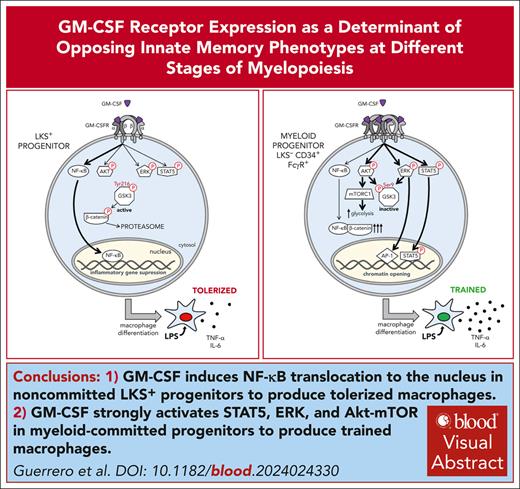
Key Points
GM-CSF strongly activates STAT5, ERK, and Akt-mTOR in myeloid-committed progenitors to produce trained macrophages.
GM-CSF induces NF-κB translocation to the nucleus in noncommitted LKS+ progenitors to produce tolerized macrophages.
Visual Abstract
Inflammatory responses must be tightly coordinated with the activation of emergency myelopoiesis to produce potent myeloid cells that fight infection without causing excessive host damage. Here, we show that granulocyte-macrophage colony-stimulating factor (GM-CSF) programs myeloid-committed progenitors to produce trained macrophages (increased cytokine response), but programs the upstream noncommitted LKS+ progenitors (defined as Lin− c-Kit+ Sca-1+ cells) to produce tolerized macrophages (decreased cytokine response). In myeloid progenitors, GM-CSF strongly activates signal transducer and activator of transcription 5 (STAT5), Ras-Raf-extracellular signal regulated kinase (ERK), and Akt-mTOR signaling pathways, which are essential to establish a training program, whereas in LKS+ progenitors, GM-CSF induces NF-κB translocation to the nucleus to establish a tolerization program. These differences arise from higher GM-CSF receptor expression in myeloid progenitors compared with LKS+ cells. We demonstrate that β-catenin regulation of NF-κB nuclear translocation is central in this process. In myeloid progenitors, glycogen synthase kinase 3 (GSK3) inactivation by strong ERK and phosphatidylinositol 3 kinase (PI3K)-Akt signaling increases cytoplasmic β-catenin levels to block NF-κB nuclear translocation. In contrast, when ERK and PI3K-Akt signaling are weak, active GSK3 causes a decrease in β-catenin, allowing NF-κB nuclear translocation in LKS+ progenitors. Finally, GM-CSF-induced LKS+ tolerization takes place in several murine models of trained immunity and in human CD34+ CD38− progenitors. Our study reveals that in addition to activating myelopoiesis, GM-CSF also programs early and immediate myeloid progenitors to produce opposing immune memory phenotypes. We propose that the inflammatory response from immediate myeloid progenitors may be balanced by the tolerized phenotype of early progenitors, thus providing a mechanism for appropriate resolution of inflammation and protection against a prolonged cytokine storm.
Introduction
Myelopoiesis is a demand-adapted process in which hematopoietic stem and progenitor cells (HSPCs) in the bone marrow manufacture myeloid cells to specifically respond to physiological challenges. During emergency myelopoiesis, granulocyte-macrophage progenitors (GMPs) expand to boost production of mature myeloid cells in the bone marrow, whereas hematopoietic stem cells (HSCs) are activated to increase myeloid-biased multipotent progenitors (MPPs), which will give rise to more GMPs.1,2
The granulocyte-macrophage colony-stimulating factor (GM-CSF), although dispensable for steady-state hematopoiesis, is produced by many immune and nonimmune cells as a mediator of emergency myelopoiesis, especially during infection, but also in some inflammatory diseases.3 It binds to its heterodimeric receptor, composed of the common β subunit CD131 and the specific α subunit CD116,4 activating multiple signaling pathways involved in proliferation, differentiation, and cell survival: Janus Kinase 2 and signal transducer and activator of transcription 5 (STAT5), Ras-Raf-extracellular signal regulated kinase (ERK1/2), phosphatidylinositol 3 kinase (PI3K)/Akt, and NF-κB pathways.4 GM-CSF instructs HSCs to become myeloid-biased progenitors by upregulating PU.1 and GMPs also respond to GM-CSF by proliferating and differentiating into granulocytes and monocytes.3,5
Besides initiating emergency myelopoiesis, GM-CSF promotes the recruitment of myeloid cells and potentiates their effector functions. GM-CSF boosts macrophage antimicrobial functions, phagocytosis, and reactive oxygen species production, and primes them to secrete proinflammatory cytokines upon a second challenge.6 However, the flip side of potentiating inflammation is that GM-CSF–activated phagocytes can also trigger tissue damage and, consequently, autoimmune, and inflammatory disorders.3
Given its importance in hematopoiesis and immunity, GM-CSF has been studied in numerous clinical contexts. It is approved for accelerating myeloid reconstitution following bone marrow transplantation, increasing survival in patients treated with myeloablative doses of radiation, and mobilization of HSPCs into peripheral blood for leukapheresis and autologous transplantation.7 GM-CSF has also been investigated as a treatment for fungal and bacterial infections and as an immune stimulant and vaccine adjuvant in cancer patients.7
Innate immune memory is defined as the ability of myeloid cells to develop a long-lasting adaptation of their inflammatory response, such that an initial encounter alters the response to subsequent unrelated challenges. The molecular mechanisms leading to this functional reprogramming of cells include metabolic and epigenetic alterations.8 Although first described in short-lived myeloid cells, inflammatory memory has been extended to HSPCs, explaining the long-term memory.8 In HSPCs, GM-CSF produced upon β-glucan administration to mice or secreted during a murine model of Candida albicans vaccination is required to establish immune memory by inducing an increased myelopoiesis and modulating the function of HSPCs and their derived myeloid cells.9,10
In this study, we show that GM-CSF signaling activates opposite inflammatory memory programs at different stages of myelopoiesis. We show that proinflammatory cytokine production is enhanced (“trained”) in macrophages derived from GM-CSF–stimulated myeloid-committed progenitors (defined as Lin− c-Kit+ Sca-1− [LKS−] CD34+ FcγR+ cells), but reduced (“tolerized”) when macrophages are derived from GM-CSF–treated noncommitted LKS+ progenitors (defined as Lin− c-Kit+ Sca-1+ cells). By probing the epigenetic changes induced by GM-CSF treatment and the signaling pathways activated by GM-CSF in both progenitor subsets, we show that the differential GM-CSF receptor (GM-CSFR) expression regulates specific signaling pathways and determines the inflammatory memory program at each stage of myelopoiesis. Thus, GM-CSF may coordinate the immediate production of trained myeloid cells to efficiently fight infection but may simultaneously limit the threat of a harmful “cytokine storm” by inducing tolerance in progenitors with the highest expansion potential.
Materials and methods
In vivo assays
For in vivo GM-CSF treatment, mice were injected IV with 1.5 μg of GM-CSF (PeproTech) in phosphate-buffered saline (PBS). For in vivo trained immune models, 300 μg of depleted zymosan (InvivoGen) or 1.5 × 106 CFUs of C albicans PCA2 strain11 (prepared as described previously10) were injected IV. Untreated animals were injected with PBS alone.
Experiments were approved by the committee on the ethics of animal experiments of the University of Valencia (permit numbers 2021/VSC/PEA/0075 and 2022/VSC/PEA/0201) and performed according to Spanish law under Real Decreto 53/2013.
Isolation of HSPCs
Mouse bone marrow cells were obtained by flushing femurs and tibias. HSPCs were isolated as lineage-negative cells (Lin− cells) by immunomagnetic cell sorting using a Miltenyi Biotec kit according to the manufacturer’s instructions. For LKS+, long-term HSC (LT-HSC), short-term HSC (ST-HSC), MPP2, MPP3, and LKS− CD34+ FcγR+ subsets, Lin− cells were stained for surface markers and sorted by flow cytometry using a BD FACS Aria Fusion cell sorter (BD Bioscience). For coculture experiments, Lin− Sca-1+ and Lin− Sca-1− cells were separated by immunomagnetic cell sorting from DsRed or C57BL/6 mice, respectively, using an anti-Sca-1 MicroBead Kit (Miltenyi Biotec).
In vitro macrophage differentiation
Murine HSPCs (10 000 LKS+ per well, 50 000 LKS− CD34+ FcγR+ per well or 200 000 Lin− per well) were cultured in complete cell culture medium (RPMI 1640 medium supplemented with 2 mM L-glutamine, 5% heat-inactivated fetal bovine serum, and 1% penicillin-streptomycin stock solution [Gibco]) in the presence of stem cell factor (SCF) and macrophage colony stimulating (M-CSF) (PreproTech) for the indicated times.
Statistical and gene expression analyses
Statistical analyses were performed using the Student t tests, the 1-way analysis of variance followed by the Dunnett test for multiple comparisons or the Gehan-Breslow-Wilcoxon tests, as indicated in the figure legends.
Results
GM-CSF stimulation of LKS+ cells tolerizes the macrophages they produce
We have previously demonstrated that trained HSPCs from C albicans–vaccinated mice play a role in protection against a secondary infection.10 Moreover, we found that GM-CSF activation of HSPCs is responsible for the trained phenotype and essential for the vaccine-induced protection (supplemental Figure 1A, available on the Blood website, and Bono et al10). Given the long-lasting durability of trained immunity, here, we decided to study the effect of GM-CSF on conferring trained immunity to LKS+ progenitors (supplemental Figure 1B), which include HSCs and MPPs, the earliest progenitors in the bone marrow probably responsible for sustaining memory over time.
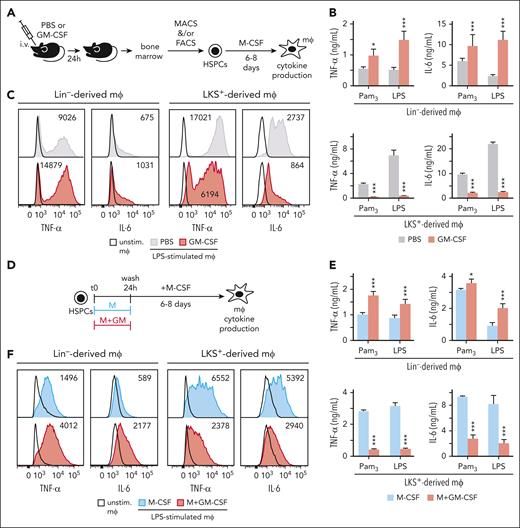
Lin−, containing all HSPCs, or LKS+ cells were isolated from the bone marrow of 24 hours PBS or GM-CSF–injected mice, differentiated into macrophages with M-CSF for 6 or 8 days respectively, and stimulated with Pam3CSK4 or lipopolysaccharide (LPS) to measure tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) production by enzyme-linked immunosorbent assay and intracellular flow cytometry (Figure 1A). As expected, Lin− cells from GM-CSF–treated mice differentiated into trained macrophages that produced more TNF-α and IL-6 than Lin−-derived macrophages from PBS-treated mice (Figure 1B-C). Surprisingly, LKS+-derived macrophages from GM-CSF–treated mice produced lower amounts of TNF-α and IL-6 than their control macrophages, indicating they are tolerized (Figure 1B-C).
Proinflammatory cytokine response of macrophages derived from GM-CSF–stimulated LKS+ or Lin− progenitors. (A) C57BL/6 mice were IV injected with PBS or 1.5 μg per mouse of GM-CSF and 24 hours later Lin− or LKS+ cells were isolated from the bone marrow and differentiated into macrophages with M-CSF (50 ng/mL) for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by enzyme-linked immunosorbent assay (ELISA) in supernatants upon Pam3CSK4 (Pam3) or LPS stimulation (B) and by intracellular flow cytometry upon LPS stimulation (C). (D) Lin− or LKS+ cells isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL). After 24 hours, cells were washed and differentiated into macrophages with M-CSF for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by ELISA in supernatants upon Pam3 or LPS stimulation (E) and by intracellular flow cytometry upon LPS stimulation (F). For the ELISA measurements triplicate samples were analyzed and expressed as means + standard deviation (SD) and for intracellular cytokine measurements mean fluorescence intensity (MFI) is indicated in the histograms; data presented are 1 representative experiment of at least 3 independent experiments. (G) Total bone marrow or LKS+ cells were isolated from 24 hours PBS- or GM-CSF–treated DsRed mice, transplanted into irradiated C57BL/6 recipients, and analyzed 16 weeks after their hematopoietic reconstitution. (H) Bone marrow from 16-week reconstituted mice was obtained, stimulated with LPS for 4 hours and with brefeldin A for the final 3 hours, and TNF-α production was measured in DsRed+ Ly6Chi monocytes (Ly6G− CD11b+ CD115+ Ly6Chi) by intracellular flow cytometry (3 representative mice are shown). (I) Bar graphs for MFIs and box plots for cell percentages are expressed as means + SD (n = 5-6 mice per condition). Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). mφ, macrophage; unstim., unstimulated.
Proinflammatory cytokine response of macrophages derived from GM-CSF–stimulated LKS+ or Lin− progenitors. (A) C57BL/6 mice were IV injected with PBS or 1.5 μg per mouse of GM-CSF and 24 hours later Lin− or LKS+ cells were isolated from the bone marrow and differentiated into macrophages with M-CSF (50 ng/mL) for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by enzyme-linked immunosorbent assay (ELISA) in supernatants upon Pam3CSK4 (Pam3) or LPS stimulation (B) and by intracellular flow cytometry upon LPS stimulation (C). (D) Lin− or LKS+ cells isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL). After 24 hours, cells were washed and differentiated into macrophages with M-CSF for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by ELISA in supernatants upon Pam3 or LPS stimulation (E) and by intracellular flow cytometry upon LPS stimulation (F). For the ELISA measurements triplicate samples were analyzed and expressed as means + standard deviation (SD) and for intracellular cytokine measurements mean fluorescence intensity (MFI) is indicated in the histograms; data presented are 1 representative experiment of at least 3 independent experiments. (G) Total bone marrow or LKS+ cells were isolated from 24 hours PBS- or GM-CSF–treated DsRed mice, transplanted into irradiated C57BL/6 recipients, and analyzed 16 weeks after their hematopoietic reconstitution. (H) Bone marrow from 16-week reconstituted mice was obtained, stimulated with LPS for 4 hours and with brefeldin A for the final 3 hours, and TNF-α production was measured in DsRed+ Ly6Chi monocytes (Ly6G− CD11b+ CD115+ Ly6Chi) by intracellular flow cytometry (3 representative mice are shown). (I) Bar graphs for MFIs and box plots for cell percentages are expressed as means + SD (n = 5-6 mice per condition). Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). mφ, macrophage; unstim., unstimulated.
To rule out indirect effects of the in vivo GM-CSF treatment, Lin− or LKS+ cells were isolated, stimulated in vitro with GM-CSF for 24 hours, washed, and differentiated into macrophages with M-CSF for 6 or 8 days, respectively, and cytokine production was measured (Figure 1D). Again, GM-CSF induced a training program in Lin− cells, but failed to do so in LKS+ cells, inducing a tolerization program instead (Figure 1E-F). GM-CSF specificity was confirmed in vivo and in vitro (supplemental Figure 1C-D). Therefore, LKS+ cells must not be contributing to the final pool of the M-CSF–derived macrophages when starting from Lin− cells. In fact, we only found 1.5% of LKS+-derived macrophages when differentiating DsRed Lin− Sca-1+ and C57BL/6 Lin− Sca-1− cell cocultures (at bone marrow proportions) with M-CSF for 6 days (supplemental Figure 1E-F).
We demonstrated that all M-CSF–derived adherent cells from both progenitors and conditions express homogeneously high levels of specific macrophage markers, possess the typical macrophage morphology, and phagocytose C albicans cells at the same rate (supplemental Figure 2A-C). No differences were found in the differentiation kinetics of progenitors following the GM-CSF treatment, but the total macrophage output was higher for both in vitro and in vivo GM-CSF–treated progenitors, probably because of a higher proliferative ability of the GM-CSF–stimulated progenitors (supplemental Figure 2D-F). Lastly, the differences in cytokine production by macrophages derived from GM-CSF–treated progenitors cannot be explained by differential Toll-like receptor (TLR) expression levels (supplemental Figure 2G).
Finally, to exclude any effects owing to in vitro differentiation, total bone marrow or LKS+ cells isolated from 24 hours PBS or GM-CSF–injected DsRed mice were adoptively transferred to C57BL/6 irradiated mice. Sixteen weeks later, bone marrow DsRed+ cell subsets were measured, and TNF-α production was quantified by intracellular flow cytometry in bone marrow DsRed-derived Ly6Chi monocytes following LPS stimulation (Figure 1G; supplemental Figure 3A). Results showed an increase in myelopoiesis by both GM-CSF–stimulated progenitors and the preservation of the trained or tolerized phenotype in monocytes derived from GM-CSF–stimulated bone marrow or LKS+ cells respectively (Figure 1H-I; supplemental Figure 3B). However, secondary reconstitution with total bone marrow from primary bone marrow–reconstituted mice did not show any differences in cell subset proportions or in the cytokine production profile of their derived monocytes (supplemental Figure 3B-C). This indicates that the immune memory program can persist after a primary adoptive transfer for a long period of time, but it vanishes after a secondary transfer.
Taken together, these data indicate that GM-CSF unexpectedly induces a tolerization program in LKS+ cells rather than the trained inflammatory program observed in Lin− cells.
Chromatin accessibility is mainly altered in GM-CSF–stimulated myeloid progenitors with AP1 and STAT5 signatures
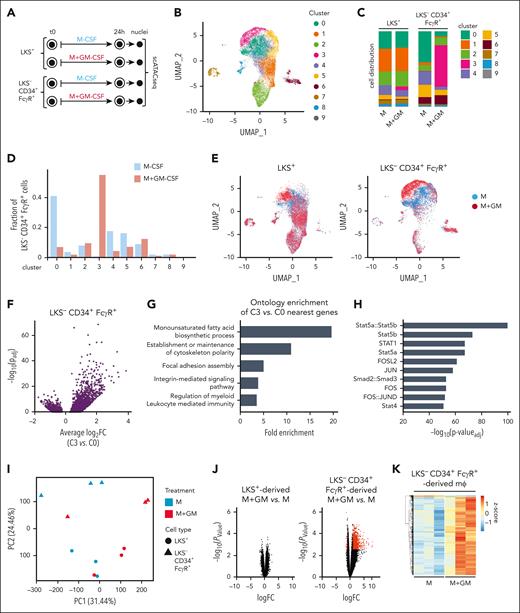
To gain a broader understanding of the mechanisms dictating immune memory in different HSPC populations, we performed single cell assay for transposase-accessible chromatin (ATAC)-seq in LKS+ cells and myeloid-committed progenitors upon GM-CSF exposure (Figure 2A). We first enriched myeloid-committed progenitors within Lin− cells by using LKS− CD34+ FcγR+ cells (supplemental Figure 4A), which are also reprogrammed by GM-CSF to produce trained macrophages (supplemental Figure 4B-C).
Chromatin accessibility in GM-CSF–stimulated LKS+ and LKS− CD34+ FcγR+ progenitors. (A) LKS− CD34+ FcγR+, or LKS+ cells were isolated from the bone marrow of 10 C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, nuclei were extracted, and single-cell assay for transposase-accessible chromatin with high-throughput sequencing (scATAC-seq) was performed. (B) Uniform manifold approximation and projection (UMAP) of scATAC-seq data with 10 clusters identified by K-nearest neighbor approach. (C) Distribution of cells in each cluster within samples. (D) Bar graph for the LKS− CD34+ FcγR+ myeloid progenitors showing the fraction of cells present in each cluster. (E) UMAP of scATAC-seq data separated by sample and treatment condition. (F) Volcano plot of regions that are differentially accessible between cluster 3 and cluster 0. (G) Gene ontology enrichment of 793 genes with 10 kb of 1269 regions with greater accessibility in cluster 3. (H) Top results from motif enrichment analysis within 1269 regions with increased accessibility in cluster 3 compared with cluster 0. (I) Principal component analysis of bulk ATAC-seq–analyzed macrophages derived from M- or M+GM–treated Lin− CD34+ FcγR+ or LKS+ progenitors. (J) Volcano plot of bulk ATAC-seq showing regions that are differentially accessible between M+GM vs M in macrophages derived from LKS− CD34+ FcγR+ or LKS+ cells; statistically significant regions (n = 1299) labeled in red. (K) Heat map of bulk ATAC-seq signal showing differentially accessible regions between macrophages derived from M+GM- vs M-treated Lin− CD34+ FcγR+ cells. Data derived from 3 biological replicates per condition.
Chromatin accessibility in GM-CSF–stimulated LKS+ and LKS− CD34+ FcγR+ progenitors. (A) LKS− CD34+ FcγR+, or LKS+ cells were isolated from the bone marrow of 10 C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, nuclei were extracted, and single-cell assay for transposase-accessible chromatin with high-throughput sequencing (scATAC-seq) was performed. (B) Uniform manifold approximation and projection (UMAP) of scATAC-seq data with 10 clusters identified by K-nearest neighbor approach. (C) Distribution of cells in each cluster within samples. (D) Bar graph for the LKS− CD34+ FcγR+ myeloid progenitors showing the fraction of cells present in each cluster. (E) UMAP of scATAC-seq data separated by sample and treatment condition. (F) Volcano plot of regions that are differentially accessible between cluster 3 and cluster 0. (G) Gene ontology enrichment of 793 genes with 10 kb of 1269 regions with greater accessibility in cluster 3. (H) Top results from motif enrichment analysis within 1269 regions with increased accessibility in cluster 3 compared with cluster 0. (I) Principal component analysis of bulk ATAC-seq–analyzed macrophages derived from M- or M+GM–treated Lin− CD34+ FcγR+ or LKS+ progenitors. (J) Volcano plot of bulk ATAC-seq showing regions that are differentially accessible between M+GM vs M in macrophages derived from LKS− CD34+ FcγR+ or LKS+ cells; statistically significant regions (n = 1299) labeled in red. (K) Heat map of bulk ATAC-seq signal showing differentially accessible regions between macrophages derived from M+GM- vs M-treated Lin− CD34+ FcγR+ cells. Data derived from 3 biological replicates per condition.
All cells were clustered based on chromatin accessibility, resulting in 10 clusters (Figure 2B). Cell distribution in each cluster strikingly showed little change in LKS+ cells between treatments (Figure 2C). However, the most prominent cluster of LKS− CD34+ FcγR+ cells in the M-CSF condition, cluster 0, strongly diminished after the GM-CSF treatment (Figure 2C-D). Conversely, cluster 3, not present in the M-CSF condition, was the most abundant in GM-CSF–treated LKS− CD34+ FcγR+ cells (Figure 2C-D). UMAP graphs separated by cell subset and treatment condition showed that C0 and C3 are nearby but are clearly distinct, although their separation is only observed in LKS− CD34+ FcγR+ cells (Figure 2E). In contrast, most LKS+ cells are overlapping between treatments, indicating no significant chromatin remodeling following GM-CSF stimulation (Figure 2E).
Analysis of the differentially open chromatin regions showed that the vast majority of differences between C3 and C0 are gains of accessibility in C3 rather than losses (Figure 2F). The top ontology terms for genes near the C3 vs C0 differential ATAC regions pointed toward lipid biosynthesis, shown to be involved in trained immunity;9 cytoskeleton polarity, and adhesion and integrin pathways, as GM-CSF induces HSPC mobilization from the bone marrow to the periphery10; and terms involved in myeloid-mediated immune responses (Figure 2G). Regarding the transcription factors that could be initiating chromatin remodeling events,12 we analyzed the DNA-binding motifs of transcription factors enriched within the regions with increased accessibility in C3 compared to C0. The top enriched motifs were for the STAT and AP1 families in cluster 3 (Figure 2H; supplemental Figure 4D). Because these are activated downstream of the GM-CSF response, we hypothesized that they may produce the epigenetic basis of trained immunity seen in LKS− CD34+ FcγR+ cells.
We next performed bulk ATAC-seq on resting macrophages differentiated from GM-CSF-treated or untreated progenitors (Figure 1D), to examine their chromatin accessibility. Similar to the progenitor analysis, we found differences in macrophages derived from GM-CSF-stimulated LKS− CD34+ FcγR+ cells (a gain of 1299 differentially accessible regions) but none in macrophages derived from GM-CSF–stimulated LKS+ cells, compared with their respective control macrophages (Figure 2I-K). However, we did not observe a strong enrichment in the ontology terms and motifs for transcription factors in macrophages derived from GM-CSF–stimulated LKS− CD34+ FcγR+ cells (supplemental Figure 4E-F), likely because of the multitude of secondary and tertiary transcription factors playing a role in shaping the epigenome downstream of GM-CSF stimulation over 7 days of differentiation. Interestingly, control macrophages derived from both progenitors also showed differences in their epigenetic states (Figure 2I; supplemental Figure 4G), perhaps related to their different ability to produce cytokines, which will require future investigations.
Strong GM-CSF activation of STAT5, ERK, and Akt-mTOR signaling pathways in myeloid progenitors
Given the results of unbiased scATAC-seq analysis, we next measured phosphorylation of STAT5 (pSTAT5), ERK (pERK), and Akt (pAkt) by intracellular flow cytometry upon 10 and 30 minutes of GM-CSF stimulation in both progenitor subsets. GM-CSF strongly activated STAT5, ERK, and Akt in LKS− CD34+ FcγR+ cells, whereas only weakly activated ERK and Akt in LKS+ cells (Figure 3A).
Signaling pathways activated in LKS+ and LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) Phosphorylated STAT5 (pSTAT5), ERK (pERK), and Akt (pAkt) were measured by intracellular flow cytometry at 10 and 30 minutes, and S6 (pS6) at 4 hours, in GM-CSF–stimulated (50 ng/mL) LKS+ and LKS− CD34+ FcγR+ progenitors. The expression of CD116 and CD131 was measured on the surface of LKS+ and LKS− CD34+ FcγR+ progenitors and Ly6Chi monocytes (Ly6G− CD11b+ CD115+ Ly6Chi) (B); and on the surface of LT-HSC, ST-HSC, MPP2, MPP3 and MPP4 progenitors (C) by flow cytometry. MFI measurements are means + SD of 3 independent experiments and histograms are from 1 representative experiment. LT-HSC, ST-HSC, MPP2, and MPP3 progenitors isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed and differentiated into macrophages with M-CSF for 8 days (MPP2 and MPP3) or 10 days (LT- and ST-HSCs); TNF-α production was measured by intracellular flow cytometry upon LPS stimulation (D) and by ELISA in supernatants upon LPS stimulation (E). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements MFI is indicated in the histograms; data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). i.c., isotype control; unstim., unstimulated.
Signaling pathways activated in LKS+ and LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) Phosphorylated STAT5 (pSTAT5), ERK (pERK), and Akt (pAkt) were measured by intracellular flow cytometry at 10 and 30 minutes, and S6 (pS6) at 4 hours, in GM-CSF–stimulated (50 ng/mL) LKS+ and LKS− CD34+ FcγR+ progenitors. The expression of CD116 and CD131 was measured on the surface of LKS+ and LKS− CD34+ FcγR+ progenitors and Ly6Chi monocytes (Ly6G− CD11b+ CD115+ Ly6Chi) (B); and on the surface of LT-HSC, ST-HSC, MPP2, MPP3 and MPP4 progenitors (C) by flow cytometry. MFI measurements are means + SD of 3 independent experiments and histograms are from 1 representative experiment. LT-HSC, ST-HSC, MPP2, and MPP3 progenitors isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed and differentiated into macrophages with M-CSF for 8 days (MPP2 and MPP3) or 10 days (LT- and ST-HSCs); TNF-α production was measured by intracellular flow cytometry upon LPS stimulation (D) and by ELISA in supernatants upon LPS stimulation (E). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements MFI is indicated in the histograms; data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). i.c., isotype control; unstim., unstimulated.
Trained immunity is associated with an mammalian target of rapamycin (mTOR)-dependent increase in glycolysis through PI3K/Akt.13 S6 (mTOR’s target) was phosphorylated in 4-hours GM-CSF–stimulated LKS− CD34+ FcγR+ cells, whereas no pS6 was observed in LKS+ cells, demonstrating a robust activation of mTOR in GM-CSF–stimulated myeloid progenitors (Figure 3A).
GM-CSF signaling strength could be related to the differential GM-CSFR expression on both progenitor subsets. Flow cytometry data showed a much higher expression of CD116 and CD131 on LKS− CD34+ FcγR+ cells compared with LKS+ cells (Figure 3B). Expression of these markers does not change 24 hours after in vivo GM-CSF treatment (supplemental Figure 5A). Mature monocytes express similar levels of CD131 in comparison with LKS+ cells and higher levels of CD116 than both progenitor subsets (Figure 3B). Additionally, all subsets within LKS+ cells (LT-HSC, ST-HSC, MPP2, MPP3, and MPP4) express similar CD116 levels, and GM-CSF treatment of the isolated subsets induced a tolerized program, which was remarkably stronger in the MPP2 and MPP3 subsets (Figure 3C-E; supplemental Figure 5B).
Altogether, these data show that GM-CSF strongly activates STAT5, ERK, and the Akt/mTOR pathways in LKS− CD34+ FcγR+ but not in LKS+ cells, which is consistent with the STAT5 and AP-1 motif enrichment observed in the scATAC-seq analysis of the GM-CSF–stimulated myeloid progenitors (Figure 2H).
GM-CSF–mediated NF-κB activity in LKS+ cells program them to produce tolerized macrophages
GM-CSF can also activate the NF-κB signaling pathway,4 which has been shown to be involved in the Pam3CSK4- or TNF-α–induced tolerization in human and mouse macrophages.14 Therefore, we measured IκBα degradation by flow cytometry in both progenitor subsets, which showed IκBα degradation 20 minutes and 40 minutes after GM-CSF stimulation, although faster and stronger in LKS+ cells (Figure 4A). Interestingly, nuclear expression of p65 was higher in 59% of GM-CSF–treated LKS+ cells, whereas in LKS− CD34+ FcγR+ cells NF-κB nuclear translocation was inhibited (Figure 4B).
NF-κB activation in LKS+ and LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) IκBα was measured by intracellular flow cytometry in LKS+ and LKS− CD34+ FcγR+ progenitors at 20 and 40 minutes upon GM-CSF (50 ng/mL) stimulation. (B) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ and LKS− CD34+ FcγR+ cells after 3 hours with or without GM-CSF (50 ng/mL) stimulation; scale bar, 2 μm. (C) LKS+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL), and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the NF-κB inhibitor BMS345541 (2 μM) was added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 8 more days. (D-E) TNF-α and IL-6 production was measured by ELISA in supernatants (D), and by intracellular flow cytometry upon LPS stimulation (E). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the heatmaps, and bar graphs for MFIs, and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way analysis of variance (ANOVA) followed by the Dunnett test for multiple comparisons (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). ab, antibody; AU, arbitrary units; BMS, BMS345541; mφ, macrophage.
NF-κB activation in LKS+ and LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) IκBα was measured by intracellular flow cytometry in LKS+ and LKS− CD34+ FcγR+ progenitors at 20 and 40 minutes upon GM-CSF (50 ng/mL) stimulation. (B) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ and LKS− CD34+ FcγR+ cells after 3 hours with or without GM-CSF (50 ng/mL) stimulation; scale bar, 2 μm. (C) LKS+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL), and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the NF-κB inhibitor BMS345541 (2 μM) was added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 8 more days. (D-E) TNF-α and IL-6 production was measured by ELISA in supernatants (D), and by intracellular flow cytometry upon LPS stimulation (E). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the heatmaps, and bar graphs for MFIs, and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way analysis of variance (ANOVA) followed by the Dunnett test for multiple comparisons (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). ab, antibody; AU, arbitrary units; BMS, BMS345541; mφ, macrophage.
To assess the effect of NF-κB activity on the tolerization of GM-CSF–stimulated LKS+ cells, we used the inhibitor of nuclear factor-κB kinase inhibitor BMS345541 (Figure 4C). NF-κB inhibition partially restored the ability of macrophages derived from GM-CSF–stimulated LKS+ cells to produce TNF-α and IL-6 (Figure 4D-E), denoting that NF-κB plays a critical role in the GM-CSF–induced tolerization program. Interestingly, the previous scATAC-seq analysis did not show any RELA motif enrichment in any of the clusters examined (supplemental Figure 5D), suggesting a secondary NF-κB–induced mechanism to establish the tolerization program.
GM-CSF–induced β-catenin restrains NF-κB–mediated tolerization in myeloid progenitors to allow for trained immunity
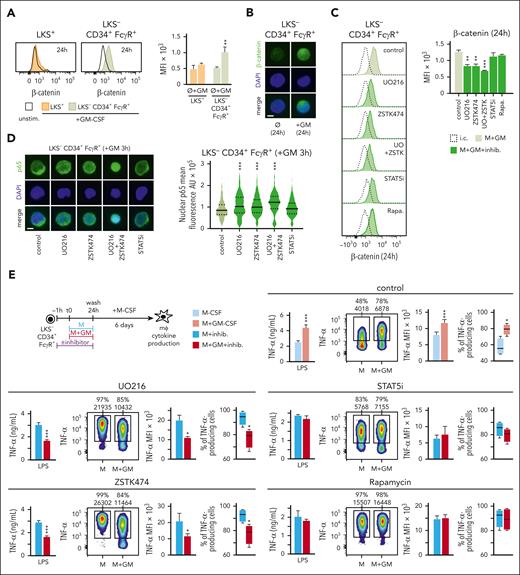
We next studied the potential mechanism preventing p65 nuclear translocation in myeloid progenitors. Previous reports demonstrated that β-catenin can inhibit NF-κB DNA binding through physical interaction15,16 and GM-CSF upregulates β-catenin in a myeloid cell line by inhibiting GSK3.17 According to this, we found upregulation of cytoplasmic β-catenin in LKS− CD34+ FcγR+, but not in LKS+ cells, upon GM-CSF stimulation (Figure 5A-B).
Signaling pathways involved in the accumulation of β-catenin in LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) β-catenin was measured by intracellular flow cytometry in LKS+ and LKS− CD34+ FcγR+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation. (B) Microphotographs of β-catenin location by immunofluorescence in LKS− CD34+ FcγR+ cells after 24 hours of GM-CSF (50 ng/mL) stimulation; scale bar, 2 μm. (C) β-catenin was measured by intracellular flow cytometry in LKS− CD34+ FcγR+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation with or without the indicated inhibitors (UO216 10 μM, ZSTK474 1 μM, STAT5i 10 μM and Rapamycin 40 ng/mL) 1 hour before and during stimulation. (D) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS− CD34+ FcγR+ cells after 3 hours of GM-CSF (50 ng/mL) stimulation with or without the indicated inhibitors 1 hour before and during stimulation; scale bar, 2 μm. (E) LKS− CD34+ FcγR+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the indicated inhibitors or dimethyl sulfoxide control solvent were added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 6 more days. TNF-α production upon LPS stimulation was measured by ELISA in supernatants and by intracellular flow cytometry. For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way ANOVA followed by the Dunnett test for multiple comparisons (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). AU, arbitrary units; i.c., isotype control; Rapa., rapamycin; unstim. or Ø, unstimulated; UO, UO216; ZSTK, ZSTK474.
Signaling pathways involved in the accumulation of β-catenin in LKS− CD34+ FcγR+ progenitors in response to GM-CSF. (A) β-catenin was measured by intracellular flow cytometry in LKS+ and LKS− CD34+ FcγR+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation. (B) Microphotographs of β-catenin location by immunofluorescence in LKS− CD34+ FcγR+ cells after 24 hours of GM-CSF (50 ng/mL) stimulation; scale bar, 2 μm. (C) β-catenin was measured by intracellular flow cytometry in LKS− CD34+ FcγR+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation with or without the indicated inhibitors (UO216 10 μM, ZSTK474 1 μM, STAT5i 10 μM and Rapamycin 40 ng/mL) 1 hour before and during stimulation. (D) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS− CD34+ FcγR+ cells after 3 hours of GM-CSF (50 ng/mL) stimulation with or without the indicated inhibitors 1 hour before and during stimulation; scale bar, 2 μm. (E) LKS− CD34+ FcγR+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the indicated inhibitors or dimethyl sulfoxide control solvent were added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 6 more days. TNF-α production upon LPS stimulation was measured by ELISA in supernatants and by intracellular flow cytometry. For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way ANOVA followed by the Dunnett test for multiple comparisons (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). AU, arbitrary units; i.c., isotype control; Rapa., rapamycin; unstim. or Ø, unstimulated; UO, UO216; ZSTK, ZSTK474.
To further dissect the mechanism that promotes cytoplasmic β-catenin accumulation, we used specific inhibitors for ERK (UO216), PI3K (ZSTK474), STAT5 (STAT5i), and mTOR (Rapamycin) (supplemental Figure 5C). We found a decrease in intracellular β-catenin levels when ERK or PI3K were inhibited, with an additive effect when both pathways were targeted. No changes were observed by inhibiting STAT5 or mTOR (Figure 5C). Consistent with β-catenin preventing NF-κB nuclear translocation, p65 nuclear levels increased when GM-CSF–stimulated LKS− CD34+ FcγR+ cells were treated with ERK, PI3K, or both inhibitors, but not when STAT5 was inhibited (Figure 5D).
Finally, we studied the contribution of GM-CSF–induced pathways to the trained inflammatory phenotype of myeloid progenitor-derived macrophages and found that this trained phenotype was lost when each individual pathway (ERK, PI3K, STAT5, or mTOR) was inhibited (Figure 5E). Interestingly, inhibition of ERK and PI3K resulted in a tolerized phenotype (Figure 5E), consistent with the higher p65 nuclear levels and the role of NF-κB in establishing a tolerization program.
Collectively, these results demonstrate that ERK- and PI3K–driven β-catenin accumulation in myeloid progenitors prevents NF-κB translocation to the nucleus and the induction of a tolerization program; whereas mTOR and STAT5 are not needed to block NF-κB activation but are required to establish a trained inflammatory memory in myeloid progenitors.
GSK3 inhibition blocks NF-κB activity and the tolerization program in GM-CSF–treated LKS+ cells
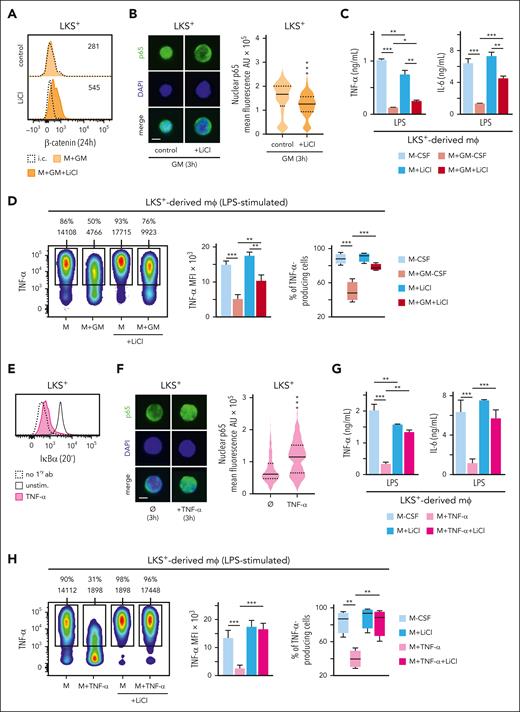
Active GSK3 phosphorylates β-catenin, resulting in its degradation in the proteasome.18,19 To address the importance of GSK3 in the GM-CSF–induced tolerization program, we inhibited GSK3 with lithium chloride (LiCl) in GM-CSF–treated LKS+ cells and found an increase in intracellular β-catenin levels (Figure 6A) and a significant reduction of p65 translocation to the nucleus (Figure 6B). More importantly, LiCl partially prevented the tolerization phenotype of LKS+-derived macrophages (Figure 6C-D).
Nuclear NF-κB levels in GM-CSF–stimulated LKS+ cells upon GSK3 inhibition. (A) β-catenin was measured by intracellular flow cytometry in LKS+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation with or without LiCl (10 mM) 1 hour before and during stimulation. (B) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ cells after 3 hours of GM-CSF (50 ng/mL) stimulation with or without LiCl 1 hour before and during stimulation; scale bar, 2 μm. (C-D) LKS+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the GSK3 inhibitor LiCl (10 mM) was added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 8 more days. TNF-α and IL-6 production by macrophages upon LPS stimulation was measured by ELISA in supernatants (C), and TNF-α by intracellular flow cytometry (D). (E) IκBα was measured by intracellular flow cytometry in LKS+ progenitors at 20 minutes upon TNF-α (100 ng/mL) stimulation. (F) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ cells after 3 hours with or without TNF-α (100 ng/mL) stimulation; scale bar, 2 μm. (G-H) LKS+ cells were treated as in panels C-D, but using TNF-α (100 ng/mL) for stimulation instead of GM-CSF, and TNF-α and IL-6 production by macrophages upon LPS stimulation was measured by ELISA in supernatants (G), and TNF-α by intracellular flow cytometry (H). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI is indicated in the heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way ANOVA followed by the Dunnett test for multiple comparisons (∗P < .05, ∗∗P < .01 and ∗∗∗P < .001). AU, arbitrary units; i.c., isotype control; mφ, macrophage.
Nuclear NF-κB levels in GM-CSF–stimulated LKS+ cells upon GSK3 inhibition. (A) β-catenin was measured by intracellular flow cytometry in LKS+ progenitors at 24 hours upon GM-CSF (50 ng/mL) stimulation with or without LiCl (10 mM) 1 hour before and during stimulation. (B) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ cells after 3 hours of GM-CSF (50 ng/mL) stimulation with or without LiCl 1 hour before and during stimulation; scale bar, 2 μm. (C-D) LKS+ cells were isolated from the bone marrow of C57BL/6 mice, cultured with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, the GSK3 inhibitor LiCl (10 mM) was added 1 hour before and during the 24 hours of GM-CSF stimulation, cells were washed, and differentiated into macrophages with M-CSF for 8 more days. TNF-α and IL-6 production by macrophages upon LPS stimulation was measured by ELISA in supernatants (C), and TNF-α by intracellular flow cytometry (D). (E) IκBα was measured by intracellular flow cytometry in LKS+ progenitors at 20 minutes upon TNF-α (100 ng/mL) stimulation. (F) Microphotographs of p65 location (left) and nuclear p65 mean fluorescence quantification (right) by immunofluorescence in LKS+ cells after 3 hours with or without TNF-α (100 ng/mL) stimulation; scale bar, 2 μm. (G-H) LKS+ cells were treated as in panels C-D, but using TNF-α (100 ng/mL) for stimulation instead of GM-CSF, and TNF-α and IL-6 production by macrophages upon LPS stimulation was measured by ELISA in supernatants (G), and TNF-α by intracellular flow cytometry (H). For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI is indicated in the heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test and the 1-way ANOVA followed by the Dunnett test for multiple comparisons (∗P < .05, ∗∗P < .01 and ∗∗∗P < .001). AU, arbitrary units; i.c., isotype control; mφ, macrophage.
To further confirm this mechanism, we used TNF-α to activate NF-κB in LKS+ cells.20 As expected, TNF-α rapidly degraded IκBα (Figure 6E) and translocated p65 to the nucleus (Figure 6F). TNF-α stimulation of LKS+ cells also tolerized the macrophages they produce, which was almost fully impeded by GSK3 inhibition with LiCl (Figure 6G-H).
These results demonstrate that active GSK3 is required in LKS+ cells to prevent β-catenin accumulation, which permits NF-κB translocation to the nucleus upon GM-CSF or TNF-α stimulation to establish a tolerization program.
The GM-CSF–driven tolerization program in LKS+ cells can be counterbalanced, occurs in other trained immunity murine models and in human CD34+ CD38− cells
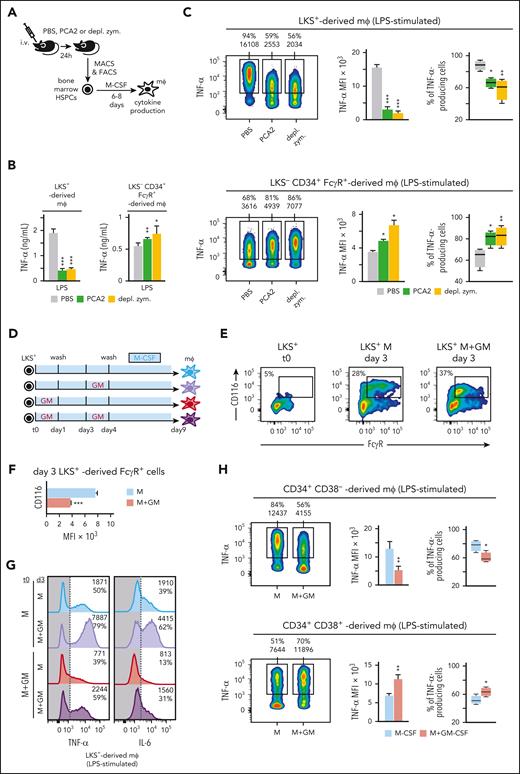
Next, we wondered whether the tolerization program observed in GM-CSF–stimulated LKS+ cells could also be found in other well-established models of trained immunity.9,10 Bone marrow LKS− CD34+ FcγR+ or LKS+ cells from C albicans (PCA2)- or β-glucan (depleted zymosan)–injected mice, were differentiated into macrophages and TNF-α production was measured (Figure 7A), showing that macrophages derived from LKS+ cells were tolerized, whereas those derived from LKS− CD34+ FcγR+ cells were trained (Figure 7B-C).
HSPC-derived macrophages phenotype in other models of trained immunity, and modulation of inflammatory tolerization. (A) C57BL/6 mice were injected IV with PBS, 1.5 million PCA2 yeasts or 300 μg per mouse of depleted zymosan and 24 hours later LKS− CD34+ FcγR+ or LKS+ cells were isolated from the bone marrow, and differentiated into macrophages with M-CSF (50 ng/mL) for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by ELISA in supernatants upon LPS stimulation (B) and TNF-α by intracellular flow cytometry upon LPS stimulation (C). (D) LKS+ cells isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed, cultured with M-CSF for 2 more days, restimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed, and differentiated into macrophages with M-CSF for 5 more days. (E) Purified LKS+ cells or day 3 LKS+-derived cells, treated or not with GM-CSF for the first 24 hours, were analyzed for FcγR and CD116 expression by flow cytometry. (F) CD116 MFI from LKS+–derived FcγR+ cells are expressed as means + SD. (G) TNF-α and IL-6 production was measured by intracellular flow cytometry in macrophages obtained as in panel D upon 4 hours of LPS stimulation and brefeldin A for the final 1 hour. (H) Human CD34+ CD38− (selected as CD11b− CD10− CD34+ CD38− cells) and CD34+ CD38+ (selected as CD11b− CD10− CD34+ CD38+ CD45RA+ cells), were isolated from human peripheral blood mononuclear cells of human bone marrow, cultured in vitro with hM-CSF (50 ng/mL) + hSCF (50 ng/mL) and stimulated with or without hGM-CSF (50 ng/mL) for 24 hours, washed, differentiated into macrophages with hM-CSF for 14 or 7 days, respectively, and TNF-α production was measured by intracellular flow cytometry upon LPS stimulation. For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the histograms or heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). depl. zym., depleted zymosan; mφ, macrophage.
HSPC-derived macrophages phenotype in other models of trained immunity, and modulation of inflammatory tolerization. (A) C57BL/6 mice were injected IV with PBS, 1.5 million PCA2 yeasts or 300 μg per mouse of depleted zymosan and 24 hours later LKS− CD34+ FcγR+ or LKS+ cells were isolated from the bone marrow, and differentiated into macrophages with M-CSF (50 ng/mL) for 6 or 8 days, respectively; TNF-α and IL-6 production was measured by ELISA in supernatants upon LPS stimulation (B) and TNF-α by intracellular flow cytometry upon LPS stimulation (C). (D) LKS+ cells isolated from the bone marrow of mice were cultured in vitro with M-CSF (50 ng/mL) + SCF (20 ng/mL) and stimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed, cultured with M-CSF for 2 more days, restimulated with or without GM-CSF (50 ng/mL) for 24 hours, washed, and differentiated into macrophages with M-CSF for 5 more days. (E) Purified LKS+ cells or day 3 LKS+-derived cells, treated or not with GM-CSF for the first 24 hours, were analyzed for FcγR and CD116 expression by flow cytometry. (F) CD116 MFI from LKS+–derived FcγR+ cells are expressed as means + SD. (G) TNF-α and IL-6 production was measured by intracellular flow cytometry in macrophages obtained as in panel D upon 4 hours of LPS stimulation and brefeldin A for the final 1 hour. (H) Human CD34+ CD38− (selected as CD11b− CD10− CD34+ CD38− cells) and CD34+ CD38+ (selected as CD11b− CD10− CD34+ CD38+ CD45RA+ cells), were isolated from human peripheral blood mononuclear cells of human bone marrow, cultured in vitro with hM-CSF (50 ng/mL) + hSCF (50 ng/mL) and stimulated with or without hGM-CSF (50 ng/mL) for 24 hours, washed, differentiated into macrophages with hM-CSF for 14 or 7 days, respectively, and TNF-α production was measured by intracellular flow cytometry upon LPS stimulation. For the ELISA measurements triplicate samples were analyzed and expressed as means + SD and for intracellular cytokine measurements, percentage of cytokine-producing cells and MFI are indicated in the histograms or heatmaps, and bar graphs for MFIs and box plots for cell percentages are expressed as means + SD. Data presented are 1 representative experiment of at least 3 independent experiments. Statistical significance was assessed by the Student t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). depl. zym., depleted zymosan; mφ, macrophage.
On the other hand, we hypothesized that once GM-CSF–stimulated LKS+ cells become myeloid progenitors, with increased GM-CSFR levels, a second challenge with GM-CSF could counterbalance the tolerized phenotype of their derived macrophages (Figure 7D). We first verified the presence of myeloid progenitors (FcγR+) with a higher expression of GM-CSFR in 3-day LKS+ cell cultures, with or without GM-CSF (Figure 7E-F). Next, these 3-day LKS+-derived cells were rechallenged with or without GM-CSF, and differentiated into macrophages for 5 more days (Figure 7D). We found that untreated LKS+ cells produced trained macrophages upon stimulation with GM-CSF at day 3 (Figure 7G). Remarkably, day 3 restimulation of GM-CSF–treated LKS+ cells gave rise to macrophages that produce similar TNF-α levels as those treated with M-CSF alone (Figure 7G). Interestingly, CD116 expression levels on LKS+-derived myeloid progenitors at day 3 correlate with cytokine production levels by their derived macrophages (Figure 7F-G). These results indicate that the tolerization program established in LKS+ cells by GM-CSF can be counterbalanced by a second GM-CSF challenge once differentiated into myeloid progenitors with higher GM-CSFR expression.
We finally tested the effects of GM-CSF on human HSPCs. We purified the human equivalent of murine LKS+ cells (CD34+ CD38−) and myeloid progenitors (CD34+ CD38+) from the bone marrow of healthy donors (supplemental Figure 5E), stimulated them with GM-CSF for 24 hours, differentiated them into macrophages with M-CSF for 14 or 7 days, respectively, and measured their ability to produce cytokines. GM-CSF–stimulated CD34+ CD38− cells produced tolerized macrophages, whereas GM-CSF–stimulated human myeloid progenitors produced trained macrophages (Figure 7H), consistent with the higher CD116 expression on human myeloid progenitors than on their upstream subsets.21 These data demonstrate that GM-CSF also induces a tolerization program in the most undifferentiated human progenitors.
Discussion
Our study reveals a novel molecular mechanism of immunomodulation by GM-CSF at different stages of myelopoiesis. We found that proinflammatory cytokine production is trained in macrophages derived from GM-CSF–stimulated myeloid-committed progenitors, but tolerized in macrophages derived from GM-CSF–treated noncommitted LKS+ progenitors. We observed that GM-CSF strongly activates STAT5, ERK, and Akt-mTOR signaling pathways in myeloid progenitors, whereas LKS+ progenitors weakly activate ERK and Akt but efficiently translocate NF-κB to the nucleus (supplemental Figure 6). These different responses to GM-CSF likely arise from the differential abundance of GM-CSFR, as myeloid-committed progenitors have a higher expression than LKS+ progenitors. Previous studies demonstrated that GM-CSF signaling depends on the receptor-ligand complex stoichiometry. The high-affinity complex of GM-CSFR is a dodecamer that enables the activation of Janus Kinase 2/STAT5, ERK, Akt, and NF-κB signaling pathways, whereas a tetramer structure only triggers Akt and NF-κB pathways, suggesting alternative receptor configurations regulate different signaling pathways.4 Although further studies are needed, we hypothesize that GM-CSF signals through dodecamers in myeloid progenitors, whereas signaling through tetramers in LKS+ progenitors.
Consistent with previous reports on trained immunity,8 we find a much stronger Akt-mTOR pathway activation in myeloid progenitors than in LKS+ cells upon GM-CSF treatment, which is essential for the establishment of the trained program. Furthermore, GM-CSF treatment enriches for open chromatin regions with STAT5 and AP-1 motifs in a subset of myeloid progenitors, whereas no substantial changes in open chromatin regions are found in LKS+ cells. Although STAT5 and AP-1 establish the trained program in myeloid progenitors, over the course of differentiation, there could be many subsequent epigenetic events that preserve this program.
A large body of studies have shown that TLR ligands or TNF-α exposure induces an NF-κB tolerant state in macrophages,14,22 and that TLR signaling in HSPCs tolerizes the cytokine response of the macrophages they produce.23 Here, we demonstrate that inhibiting NF-κB in LKS+ cells blocks the tolerization program induced by GM-CSF. Interestingly, IκBα is degraded in both GM-CSF-stimulated LKS+ and myeloid progenitors but NF-κB is translocated to the nucleus only in LKS+ cells. Similarly, TNF-α was previously shown to strongly activate NF-κB nuclear translocation in HSCs, but not GMPs, to promote HSC survival and myeloid differentiation.20 Here, we demonstrate that GSK3/β-catenin regulation of NF-κB translocation to the nucleus determines the type of inflammatory memory in HSPCs. Previous reports found that β-catenin can physically complex with NF-κB, resulting in a reduction of NF-κB DNA binding and an inhibition of NF-κB target genes.15 GSK3 is a negative regulator of β-catenin stability, so its inhibition causes constitutive β-catenin protein stabilization.18,19 Previous reports proved that PI3K/Akt or ERK activation inhibits GSK3, resulting in β-catenin upregulation.24,25 We found that β-catenin increases in the cytoplasm of myeloid progenitors upon GM-CSF stimulation, indicating the inhibition of GSK3 by GM-CSF signaling in these cells. Moreover, inhibiting PI3K and ERK during the GM-CSF treatment reduces β-catenin levels and causes NF-κB translocation to the nucleus. In fact, this inhibition not only blocks the trained program in myeloid progenitors, but also establishes a tolerized program.
In GM-CSF-stimulated LKS+ progenitors, constitutively active GSK3 maintains low levels of β-catenin allowing for NF-κB translocation to the nucleus. Therefore, inhibition of GSK3 activity with LiCl increases β-catenin levels and prevents NF-κB translocation to the nucleus, blocking the tolerization program. This is consistent with a previous report in which inhibition of GSK3 reverses TNF-α–induced cross tolerance in macrophages.14 Other reports also show that β-catenin activation reduces the DNA-binding activity of RelA and the expression of proinflammatory NF-κB target genes.16,26
Numerous molecular mechanisms in macrophage tolerance have been described.27 Although we do not observe open chromatin differences in GM-CSF–stimulated LKS+ progenitors or their derived macrophages, NF-κB may be indirectly controlling gene transcription by enhancing the expression of negative regulators of pattern recognition receptor signaling or leading to other epigenetic alterations to repress gene transcription, such as changes in DNA methylation patterns or histones modifications.28 For instance, it has been shown that changes in DNA methylation at enhancers correlate with disrupted immune gene expression, which underlies monocyte dysregulation and innate exhaustion memory in a mouse sepsis model.29 Moreover, active transcription factors can also recruit epigenetic repressors to restrain transcription of their target gene. For example, p65 recruits Sirtuin 6 (SIRT6) to promote histone deacetylation of its target promoters.30 Finally, we confirm that the GM-CSF–induced tolerization program in LKS+ progenitors also occurs in other models of murine trained immunity and in their equivalent in human bone marrow, which highlights the relevance of GM-CSF as a therapeutic strategy.
The immune system is constantly seeking balance to avoid tissue damage by an inappropriate inflammatory response. Our study reveals another level of immunomodulation by GM-CSF at different stages of myelopoiesis, suggesting that the more immediate progenitors to produce myeloid cells establish a trained program for cytokine production to overcome infection, whereas progenitors with higher expansion potential become tolerized to protect against the deleterious effects of a cytokine storm. These functional differences in monocytes and macrophages based on their origin are in line with recent studies, which demonstrate functionally diverse monocyte subtypes defined by the progenitor epigenome they are originated from.31,32
Our results also demonstrate the long-lasting effects of GM-CSF on progenitors, as primary transplantation into irradiated mice preserve the trained or tolerized phenotype in monocytes for at least 16 weeks. Interestingly, secondary reconstituted mice do not display trained monocytes, suggesting that self-renewing HSCs, acting during serial transplantation, are protected from the GM-CSF effects.
These results have significant implicants for the clinical applications of GM-CSF, revealing the molecular pathways that could be manipulated in hematopoietic progenitors to obtain the desired effects on the inflammatory responses of the innate immune system.
Further investigation will be needed to understand the mechanisms by which NF-κB establishes a tolerization program in LKS+ progenitors, and the study of other immunological features in their progeny will contribute to the development of therapeutic interventions to precisely modulate inflammatory responses.
Acknowledgments
The authors thank the Servicio Central de Soporte a la Investigación Experimental of the University of Valencia for technical assistance, and the 10x Genomics and the Bonsai Lab Core Grant Program at the Instituto de Investigación Sanitaria de Valencia for performing the scATAC-seq.
This work was supported by funds from the Ministerio de Ciencia, Innovación y Universidades from Spain, and the European Fund for Regional Development (grant PID2021-124937NB-I00 [A.Y.; M.L.G.]); Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital, Generalitat Valenciana (grant AICO/2021/350 [A.Y.; M.L.G.]); and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant K08-AI168567 [Q.J.C.]). A.Y. is the recipient of a Ramón y Cajal contract (RYC-2017-22895) from the “Ministerio de Ciencia, Innovación y Universidades,” Spain. P.G. is a recipient of the fellowship “Atracció de talent” from the University of Valencia. M.S. is a recipient of the fellowship “CIACIF” from Generalitat Valenciana.
Authorship
Contribution: A.Y. and M.L.G. designed experiments and discussed results; Q.J.C. performed the ATAC-seq data analysis and discussed results; P.G., C.B., M.S., and A.G. performed the experiments and analyzed the data; A.Y. wrote the manuscript and the remaining authors helped in editing; and all authors contributed to and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Yáñez, Instituto de Biotecnología y Biomedicina (BIOTECMED) and Departamento de Microbiología y Ecología, Facultat de Ciències Biològiques, Universitat de València, 46100 Burjassot, Spain; email: alberto.yanez@uv.es.
References
Author notes
The ATAC-seq data have been deposited in the Gene Expression Omnibus database (accession number GSE239650).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal