) to deplete macrophages; maximal depletion occurs within 24 hours, and recovery occurs after ∼5 days in the liver and 1 to 2 weeks in the spleen.37,38 Splenectomized mice were generated by surgical ligation of the attachments and vessels to the hilum and allowed to recover for 1 week before use.
EP studies
EP studies in mice were performed according to previously described methods,39 in which isolated mouse RBCs labeled with the red-fluorescent lipophilic membrane dye PKH26 were transfused into recipients (with or without addition of platelet-labeling antibody, X649), and blood, spleen, and liver phagocytes were assessed for labeled RBCs and platelets by flow cytometry or by immunostaining frozen sections from fixed organs. See supplemental Methods for details.
Studies of patients who underwent splenectomy and those with ITP
P-RBC complexes were measured by flow cytometry (CytoFLEX, Beckman Coulter) on archived venous blood samples (paraformaldehyde fixed) from a longitudinal cohort of 16 patients undergoing splenectomy for trauma-related incidents40 and a second cohort of 20 spleen-intact healthy controls,8 all residing in Timika, Papua, Indonesia. See supplemental Figure 4 for the gating strategy. This study was approved by the Human Research Ethics Committees of Menzies School of Health Research, Australia (10-1397) and Gadjah Mada University, Indonesia (KE/FK/0912/EC). P-RBC complexes and markers of aged RBCs were measured in freshly collected trisodium citrate anticoagulated whole blood, after provision of informed consent, from healthy donors and patients with clinically diagnosed immune thrombocytopenia (ITP). ITP diagnosis was based on the exclusion of alternative causes for thrombocytopenia and otherwise meeting international consensus criteria.41 This study was approved by the Australian University Human Research Ethics Committee (2017/924).
Statistics
Data were analyzed using GraphPad Prism software. Unless stated otherwise, statistical comparisons and P values were determined using 2-way analysis of variance with Sidak multiple comparison. Curves were fitted to data using nonlinear regression (least squares method with medium convergence and no weighting), unless stated otherwise.
Results
Platelets form P-RBC complexes in the bloodstream with the most chronologically aged RBCs
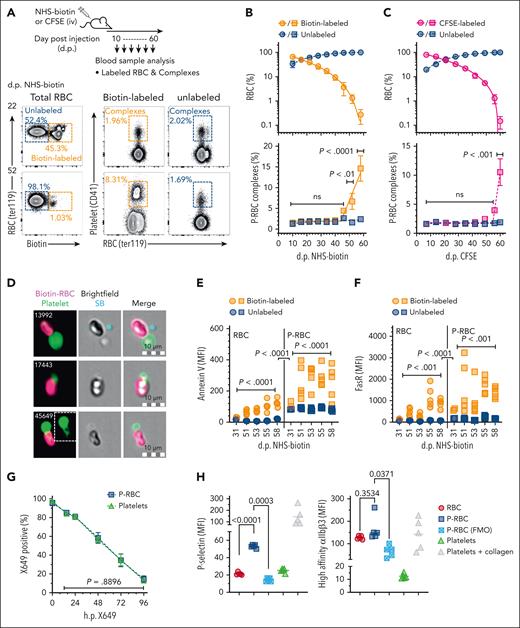
Mice were subjected to a pulse-chase labeling protocol, in which, after an IV bolus of NHS-biotin or CFSE to uniformly label all blood cells, RBCs were sampled and analyzed according to their chronological age and platelet binding propensity (Figure 1A). Over 60 days, the proportions of labeled RBCs steadily declined consistent with the production of new (unlabeled) and removal of old (labeled) cells (Figure 1B-C, top). The oldest cells detected were aged between 58 and 60 days, which agreed with other reports of the maximal RBC life span in this species,31,32 as did our estimates of RBC half-life (18.3 days; 95% confidence interval, 14.3-25.0).42,43 As expected, the concentrations of total (unlabeled and labeled) P-RBCs were relatively consistent and unchanged during the entire 60 days, ranging from ∼80 million/mL to 150 million/mL and comprising 1.5% to 2.7% of the RBCs (supplemental Figure 1A). In conjunction with the loss of labeled RBCs, concentrations of labeled P-RBCs similarly declined from ∼60 million/mL on day 10 to 1.5 million/mL by day 58 in the NHS-biotin–treated mice (supplemental Figure 1B). However, comparing the relative proportions of P-RBCs in the labeled vs unlabeled RBCs revealed they were similar up until 45 to 50 days but were then significantly higher in the labeled (older) cells at later time points, including 52 days (average ± standard error of the mean = 6.7% ± 5.1%; P < .01) and 58 days (15% ± 8.1%; P < .0001) in the NHS-biotin–treated mice and 60 days (11% ± 5.6%; P < .0001) in the CFSE-treated mice (Figure 1B-C, bottom). Overall, both cell labeling protocols showed the proportion of P-RBC complexes were 2 to 10 times greater in the old (50-60 days) than in younger (<50 day) RBCs. Imaging flow cytometry showed P-RBC complexes were composed of single platelets that appeared physically connected to individual RBCs (Figure 1D; supplemental Figure 2). Samples from a cohort of NHS-biotin–pulsed mice were analyzed for markers of senescent RBCs to verify the aged RBC phenotype of the complexes. As expected, the oldest RBC (aged ≥51 days), compared with younger and unlabeled RBC, contained significantly higher levels of both annexin V, which indicates amounts of membrane-exposed PS,44-46 and Fas receptor (FasR), which is expressed on RBCs undergoing senescence and apoptosis47 (Figure 1E-F, RBC panels). Similar relationships were observed in the P-RBC complexes (Figure 1E-F, P-RBC panels), collectively indicating that annexin V and FasR staining predicted the chronological age of RBCs and P-RBC complexes independent of the pulse-chase label. Furthermore, the levels of both markers were significantly higher in the platelet-bound vs platelet-free RBCs (Figure 1E and F, comparing P-RBC vs RBC panels). These differences confirm the aforementioned observations that P-RBC complexes are mostly restricted to older-aged RBCs and, by deduction, indicate that RBC chronological age is an important determinant in platelet-cell binding. Pulse-chase labeling of mice with a platelet-specific anti-GPIbβ antibody (X649), which does not cause or interfere with platelet activation or adhesion in vivo,33 revealed no bias in the chronological age of the RBC-bound platelets (Figure 1G). One marker of platelet activation, P-selectin, which is exposed on the surface of degranulated platelets, was increased on P-RBCs compared with RBCs, although a second marker, high-affinity αIIbβ3 integrin, which binds fibrin, was not different (noting its relatively high background on RBC; Figure 1H), indicating a modest activated state of the RBC-bound platelets.
Identification and characterization of P-RBC complexes in chronologically aged RBC. (A) Mice were pulse-labeled with NHS-biotin or CFSE (IV delivery), and blood samples collected from the same mice after several intervals were analyzed by flow cytometry using the method depicted. Proportions of total RBCs (ter119+) labeled with and without biotin or CFSE were quantified and gated separately, and each population analyzed for proportions of P-RBC complexes (CD41+). Each row of panels shows the proportions of biotin-labeled and unlabeled RBC and complexes in the same mouse 22 and 52 days after NHS-biotin. (B-C) Proportions of labeled and unlabeled RBCs (top, circles) and labeled and unlabeled P-RBC complexes (bottom, squares) determined at indicated times after NHS-biotin (B) (7 mice and 2 independent experiments) or CFSE (C) (6 mice). Symbols and bars indicate means and standard error of the mean (SEM). Comparisons shown between labeled and unlabeled complexes at indicated time points using 2-way analysis of variance (ANOVA) after Tukey correction for multiple testing (ns; P > .05). (D) Photomicrographs of P-RBC complexes identified in NHS-biotin–injected mice using imaging flow cytometry analysis. Single platelets are observed physically connected to RBCs and coating of RBC with platelet material (event 45 649, inset). (E-F) Levels of annexin V and FasR, respectively, on biotin-labeled and unlabeled RBCs (circles, left panels) and biotin-labeled and unlabeled P-RBC complexes (squares, right panels) at indicated times after NHS-biotin injection. Comparisons shown between labeled and unlabeled cells or complexes using 2-way ANOVA, and between labeled cells and labeled complexes using 3-way ANOVA, after Sidak correction for multiple testing. (G) Mean proportions of platelets and P-RBC labeled with anti-GPIbβ-DyLight649 (X649 positive) in blood samples from 4 mice collected at the indicated times after X649 administration (IV), compared using 2-way ANOVA. Error bars indicate SD. (H) Levels of P-selectin and high-affinity αIIbβ3 integrin on RBCs and P-RBC complexes in blood samples from 5 mice, along with levels measured in the absence of staining antibody (FMO) and in platelets either untreated or treated with 10 μg/mL collagen, as respective negative and positive stain controls. Comparisons indicated using 1-way ANOVA. dp, days past treatment; FMO, fluorescence minus one control; hp, hours past treatment; ns, not significant P > .05; SB, speed bead calibration bead; SD, standard deviation.
Identification and characterization of P-RBC complexes in chronologically aged RBC. (A) Mice were pulse-labeled with NHS-biotin or CFSE (IV delivery), and blood samples collected from the same mice after several intervals were analyzed by flow cytometry using the method depicted. Proportions of total RBCs (ter119+) labeled with and without biotin or CFSE were quantified and gated separately, and each population analyzed for proportions of P-RBC complexes (CD41+). Each row of panels shows the proportions of biotin-labeled and unlabeled RBC and complexes in the same mouse 22 and 52 days after NHS-biotin. (B-C) Proportions of labeled and unlabeled RBCs (top, circles) and labeled and unlabeled P-RBC complexes (bottom, squares) determined at indicated times after NHS-biotin (B) (7 mice and 2 independent experiments) or CFSE (C) (6 mice). Symbols and bars indicate means and standard error of the mean (SEM). Comparisons shown between labeled and unlabeled complexes at indicated time points using 2-way analysis of variance (ANOVA) after Tukey correction for multiple testing (ns; P > .05). (D) Photomicrographs of P-RBC complexes identified in NHS-biotin–injected mice using imaging flow cytometry analysis. Single platelets are observed physically connected to RBCs and coating of RBC with platelet material (event 45 649, inset). (E-F) Levels of annexin V and FasR, respectively, on biotin-labeled and unlabeled RBCs (circles, left panels) and biotin-labeled and unlabeled P-RBC complexes (squares, right panels) at indicated times after NHS-biotin injection. Comparisons shown between labeled and unlabeled cells or complexes using 2-way ANOVA, and between labeled cells and labeled complexes using 3-way ANOVA, after Sidak correction for multiple testing. (G) Mean proportions of platelets and P-RBC labeled with anti-GPIbβ-DyLight649 (X649 positive) in blood samples from 4 mice collected at the indicated times after X649 administration (IV), compared using 2-way ANOVA. Error bars indicate SD. (H) Levels of P-selectin and high-affinity αIIbβ3 integrin on RBCs and P-RBC complexes in blood samples from 5 mice, along with levels measured in the absence of staining antibody (FMO) and in platelets either untreated or treated with 10 μg/mL collagen, as respective negative and positive stain controls. Comparisons indicated using 1-way ANOVA. dp, days past treatment; FMO, fluorescence minus one control; hp, hours past treatment; ns, not significant P > .05; SB, speed bead calibration bead; SD, standard deviation.
P-RBC complexes are rapidly and selectively removed from circulation via phagocyte-dependent functions
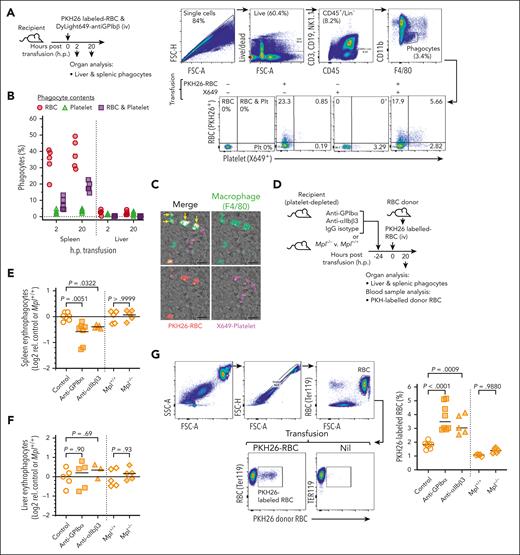
Aged and damaged RBCs are efficiently removed from circulation by the mononuclear phagocytic system. We hypothesized that complexes are similarly cleared and, to investigate this, compared the circulation lifespans of P-RBC complexes, RBCs, and platelets that were labeled ex vivo and transfused into recipient mice. The donor platelets were labeled with CFSE and the donor RBCs with Atto633, and, after coincubation, produced a mixture of colabeled P-RBC complexes as well as single-labeled platelets and RBCs (Figure 2A; supplemental Figure 3). After transfusion, the proportions of donor complexes (relative to donor and recipient complexes combined) decreased significantly more rapidly than either donor RBCs or donor platelets (P < .0001), and by 24 hours, <5% of the initial complexes remained compared with 20% of platelets and 60% of RBCs (Figure 2B, left). The corresponding clearance rate of the complexes was, on average, 7 times faster than platelet-free RBCs (P < .0001) and 3 times faster than cell-free platelets (P < .001; Figure 2B, right). Therefore, the complexes were cleared more rapidly from the circulation than both RBCs and platelets. Cohorts of mice were also treated before transfusion with clodronate liposomes to systemically deplete phagocytes and, thus, determine their importance. Clearance of the donor complexes was significantly reduced by almost fivefold compared with control mice (P < .0001), whereas the clearance of the platelets and RBCs were not obviously affected (Figure 2C). Thus, phagocytes are required to mediate the clearance of P-RBC complexes. In splenectomized mice, the clearance rates of transfused complexes were slightly but not significantly reduced compared with spleen-intact controls (Figure 2D); however, the proportions of endogenous complexes were significantly higher (P = .009; Figure 2E). This increase, although potentially masking any changes in the donor complexes, suggests the spleen is also required for complex removal. Subsequently, a longitudinal cohort of 16 patients referred for splenectomy and 20 population-matched healthy spleen-intact control individuals were studied for P-RBC complex levels (Table 1; supplemental Figure 4; supplemental Methods). Before surgery, the patients had significantly lower RBCs (P = .0011), hematocrit (P < .0001), and hemoglobin (P = .021) than the healthy control group, consistent with their splenic RBC congestion.40 Platelets were significantly elevated on day 60 (P < .0001) and day 360 after surgery (P = .023), indicative of splenectomy-induced thrombocytosis, which is widely reported,48,49 along with increases in RBC indices suggesting a recovery-driven erythropoiesis and/or a lack of retention of RBCs by a congested spleen (Table 1).50 As collective groups, P-RBC complexes in patients before surgery were comparable with those in healthy controls (mean ± standard error of the mean = 0.8 × 103/mL ± 0.1 × 103/mL vs 1.1 × 103/mL ± 0.5 × 103/mL; P = .98) but were significantly higher on day 60 (11.6 ± 1.5; P < .0001) and marginally but not significantly higher on day 360 (4.1% ± 1.1%; P = .103; Figure 2F). Pairwise comparison of the platelet-corrected P-RBC levels confirmed that the complexes were significantly elevated 60 days after surgery (P < .001) by an average 21.5-fold increase (Figure 2G). Thus, in both mice and humans, the clearance of P-RBC complexes in the circulation is mediated by the spleen.
Characterization of P-RBC complex clearance from the bloodstream. (A) Platelets and RBCs were separately isolated from donor mice, labeled with CFSE and atto633, respectively, coincubated to generate P-RBC complexes (inset image; platelet, green; RBC, red), and then transfused into recipient mice, either untreated, pretreated with clodronate or phosphate-buffered saline (PBS) liposomes (phagocyte depletion), or splenectomized. Blood samples collected at indicated times after transfusion (hours posttransfusion [h.p.]) were analyzed by flow cytometry using the method depicted. Total (recipient plus donor) populations of RBCs (ter119+), platelets (CD41+), and P-RBC complexes (ter119+ CD41+) were gated and then separately analyzed for proportions of respective donor cells (atto633+, CFSE+, or atto633+ and CFSE+). The panels show the same mouse analyzed 0.17 (10 minutes) and 18 hp. (B) Proportions of transfused RBCs, platelets, and P-RBC complexes in the bloodstream of recipient mice measured up to 24 h.p. transfusion (left) and the clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly lower than those of platelets and RBCs (P < .0001), and platelet levels were significantly lower than RBC numbers at indicated time points (P < .001). Nine mice and 3 independent experiments. (C) Proportions of transfused RBCs, platelets, and P-RBC complexes in recipient mice treated 24 hours prior with clodronate or PBS (control) liposomes (left) and clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly higher in clodronate vs control mice (P < .001 and .0001, respectively); 10 mice and 2 experiments. (D) Proportions of transfused RBC, platelet, and P-RBC complex levels in recipient mice previously splenectomized (splenec.) compared with nonsplenectomized (intact) mice (left), clearance rates derived from these data (right), and (E) relative proportions of recipient (unlabeled) RBCs, platelets, and complexes; 3 and 4 mice, respectively. (B-D) Dashed lines indicate decay curves fitted using nonlinear regression. (B-E) Symbols and bars indicate means and SEM or individuals; comparisons shown using ANOVA. (F) P-RBC complexes in 20 population-matched spleen-intact individuals (healthy) and 16 patients measured before (pre) and 60 and 360 days (6 of 16 patients) after surgical removal of their spleen (mean, 95% confidence interval, and minimum and maximum values; comparisons shown using ANOVA), and (G) the P-RBC:platelet ratio for the same patients plotted longitudinally. Comparisons shown using Kruskal-Wallis test. Plt, platelet.
Characterization of P-RBC complex clearance from the bloodstream. (A) Platelets and RBCs were separately isolated from donor mice, labeled with CFSE and atto633, respectively, coincubated to generate P-RBC complexes (inset image; platelet, green; RBC, red), and then transfused into recipient mice, either untreated, pretreated with clodronate or phosphate-buffered saline (PBS) liposomes (phagocyte depletion), or splenectomized. Blood samples collected at indicated times after transfusion (hours posttransfusion [h.p.]) were analyzed by flow cytometry using the method depicted. Total (recipient plus donor) populations of RBCs (ter119+), platelets (CD41+), and P-RBC complexes (ter119+ CD41+) were gated and then separately analyzed for proportions of respective donor cells (atto633+, CFSE+, or atto633+ and CFSE+). The panels show the same mouse analyzed 0.17 (10 minutes) and 18 hp. (B) Proportions of transfused RBCs, platelets, and P-RBC complexes in the bloodstream of recipient mice measured up to 24 h.p. transfusion (left) and the clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly lower than those of platelets and RBCs (P < .0001), and platelet levels were significantly lower than RBC numbers at indicated time points (P < .001). Nine mice and 3 independent experiments. (C) Proportions of transfused RBCs, platelets, and P-RBC complexes in recipient mice treated 24 hours prior with clodronate or PBS (control) liposomes (left) and clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly higher in clodronate vs control mice (P < .001 and .0001, respectively); 10 mice and 2 experiments. (D) Proportions of transfused RBC, platelet, and P-RBC complex levels in recipient mice previously splenectomized (splenec.) compared with nonsplenectomized (intact) mice (left), clearance rates derived from these data (right), and (E) relative proportions of recipient (unlabeled) RBCs, platelets, and complexes; 3 and 4 mice, respectively. (B-D) Dashed lines indicate decay curves fitted using nonlinear regression. (B-E) Symbols and bars indicate means and SEM or individuals; comparisons shown using ANOVA. (F) P-RBC complexes in 20 population-matched spleen-intact individuals (healthy) and 16 patients measured before (pre) and 60 and 360 days (6 of 16 patients) after surgical removal of their spleen (mean, 95% confidence interval, and minimum and maximum values; comparisons shown using ANOVA), and (G) the P-RBC:platelet ratio for the same patients plotted longitudinally. Comparisons shown using Kruskal-Wallis test. Plt, platelet.
Baseline characteristics of spleen-intact controls and patients undergoing splenectomy
| Parameter . | Spleen-intact controls (n = 20) . | Patients undergoing splenectomy (n = 16) . | ||
|---|---|---|---|---|
| Day 0 . | Day 60 . | Day 360∗ . | ||
| Age, y | 28 (26-31) | 27 (17-35) | ||
| Males, % (n/N) | 40 (8/20) | 81 (13/16) | ||
| Papuan, % (n/N) | 95 (19/20) | 25 (4/16) | ||
| Splenectomy due to trauma, % (n/N) | — | 88 (14/16) | ||
| Platelet counts, ×103/μL blood | 194 (156-228) | 221 (111-279) P = .99† | 612 (444-708) P < .0001‡ | 379 (262-541) P = .023‡ |
| Hemoglobin, g/dL | 11.9 (11-15) | 11.3 (8.3-12.6) P = .021† | 13 (12.4-14.3) P = .012‡ | 14.8 (12.6-15.7) P = .003‡ |
| Hematocrit, % | 36.4 (32.9-43.6) | 32.3 (24.3-37.8) P < .0001† | 39.6 (38.1-41.8) P = .002‡ | 43.5 (39.1-46.3) P = .018‡ |
| RBC count, ×109/μL blood | 5.5 (4.9-5.8)§ | 4.0 (3.2-4.7) P = .0011† | 5.0 (4.7-5.4) P = .0002‡ | 5.5 (4.7-5.8) P = .0007‡ |
| Peripheral microscopic Plasmodium parasitemia, % (n/N) | 0 | 31 (5/16) | 0 | 33 (2/6) |
| Parameter . | Spleen-intact controls (n = 20) . | Patients undergoing splenectomy (n = 16) . | ||
|---|---|---|---|---|
| Day 0 . | Day 60 . | Day 360∗ . | ||
| Age, y | 28 (26-31) | 27 (17-35) | ||
| Males, % (n/N) | 40 (8/20) | 81 (13/16) | ||
| Papuan, % (n/N) | 95 (19/20) | 25 (4/16) | ||
| Splenectomy due to trauma, % (n/N) | — | 88 (14/16) | ||
| Platelet counts, ×103/μL blood | 194 (156-228) | 221 (111-279) P = .99† | 612 (444-708) P < .0001‡ | 379 (262-541) P = .023‡ |
| Hemoglobin, g/dL | 11.9 (11-15) | 11.3 (8.3-12.6) P = .021† | 13 (12.4-14.3) P = .012‡ | 14.8 (12.6-15.7) P = .003‡ |
| Hematocrit, % | 36.4 (32.9-43.6) | 32.3 (24.3-37.8) P < .0001† | 39.6 (38.1-41.8) P = .002‡ | 43.5 (39.1-46.3) P = .018‡ |
| RBC count, ×109/μL blood | 5.5 (4.9-5.8)§ | 4.0 (3.2-4.7) P = .0011† | 5.0 (4.7-5.4) P = .0002‡ | 5.5 (4.7-5.8) P = .0007‡ |
| Peripheral microscopic Plasmodium parasitemia, % (n/N) | 0 | 31 (5/16) | 0 | 33 (2/6) |
Median (interquartile range) shown, unless specified otherwise.
Splenectomized patients on day 360 restricted to n = 6.
Comparison spleen-intact controls vs day 0 patients (1-way analysis of variance).
Comparison day 0 vs day 60 or day 360 patients (1-way analysis of variance).
RBC counts missing for 16 spleen-intact individuals.
P-RBC complexes are phagocytosed by splenic phagocytes
The results obtained prompted us to investigate whether splenic red pulp macrophages (RPMs), the major site of RBC turnover, and/or hepatic phagocytes, which also consume RBC under certain conditions,51 were able to phagocytose complexes. In vivo phagocytosis assays were conducted, in which mice were transfused with PKH26-labeled RBCs and a platelet-specific fluorophore–conjugate antibody (X649) to generate detectable P-RBC complexes composed of recipient platelets and donor RBCs; these occurred in similar proportions to endogenous levels (supplemental Figure 5). After transfusion, RPMs and liver phagocytes were analyzed to identify those containing only donor RBCs (erythrophagocytes) or both RBCs and platelets (platelet-containing erythrophagocytes; Figure 3A). After 2 and 20 hours, the greatest proportions of erythrophagocytes were observed in RPMs (20%-50%) compared with liver phagocytes (1%-4%), consistent with the spleen’s dominant role in RBC consumption. However, an additional 4% to 13% (2 hours) and 14% to 22% (20 hours) of erythrophagocytes also contained platelets, indicative of phagocytosis of P-RBC complexes. The alternative explanation that these resulted from bystander ingestion of platelets was considered less likely because the frequency of phagocytes containing platelets without RBC was only 1% to 5% (Figure 3B). As confirmation, immunostaining of sections of spleen from mice that received a transfusion showed frequent examples of RPM containing PKH26- and X649-labeled materials (Figure 3C; supplemental Figure 6), indicative of phagocytosed complexes. The flow cytometry results also revealed a significant disparity between the relatively large proportions of platelet-containing erythrophagocytes (at least 25%) and much smaller proportions of P-RBC complexes in the bloodstream available for phagocytosis (1%; supplemental Figure 5B). This suggested platelets in some way enhance EP, and to investigate this, mice were depleted of platelets before being transfused with the PKH26-labeled RBCs. Two different platelet depletion antibodies were used, anti-GPIbα and anti-αIIbβ3; each is known to induce severe platelet depletion (<1% normal platelet levels) lasting 2 to 3 days,34-36 which we confirmed (supplemental Figure 7). In additional experiments, PKH26-labeled RBCs were transferred to mice lacking the thrombopoietin receptor gene Mpl (Mpl−/−). These mice have ∼10% of normal platelet numbers, which are sufficient to maintain hemostasis and prevent active bleeding.52 In the antibody platelet-depleted mice, splenic erythrophagocytes were significantly reduced by 25% to 35% compared with control mice (P < .01 and P < .05 for each respective antibody), but there were no differences between the Mpl−/− and control wild-type mice (Figure 3E). None of the platelet depletions caused changes in the liver erythrophagocyte levels (Figure 3F). In addition to reduced EP, levels of PKH26-labeled RBCs in the bloodstream of the antibody-treated mice were almost twice as those of controls (P < .0001 and P < .001 for each respective antibody; Figure 3G). Thus, under conditions of severe but not mild reductions in platelet count, EP and the clearance of RBCs were perturbed. Collectively, this suggests that not only are P-RBC complexes phagocytosed by RPM, but platelets are also required for efficient EP.
Characterization of phagocytosis of P-RBC complexes. (A) Mice were transfused with PKH26-labeled (donor) RBCs and platelet-labeling antibody (anti-GPIbβ-DyLight649, X649). Single-cell liver and spleen preparations harvested 2 or 20 hp were stained and analyzed by flow cytometry using the method depicted. Phagocytes were defined as CD45+, CD3ε–, CD19–, and NK1.1– (Lin–); CD11blo and F4/80hi and subsequently classified as those containing only donor RBCs (PKH26+, X649–), only platelets (PKH26−, X649+), or both RBCs and platelets (PKH26+, X649+). Bottom panels show proportions of the different phagocyte types in spleens from nontransfused mice and those transfused with only donor RBC or only X649 (as controls) or both RBC and X649. (B) Proportions of phagocytes in spleen and liver containing donor RBCs, platelets, or both RBCs and platelets; 5 mice and 1 experiment. (C) Fluorescence microscopy images of spleen collected 20 hp showing macrophages (F4/80-stained) containing donor-derived RBCs (PKH26-labeled, arrowheads) and both donor RBCs and X649-labeled platelets (arrows); Scale bar, 10 μm. Additional images from other mice shown in supplemental Figure 6. (D-G) Cohorts of mice were pretreated 24 hours prior with platelet-depleting (anti-GPIbα or anti-αIIbβ3) or IgG isotype (control) antibodies and along with Mpl−/− and Mpl+/+ mice, transfused with PKH26-labeled donor RBCs (D) and analyzed after 20 hours for proportions of splenic (E) and liver (F) erythrophagocytes. (G) Blood samples collected at take down were analyzed for PKH26-labeled RBC levels in bloodstream using the gating strategy depicted. Singlet RBC were identified by anti-ter119 staining. A nontransfused mouse (nil) is shown for comparison; 5 to 8 mice per group, 3 independent experiments. Symbols and horizontal bars indicate individual mice and means, respectively. Comparisons shown using ANOVA. IgG, immunoglobulin G.
Characterization of phagocytosis of P-RBC complexes. (A) Mice were transfused with PKH26-labeled (donor) RBCs and platelet-labeling antibody (anti-GPIbβ-DyLight649, X649). Single-cell liver and spleen preparations harvested 2 or 20 hp were stained and analyzed by flow cytometry using the method depicted. Phagocytes were defined as CD45+, CD3ε–, CD19–, and NK1.1– (Lin–); CD11blo and F4/80hi and subsequently classified as those containing only donor RBCs (PKH26+, X649–), only platelets (PKH26−, X649+), or both RBCs and platelets (PKH26+, X649+). Bottom panels show proportions of the different phagocyte types in spleens from nontransfused mice and those transfused with only donor RBC or only X649 (as controls) or both RBC and X649. (B) Proportions of phagocytes in spleen and liver containing donor RBCs, platelets, or both RBCs and platelets; 5 mice and 1 experiment. (C) Fluorescence microscopy images of spleen collected 20 hp showing macrophages (F4/80-stained) containing donor-derived RBCs (PKH26-labeled, arrowheads) and both donor RBCs and X649-labeled platelets (arrows); Scale bar, 10 μm. Additional images from other mice shown in supplemental Figure 6. (D-G) Cohorts of mice were pretreated 24 hours prior with platelet-depleting (anti-GPIbα or anti-αIIbβ3) or IgG isotype (control) antibodies and along with Mpl−/− and Mpl+/+ mice, transfused with PKH26-labeled donor RBCs (D) and analyzed after 20 hours for proportions of splenic (E) and liver (F) erythrophagocytes. (G) Blood samples collected at take down were analyzed for PKH26-labeled RBC levels in bloodstream using the gating strategy depicted. Singlet RBC were identified by anti-ter119 staining. A nontransfused mouse (nil) is shown for comparison; 5 to 8 mice per group, 3 independent experiments. Symbols and horizontal bars indicate individual mice and means, respectively. Comparisons shown using ANOVA. IgG, immunoglobulin G.
Platelets are required to maintain senescent RBC clearance
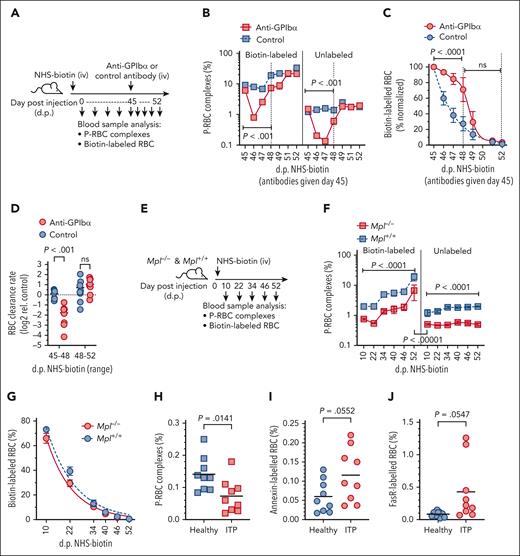
A requirement for phagocytes in senescent RBC clearance has been demonstrated previously,53 and we confirmed these findings, showing that systemic phagocyte depletion (clodronate treatment) significantly impeded the clearance of aged (>42 days) RBCs in mice subjected to NHS-biotin pulse-chase labeling (supplemental Figure 8). As our observations suggested that platelet binding to RBC promotes RBC clearance through ingestion by phagocytes and that P-RBC complexes comprise predominantly senescent RBC, we hypothesized that platelets were required for senescent RBC clearance. To test this, NHS-biotin–pulsed mice were subjected to platelet depletion (anti-GPIbα treatment) at the point when increased P-RBC generation to aged RBCs was first observed (day 45). The levels of P-RBCs and rates of RBC clearance before and after platelet depletion were compared with those of platelet-replete control mice (Figure 4A). On day 1 after platelet depletion, P-RBC levels were reduced more than tenfold and remained significantly depressed for 3 days before recovering to normal levels on days 6 to 7 (Figure 4B). These kinetics were comparable with the drop and recovery of platelets after either anti-GPIbα or -αIIbβ3 antibody treatment (supplemental Figure 7), indicating that complex formation requires a constant supply of platelets. Strikingly, the reductions in platelets and complexes coincided with a marked retention of biotin-labeled RBCs; this difference lasted for several days and was reproducible in multiple experiments (supplemental Figure 9). Regression analysis showed the effect was most significant for the first 3 days after platelet depletion (Figure 4C) and corresponded to an average 3.4-fold decrease in the rate of RBC clearance (Figure 4D). In another platelet-depleted cohort, we found that aged RBCs (identified by annexin V staining) were increased in the bloodstream during the platelet nadir and recovery (days 2-7 after anti-GPIbα treatment; supplemental Figure 10). As a comparison, P-RBC complexes and RBC clearance were measured in Mpl−/− mice that were treated with NHS-biotin. Consistent with their reduced platelet count, Mpl−/− mice contained ∼50% fewer complexes than wild-type littermates but still showed an equivalent preference for P-RBC formation with older RBCs (aged >45 days; Figure 4F). However, the RBC half-life and, by deduction, clearance rate were not different between Mpl−/− and wild-type strains (Figure 4G). Thus, only the severe thrombocytopenia conditions affected cell clearance. To extend these findings to humans, blood samples from 9 patients with clinically well-defined ITP along with matched healthy controls were analyzed for P-RBC complexes and aged RBC levels. Patients exhibited significantly depressed platelet count and elevated mean platelet volume but minimal differences in other hematological parameters (Table 2) and, as expected, had significantly lower proportions of P-RBC complexes compared with controls (Figure 4H). Analysis of the RBCs showed increased levels of the age-defining markers, annexin V and FasR, on the surface of the cells (Figure 4I-J). These results indicate that patients with ITP have a greater circulating burden of aged RBCs, which is related to their platelet deficiency.
Effects of platelet depletion on senescent RBC retention in the circulation. (A-D) Cohorts of mice were pulse-labeled with NHS-biotin (IV, day 0) and then treated with platelet-depleting anti-GPIbα or nonimmune isotype (control) antibodies on day 45, at which time the mean proportions of P-RBC complexes in the labeled RBCs were significantly higher than in the unlabeled RBCs (mean ± SEM: 7.6% ± 1.2% vs 1.36% ± 0.17%; P < .0001). Blood samples were taken at indicated intervals and analyzed for percentages of biotin-labeled and unlabeled P-RBC complexes (B), biotin-labeled RBCs (normalized to day 45 levels) (C), and the relative RBC clearance rates (D). Clearance rates (determined by nonlinear regression) are shown separately for the days 45 to 48 and days 48 to 52 periods (indicated by vertical dashed lines in panel C). Comparisons shown between treatment groups using ANOVA; 8 mice per group, 2 independent experiments. (E-G) Mpl−/− and Mpl+/+ mice were pulse-labeled with NHS-biotin and blood samples taken at indicated intervals and analyzed for percentages of biotin-labeled and unlabeled P-RBC complexes (F) and biotin-labeled RBCs (G). Comparisons shown between treatment groups using ANOVA; 5 mice per group. (H-J) Proportions of P-RBC complexes (H) and annexin V–stained (I) and anti-FasR–stained (J) RBCs in blood samples collected from 9 patients with primary ITP and 9 healthy controls. Comparisons shown between groups using Student t test. Bars indicate means.
Effects of platelet depletion on senescent RBC retention in the circulation. (A-D) Cohorts of mice were pulse-labeled with NHS-biotin (IV, day 0) and then treated with platelet-depleting anti-GPIbα or nonimmune isotype (control) antibodies on day 45, at which time the mean proportions of P-RBC complexes in the labeled RBCs were significantly higher than in the unlabeled RBCs (mean ± SEM: 7.6% ± 1.2% vs 1.36% ± 0.17%; P < .0001). Blood samples were taken at indicated intervals and analyzed for percentages of biotin-labeled and unlabeled P-RBC complexes (B), biotin-labeled RBCs (normalized to day 45 levels) (C), and the relative RBC clearance rates (D). Clearance rates (determined by nonlinear regression) are shown separately for the days 45 to 48 and days 48 to 52 periods (indicated by vertical dashed lines in panel C). Comparisons shown between treatment groups using ANOVA; 8 mice per group, 2 independent experiments. (E-G) Mpl−/− and Mpl+/+ mice were pulse-labeled with NHS-biotin and blood samples taken at indicated intervals and analyzed for percentages of biotin-labeled and unlabeled P-RBC complexes (F) and biotin-labeled RBCs (G). Comparisons shown between treatment groups using ANOVA; 5 mice per group. (H-J) Proportions of P-RBC complexes (H) and annexin V–stained (I) and anti-FasR–stained (J) RBCs in blood samples collected from 9 patients with primary ITP and 9 healthy controls. Comparisons shown between groups using Student t test. Bars indicate means.
Baseline characteristics of patients with ITP and healthy controls
| . | ITP . | Healthy control . |
|---|---|---|
| Number of individuals | 9 | 9 |
| Age, y | 62 (51-71) | ∗ |
| Males/females (n/n) | 8/1 | ∗ |
| ITP category (chronic/new diagnosis) | 4/5 | |
| Platelet count, ×103/μL blood | 8.5 (1-45) P < .00001† | 194 (173-218) |
| Mean platelet volume (fL) | 8.91 (7.60- 9.75) P = .013† | 7.07 (7.0-8.0) |
| RBC count, ×109/μL blood | 4.14 (3.94-4.45) | 4.22 (3.64-4.58) |
| Hemoglobin, g/dL | 132 (127-138) | 134 (121-146) |
| Hematocrit, % | 0.376 (0.351-0.399) | 0.377 (0.338-0.412) |
| White blood cell count, ×103/μL blood | 7.93 (5.85-7.75) | 5.33 (4.35-6.50) |
| . | ITP . | Healthy control . |
|---|---|---|
| Number of individuals | 9 | 9 |
| Age, y | 62 (51-71) | ∗ |
| Males/females (n/n) | 8/1 | ∗ |
| ITP category (chronic/new diagnosis) | 4/5 | |
| Platelet count, ×103/μL blood | 8.5 (1-45) P < .00001† | 194 (173-218) |
| Mean platelet volume (fL) | 8.91 (7.60- 9.75) P = .013† | 7.07 (7.0-8.0) |
| RBC count, ×109/μL blood | 4.14 (3.94-4.45) | 4.22 (3.64-4.58) |
| Hemoglobin, g/dL | 132 (127-138) | 134 (121-146) |
| Hematocrit, % | 0.376 (0.351-0.399) | 0.377 (0.338-0.412) |
| White blood cell count, ×103/μL blood | 7.93 (5.85-7.75) | 5.33 (4.35-6.50) |
Median (interquartile range) shown, unless specified otherwise.
Ages and sex not available. Recruitment age range, from 18 to 70 years.
Comparison of ITP patients vs healthy controls (1-way analysis of varaiance).
Discussion
The existence of platelet-bound RBCs as an integral component of the bloodstream has been recognized,3-5 but until this study, their functional significance was not known. Prior work showing platelets binding to diseased RBCs led us to investigate whether chronologically old or senescent RBCs were also a target of platelet binding. Senescent RBCs constitute a constant and numerically significant fraction of the circulation, and unlike young healthy cells, the membrane of old RBCs mimics that of platelet-adherent cells. By studying mice with pulse-labeled RBCs, we could evaluate P-RBC complexes formed in vivo with physiologically aged RBCs. We found the oldest cells (aged 55-60 days) contained ∼10 times more complexes than the younger cells. This indicates that platelets in the circulation preferentially bind to old and senescent RBCs. The apparent selectivity of the binding is likely to be complex because old and damaged RBCs exhibit various modifications that could promote platelet binding.54 Our studies suggest roles for PS and FasR because both are more highly expressed on the older and platelet-bound RBCs. Previous work has shown that RBC-expressed PS interacts with platelet molecules CXCL16 and CD36,16 and FasR on RBCs can bind Fas ligand on platelets15; P-RBC interaction also stimulates additional FasR expression on RBCs.15 P-selectin, which was increased on RBC-bound platelets, can bind sialyl Lewis X antigen55 and is found on sickle RBCs.56 In other studies, CD36 on platelets also enables binding to Plasmodium-infected RBCs,13 and interactions between platelet αIIbβ3 integrin and RBC ICAM414 are important for P-RBC complex generation in SCD.57 Collectively, these findings support the concept that aged, diseased, or otherwise damaged RBCs may act as substrates for platelet binding.
Senescent RBCs, cell aggregates, and debris produced in circulation are efficiently removed by the mononuclear phagocytic system. In our study, we demonstrated that P-RBC complexes undergo a similar fate. Ex vivo–generated complexes that were returned to the circulation via transfusion were almost completely cleared within 24 hours, with a removal rate 7 and 3 times faster, respectively, than either RBCs or platelets not bound into complexes. Although these may not represent physiologically formed complexes, the overall observations suggest that platelet binding is the critical factor driving their removal. If endogenous complexes are cleared at equally accelerated rates, their existence in the bloodstream is probably relatively transient. The physical presence of platelets may hinder the ability of an RBC to pass through the splenic red pulp cords and become entrapped. Our observations showing elevated levels of complexes in the bloodstream of both splenectomized mice and human patients indicates the importance of this organ for clearance of complexes, although steric hindrance and phagocytosis in other tissues may also contribute to their overall removal. Additionally, a significant proportion of erythrophagocytes in the splenic red pulp contained ingested complexes, with a ratio of phagocytosed complexes to platelet-free RBCs (∼1:3) exceeding that in the circulation (∼1:100). This suggests that P-RBCs may also be selectively phagocytosed, and platelets, upon binding to RBCs, enhance their recognition and/or ingestion. This could occur because platelets provide greater PS (observed in Figure 1E), which would strengthen the phagocyte “eat-me” signal.17 Platelets may also support the RBC-phagocyte interaction through upregulation of cell-adhesive receptors58 and release of a range of prophagocytic molecules.59,60 Additionally, platelets are a major source of thrombospondin, a dimeric molecule that can bridge oxidized CD47 (expressed on senescent RBC) and the phagocytic SIRP-α receptor.21 Investigating whether binding to RBC stimulates these prophagocytic properties in platelets and how this drives erythrocyte clearance are important questions for future studies.
Our results also identified an important relationship between platelet count, P-RBC complex formation, and the rate at which senescent RBCs are normally removed from the circulation. Severe depletion of platelets (<1% of normal levels) in mice reduced the proportion of platelet-bound senescent RBC by more than 90% and resulted in their retention in the circulation, and in a cohort of ITP patients, equivalent reductions in platelet count and P-RBC complexes were associated with a greater burden of aged RBC. Our mouse studies also showed that platelet depletion reduced EP and that phagocyte depletion caused a similar retention of aged RBCs. However, in Mpl-deficient mice, which have a relatively mild platelet deficiency (10% of normal levels), neither RBC clearance nor EP were affected, indicating that complex-mediated cell clearance was only compromised by severe losses of platelets. Therefore, a healthy platelet count seems sufficient to meet the normal demands of RBC clearance, and only a severe thrombocytopenic state disrupts this relationship. Alternatively, the chronic platelet deficiency in Mpl−/− mice may have compensatory effects on RBC turnover, which could also explain why the buildup of complexes observed in patients who underwent splenectomy was temporary. Compensation may be provided by specialized monocyte recruitment in the liver, which has been shown under conditions of erythrocyte stress to be an important support for RBC removal and iron recycling.61
Relationships between platelets, complex formation, and senescent RBC clearance observed in the mouse studies likely extend to humans. We were able to confirm the occurrence and levels of complexes observed in previous human studies.4,8,9 Additionally, we observed the effects of spleen removal (elevated complexes) and acute suppression of platelet count (reduced complexes and elevated aged RBCs) for the first time, to our knowledge, and these findings were largely consistent with the mouse studies. Considering the relative levels of platelets, RBCs, and complexes, this mechanism of complex production and aged RBC disposal may be a significant factor in the maintenance of blood homeostasis. In mice ∼160 million RBCs per mL per day are cleared under normal physiological conditions (assuming a 10 000 million per mL count and 60-day lifespan). Based on our estimates of P-RBC lifespan (7 times shorter than RBC) and bloodstream levels (1.5%-2.7% or 80 million-150 million per mL), P-RBC clearance rates may range between 9.5 million to 18 million P-RBCs per mL per day, which constitutes 6% to 11% of the total RBC clearance. Translating this to humans, the fraction of RBC clearance attributable to P-RBCs may be somewhat less at 0.7% to 2%, assuming RBC turnover is 42 million/mL per day (5000 million/mL RBC count and 120-day lifespan) vs P-RBC turnover of 0.3 million/mL to 0.9 million/mL per day (based on 0.1%-0.3% or 5 million/mL-15 million/mL P-RBC levels and a sevenfold reduced lifespan). However, these calculations may underestimate the possible synergistic effects of platelet binding on the already clearance-prone senescent cells. In addition, if each complex contains at least 1 platelet, these levels of complexes in humans would consume 2% to 6% of the total platelet pool (250 million/mL); increased complex formation would consume even greater platelet numbers. Such a relationship is exemplified in malaria in which platelet binding to Plasmodium-infected RBC is associated with thrombocytopenia.8
Our findings also predict that conditions in which complex generation is reduced (ITP) or when the clearance of complexes is impaired (splenectomy) will cause the inappropriate accumulation of old RBCs in the bloodstream and, as a consequence, increase the risk of coagulation-related outcomes. Thromboembolic events are widely reported after splenectomy62-65 and in ITP,66-69 and we speculate the buildup of aged RBCs and complexes, as well as increased RBC-endothelial interactions and platelet activation, are contributing to the hypercoagulable conditions proposed to explain this problem.70 Similarly, the excessive levels of complexes observed in SCD may contribute to imbalances in coagulant function characteristic of the condition.10,11 Future studies will explore the effect of antiplatelet or anticoagulant therapies on the stability of P-RBC complexes. Many other diseases including myeloproliferative neoplasms, chronic systemic inflammation, autoimmune disease, and iron deficiency are accompanied by abnormal production and/or compromised integrity of RBCs and platelets, which could disrupt the balance between platelet count, complex formation, and RBC turnover. Furthermore, individuals with high platelet and RBC counts have significantly elevated risks of thrombosis.71,72 Therefore, a better understanding of the physiological effects of P-RBC complexes in disease is warranted.
Acknowledgments
The authors thank Lucy Coupland for advice, Daryl Webb for technical assistance, Warren Alexander for providing the Mpl−/− mouse line, the Australian Phenomics Facility and John Curtin School of Medical Research Imaging and Flow Cytometry Facility for technical support, the Spleen Study team at the Papuan Health and Community Development Foundation for their support in Timika, and staff at the Canberra Hospital Department of Clinical Haematology. The authors acknowledge the facilities of Microscopy Australia at the Centre for Advanced Microscopy, Australian National University, funded by the University and the Australian Government.
The work was supported by grants from the National Health and Medical Research Council (APP1066502 and APP1183927, program grant 1037304), the Australian Research Council (DP120100061), The Bootes Foundation, Australian Capital Territory Health, a Menzies Future Leaders Fellowship (S.K.), and the National Collaborative Research Infrastructure via the Australian Phenomics Network.
The funders had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Authorship
Contribution: B.J.M. conceived the project; D.C.N., F.L., H.S., Y.L.T., S.A., M.D., K.J., S.K., J.L., and B.J.M. performed the experiments; D.C.N., F.L., M.R., S.K., and B.J.M. designed and analyzed the experiments; S.M.H., Y.L.T., E.K., and J.R.P. collected patient samples; P.Y.-I.C. and N.M.A. founded the patient studies; E.K., J.R.P., M.R., E.E.G., and B.J.M. provided supervision; F.L., S.K., E.E.G., and B.J.M. prepared the figures and wrote the paper; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brendan J. McMorran, Division of Immunology and Infectious Disease, John Curtin School of Medical Research, The Australian National University, Canberra, ACT 2601, Australia; email: brendan.mcmorran@anu.edu.au.
References
Author notes
Original data are available on request from the corresponding author, Brendan J. McMorran (brendan.mcmorran@anu.edu.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Characterization of P-RBC complex clearance from the bloodstream. (A) Platelets and RBCs were separately isolated from donor mice, labeled with CFSE and atto633, respectively, coincubated to generate P-RBC complexes (inset image; platelet, green; RBC, red), and then transfused into recipient mice, either untreated, pretreated with clodronate or phosphate-buffered saline (PBS) liposomes (phagocyte depletion), or splenectomized. Blood samples collected at indicated times after transfusion (hours posttransfusion [h.p.]) were analyzed by flow cytometry using the method depicted. Total (recipient plus donor) populations of RBCs (ter119+), platelets (CD41+), and P-RBC complexes (ter119+ CD41+) were gated and then separately analyzed for proportions of respective donor cells (atto633+, CFSE+, or atto633+ and CFSE+). The panels show the same mouse analyzed 0.17 (10 minutes) and 18 hp. (B) Proportions of transfused RBCs, platelets, and P-RBC complexes in the bloodstream of recipient mice measured up to 24 h.p. transfusion (left) and the clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly lower than those of platelets and RBCs (P < .0001), and platelet levels were significantly lower than RBC numbers at indicated time points (P < .001). Nine mice and 3 independent experiments. (C) Proportions of transfused RBCs, platelets, and P-RBC complexes in recipient mice treated 24 hours prior with clodronate or PBS (control) liposomes (left) and clearance rates derived from these data (right). Levels and clearance rates of P-RBC complexes were significantly higher in clodronate vs control mice (P < .001 and .0001, respectively); 10 mice and 2 experiments. (D) Proportions of transfused RBC, platelet, and P-RBC complex levels in recipient mice previously splenectomized (splenec.) compared with nonsplenectomized (intact) mice (left), clearance rates derived from these data (right), and (E) relative proportions of recipient (unlabeled) RBCs, platelets, and complexes; 3 and 4 mice, respectively. (B-D) Dashed lines indicate decay curves fitted using nonlinear regression. (B-E) Symbols and bars indicate means and SEM or individuals; comparisons shown using ANOVA. (F) P-RBC complexes in 20 population-matched spleen-intact individuals (healthy) and 16 patients measured before (pre) and 60 and 360 days (6 of 16 patients) after surgical removal of their spleen (mean, 95% confidence interval, and minimum and maximum values; comparisons shown using ANOVA), and (G) the P-RBC:platelet ratio for the same patients plotted longitudinally. Comparisons shown using Kruskal-Wallis test. Plt, platelet.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/6/10.1182_blood.2023021611/1/m_blood_bld-2023-021611-gr2.jpeg?Expires=1769690237&Signature=fyxvIuUli7v8K0Ojlpl-mL~lSieiA8mxXJnbchz~CajLsJCsgu5ubPOhB6BHB2kDZErvGWoOroPKJA6bDnrc0v582W95lkQiYmA8qRmSw1w0YgHInvgzuNTqwZPwJ4NFLuCFEVqyg7xQtoLqDlBUJv8sFZOKqpwEKWGc7e1-fDYDanqyut93lO-CfSpUsmTAmcNeBitbwN~hQotSGkTcdS2nta2wKQ5qZ8-aperk55gAGdWjG~nuTm6K6RqiPsPWPKdqkDkRee88ZGD8gBiW5oxKsM~zU9D5WwfRDdaPRASJLJEE5m0yHZidUwUFg6VDA7-k3n~cvZWpJKZWy1Praw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal