Second primary malignancies were reported in 536 of 12 394 (4.3%) adverse event reports following chimeric antigen receptor T-cell therapies in the Food and Drug Administration Adverse Event Reporting System. Myeloid and T-cell neoplasms were disproportionately more frequently reported, warranting further follow-up.
TO THE EDITOR:
Chimeric antigen receptor T-cell (CAR T) therapies have emerged as groundbreaking treatments for different hematologic malignancies.1 To date, the Food and Drug Administration (FDA) has approved 6 CAR T products for relapsed or refractory B-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, and multiple myeloma. CAR T–eligible patients are often heavily pretreated with a higher risk of treatment-related adverse events (AEs), including second primary malignancies (SPMs).2,3 Recently, the FDA received reports of CAR-positive lymphomas in patients treated with CAR T products.4 Such concerns highlight the need for better characterization of SPM risk after CAR T therapy. Herein, we analyzed the FDA Adverse Events Reporting System (FAERS) database to quantify the CAR T reports with SPMs. Detailed methods can be found in the supplemental Appendix, available on the Blood website.
We identified 12 394 unique CAR T AE reports, of which 2225 were associated with the system organ class “Neoplasms benign, malignant and unspecified.” After applying exclusion criteria, 536 of 12 394 (4.3%) SPM reports were included. Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) comprised most of the reports (277 of 536, 51.7% and 177 of 536, 33.0%, respectively). Characteristics of the AE reports are detailed in Table 1.
Characteristics of FAERS CAR T reports
| . | All CAR T reports . | All SPM CAR T reports . | Axi-cel SPM reports . | Tisa-cel SPM reports . | Brexu-cel SPM reports . | Liso-cel SPM reports . | Ide-cel SPM reports . | Cilta-cel SPM reports . |
|---|---|---|---|---|---|---|---|---|
| N | 12 394 | 536 | 277 | 177 | 20 | 23 | 15 | 24 |
| Age, y | ||||||||
| Mean (SD) | 53.8 (20.5) | 58.7 (18.2) | 61.3 (11.1) | 50.1 (26.2) | 58.4 (18.8) | 69.4 (9.9) | 65.0 (8.3) | 68.5 (7.8) |
| Median (IQR) | 60.0 (45.0-68.0) | 63.0 (56.0-70.0) | 62.0 (56.0-68.0) | 62.0 (20.0-70.8) | 62.0 (60.3-70.3) | 71.0 (67.0-76.0) | 66.5 (56.0-70.0) | 71.0 (63.0-75.0) |
| Missing | 3 884 | 131 | 62 | 55 | 6 | 2 | 3 | 3 |
| Sex (%) | ||||||||
| Female | 3 813 (38.1) | 181 (36.8) | 91 (34.5) | 68 (44.2) | 3 (16.7) | 10 (45.5) | 3 (23.1) | 6 (28.6) |
| Male | 6 182 (61.9) | 311 (63.2) | 173 (65.5) | 86 (55.8) | 15 (83.3) | 12 (54.5) | 10 (76.9) | 15 (71.4) |
| Missing | 2 399 | 44 | 13 | 23 | 2 | 1 | 2 | 3 |
| Mean weight (SD), kg | 75.78 (24.6) | 74.21 (24.1) | 75.73 (18.9) | 68.73 (28.4) | 74.5 (23.9) | 84.5 (27.1) | 78.9 (8.8) | 76.9 (17.0) |
| Reporter (%) | ||||||||
| Consumer | 1 274 (11.4) | 24 (4.6) | 10 (3.7) | 13 (7.6) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) |
| Health care practitioner | 4 844 (43.3) | 220 (42.3) | 153 (56.9) | 53 (30.8) | 9 (50.0) | 3 (13.6) | 0 (0.0) | 2 (8.3) |
| Physician | 5 075 (45.3) | 276 (53.1) | 106 (39.4) | 106 (61.6) | 9 (50.0) | 19 (86.4) | 14 (93.3) | 22 (91.7) |
| Missing | 1 201 | 16 | 8 | 5 | 2 | 1 | 0 | 0 |
| Reporting region (%) | ||||||||
| North America | 7 530 (66.7) | 346 (66.3) | 201 (73.6) | 95 (54.0) | 11 (64.7) | 16 (84.2) | 7 (50.0) | 16 (69.6) |
| Europe | 2 890 (25.6) | 151 (28.9) | 70 (25.6) | 62 (35.2) | 6 (35.3) | 1 (5.3) | 7 (50.0) | 5 (21.7) |
| Asia | 613 (5.4) | 17 (3.3) | 1 (0.4) | 14 (8.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) |
| Africa | 1 (0.01) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 261 (2.3) | 8 (1.5) | 1 (0.4) | 5 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) |
| Missing | 1 099 | 14 | 4 | 1 | 3 | 4 | 1 | 1 |
| Report year (%) | ||||||||
| 2017 (October-December) | 72 (0.6) | 1 (0.2) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2018 | 833 (6.7) | 22 (4.1) | 9 (3.3) | 12 (6.8) | 0 (0.0) | 1 (4.4) | 0 (0.0) | 0 (0.0) |
| 2019 | 1 652 (13.3) | 43 (8.0) | 17 (6.1) | 26 (14.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2020 | 1 752 (14.1) | 80 (14.9) | 52 (18.8) | 22 (12.4) | 0 (0.0) | 4 (17.4) | 2 (13.3) | 0 (0.0) |
| 2021 | 1 927 (15.6) | 114 (21.3) | 59 (21.3) | 41 (23.2) | 4 (20.0) | 9 (39.1) | 1 (6.7) | 0 (0.0) |
| 2022 | 2 611 (21.1) | 129 (24.1) | 80 (28.9) | 22 (12.4) | 5 (25.0) | 5 (21.7) | 6 (40.0) | 11 (45.8) |
| 2023 | 3 547 (28.6) | 147 (27.4) | 60 (21.7) | 53 (30.0) | 11 (55.0) | 4 (17.4) | 6 (40.0) | 13 (54.2) |
| Outcome specified as serious (%) | 11 571 (93.4%) | 530 (98.9) | 276 (99.6) | 176 (99.4) | 19 (95.0) | 23 (100.0) | 15 (100.0) | 21 (87.50) |
| Outcome (%) | ||||||||
| Death | 2 861 (24.7) | 207 (39.1) | 124 (44.9) | 67 (38.1) | 6 (31.6) | 4 (17.4) | 4 (26.7) | 2 (9.5) |
| Disability | 94 (0.8) | 17 (3.2) | 4 (1.5) | 11 (6.3) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (4.8) |
| Hospitalization | 1 860 (16.1) | 37 (7.0) | 14 (5.1) | 13 (7.4) | 5 (26.3) | 0 (0.0) | 2 (13.3) | 3 (14.3) |
| Life-threatening | 673 (5.8) | 15 (2.8) | 4 (1.5) | 9 (5.1) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (4.8) |
| Required intervention | 28 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other serious | 6 055 (52.3) | 254 (47.9) | 130 (47.1) | 76 (43.2) | 8 (42.1) | 19 (82.6) | 7 (46.7) | 14 (66.7) |
| Missing | 823 | 6 | 1 | 1 | 1 | 0 | 0 | 3 |
| . | All CAR T reports . | All SPM CAR T reports . | Axi-cel SPM reports . | Tisa-cel SPM reports . | Brexu-cel SPM reports . | Liso-cel SPM reports . | Ide-cel SPM reports . | Cilta-cel SPM reports . |
|---|---|---|---|---|---|---|---|---|
| N | 12 394 | 536 | 277 | 177 | 20 | 23 | 15 | 24 |
| Age, y | ||||||||
| Mean (SD) | 53.8 (20.5) | 58.7 (18.2) | 61.3 (11.1) | 50.1 (26.2) | 58.4 (18.8) | 69.4 (9.9) | 65.0 (8.3) | 68.5 (7.8) |
| Median (IQR) | 60.0 (45.0-68.0) | 63.0 (56.0-70.0) | 62.0 (56.0-68.0) | 62.0 (20.0-70.8) | 62.0 (60.3-70.3) | 71.0 (67.0-76.0) | 66.5 (56.0-70.0) | 71.0 (63.0-75.0) |
| Missing | 3 884 | 131 | 62 | 55 | 6 | 2 | 3 | 3 |
| Sex (%) | ||||||||
| Female | 3 813 (38.1) | 181 (36.8) | 91 (34.5) | 68 (44.2) | 3 (16.7) | 10 (45.5) | 3 (23.1) | 6 (28.6) |
| Male | 6 182 (61.9) | 311 (63.2) | 173 (65.5) | 86 (55.8) | 15 (83.3) | 12 (54.5) | 10 (76.9) | 15 (71.4) |
| Missing | 2 399 | 44 | 13 | 23 | 2 | 1 | 2 | 3 |
| Mean weight (SD), kg | 75.78 (24.6) | 74.21 (24.1) | 75.73 (18.9) | 68.73 (28.4) | 74.5 (23.9) | 84.5 (27.1) | 78.9 (8.8) | 76.9 (17.0) |
| Reporter (%) | ||||||||
| Consumer | 1 274 (11.4) | 24 (4.6) | 10 (3.7) | 13 (7.6) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) |
| Health care practitioner | 4 844 (43.3) | 220 (42.3) | 153 (56.9) | 53 (30.8) | 9 (50.0) | 3 (13.6) | 0 (0.0) | 2 (8.3) |
| Physician | 5 075 (45.3) | 276 (53.1) | 106 (39.4) | 106 (61.6) | 9 (50.0) | 19 (86.4) | 14 (93.3) | 22 (91.7) |
| Missing | 1 201 | 16 | 8 | 5 | 2 | 1 | 0 | 0 |
| Reporting region (%) | ||||||||
| North America | 7 530 (66.7) | 346 (66.3) | 201 (73.6) | 95 (54.0) | 11 (64.7) | 16 (84.2) | 7 (50.0) | 16 (69.6) |
| Europe | 2 890 (25.6) | 151 (28.9) | 70 (25.6) | 62 (35.2) | 6 (35.3) | 1 (5.3) | 7 (50.0) | 5 (21.7) |
| Asia | 613 (5.4) | 17 (3.3) | 1 (0.4) | 14 (8.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) |
| Africa | 1 (0.01) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 261 (2.3) | 8 (1.5) | 1 (0.4) | 5 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) |
| Missing | 1 099 | 14 | 4 | 1 | 3 | 4 | 1 | 1 |
| Report year (%) | ||||||||
| 2017 (October-December) | 72 (0.6) | 1 (0.2) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2018 | 833 (6.7) | 22 (4.1) | 9 (3.3) | 12 (6.8) | 0 (0.0) | 1 (4.4) | 0 (0.0) | 0 (0.0) |
| 2019 | 1 652 (13.3) | 43 (8.0) | 17 (6.1) | 26 (14.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2020 | 1 752 (14.1) | 80 (14.9) | 52 (18.8) | 22 (12.4) | 0 (0.0) | 4 (17.4) | 2 (13.3) | 0 (0.0) |
| 2021 | 1 927 (15.6) | 114 (21.3) | 59 (21.3) | 41 (23.2) | 4 (20.0) | 9 (39.1) | 1 (6.7) | 0 (0.0) |
| 2022 | 2 611 (21.1) | 129 (24.1) | 80 (28.9) | 22 (12.4) | 5 (25.0) | 5 (21.7) | 6 (40.0) | 11 (45.8) |
| 2023 | 3 547 (28.6) | 147 (27.4) | 60 (21.7) | 53 (30.0) | 11 (55.0) | 4 (17.4) | 6 (40.0) | 13 (54.2) |
| Outcome specified as serious (%) | 11 571 (93.4%) | 530 (98.9) | 276 (99.6) | 176 (99.4) | 19 (95.0) | 23 (100.0) | 15 (100.0) | 21 (87.50) |
| Outcome (%) | ||||||||
| Death | 2 861 (24.7) | 207 (39.1) | 124 (44.9) | 67 (38.1) | 6 (31.6) | 4 (17.4) | 4 (26.7) | 2 (9.5) |
| Disability | 94 (0.8) | 17 (3.2) | 4 (1.5) | 11 (6.3) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (4.8) |
| Hospitalization | 1 860 (16.1) | 37 (7.0) | 14 (5.1) | 13 (7.4) | 5 (26.3) | 0 (0.0) | 2 (13.3) | 3 (14.3) |
| Life-threatening | 673 (5.8) | 15 (2.8) | 4 (1.5) | 9 (5.1) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (4.8) |
| Required intervention | 28 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other serious | 6 055 (52.3) | 254 (47.9) | 130 (47.1) | 76 (43.2) | 8 (42.1) | 19 (82.6) | 7 (46.7) | 14 (66.7) |
| Missing | 823 | 6 | 1 | 1 | 1 | 0 | 0 | 3 |
IQR, interquartile range; SD, standard deviation.
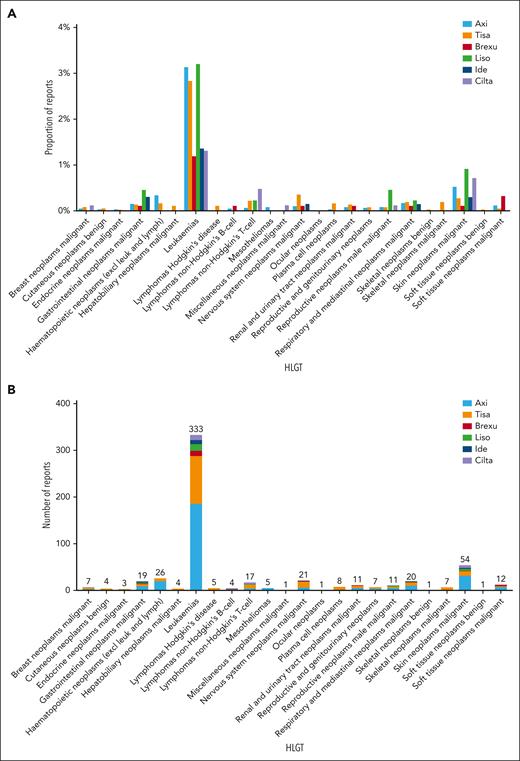
The most frequent SPMs by high-level group term were leukemias (333 of 536, 62.1%) representing 2.7% (333 of 12 394) of all CAR T reports. Leukemias included myelodysplastic syndromes (208 of 536, 38.8%; 208 of 12 394, 1.7%), acute myeloid leukemias (106 of 536, 19.8%; 106 of 12 394, 0.9%), and 2 cases of T-cell large granular lymphocytic leukemia. Skin neoplasms were the second most frequent SPM (54 of 536, 10.1%; 54 of 12 394, 0.4%), which included nonmelanoma skin neoplasms (42 of 536, 7.8%; 42 of 12 394, 0.3%) and skin melanomas (12 of 536, 2.2%; 12 of 12 394, 0.1%). Hematopoietic neoplasms excluding leukemias and lymphomas were reported in (26 of 536, 4.9%; 26 of 12 394, 0.2%) including lymphoproliferative disorder not elsewhere classified (NEC) (n = 15), myeloproliferative neoplasms (n = 7), and histiocytoses (n = 4). Nervous system tumors were reported in (21 of 536, 3.9%; 21 of 12 394, 0.2%), and respiratory neoplasms were reported in (20 of 536, 3.7%; 20 of 12 394, 0.2%) (Figure 1; supplemental Tables 6-11).
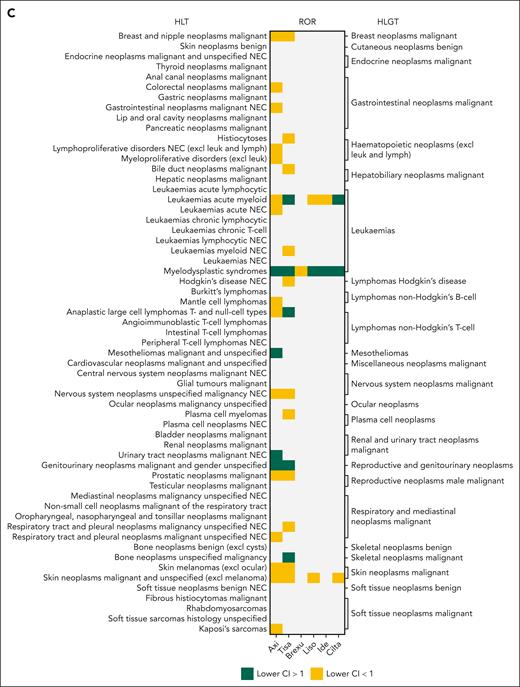
Frequency and disproportionality of reporting for second primary malignancies (SPMs) in different CAR T products. (A) Proportion of reports for each SPM (in high-level group terms) within each product, realtive to the number of AE reports in the respective product. (B) Absolute number of reports for each SPM (in high-level group terms) in CAR T products. (C) Disproportionality of reporting measured as the ROR, compared with non-CAR T drugs administered for the respective indication. Gray areas reflect insufficient number of reports (<3 reports) and thus ROR was not calculated. Green areas reflect significant ROR, defined as lower bound of the 95% CI of >1. Yellow areas reflect nonsignificant ROR signal. Axi, axicabtagene ciloleucel; Brexu, brexucabtagene autoleucel; Cilta, ciltacabtagene autoleucel; excl, excluding; HLGT, high-level group term; Ide, idecabtagene vicleucel; leuk, leukemia; Liso, lisocabtagene maraleucel; lymph, lymphoma; NEC, not elsewhere classified; Tisa, tisagenlecleucel.
Frequency and disproportionality of reporting for second primary malignancies (SPMs) in different CAR T products. (A) Proportion of reports for each SPM (in high-level group terms) within each product, realtive to the number of AE reports in the respective product. (B) Absolute number of reports for each SPM (in high-level group terms) in CAR T products. (C) Disproportionality of reporting measured as the ROR, compared with non-CAR T drugs administered for the respective indication. Gray areas reflect insufficient number of reports (<3 reports) and thus ROR was not calculated. Green areas reflect significant ROR, defined as lower bound of the 95% CI of >1. Yellow areas reflect nonsignificant ROR signal. Axi, axicabtagene ciloleucel; Brexu, brexucabtagene autoleucel; Cilta, ciltacabtagene autoleucel; excl, excluding; HLGT, high-level group term; Ide, idecabtagene vicleucel; leuk, leukemia; Liso, lisocabtagene maraleucel; lymph, lymphoma; NEC, not elsewhere classified; Tisa, tisagenlecleucel.
T-cell non-Hodgkin lymphomas were identified in 17 of 536 (3.2%) reports, representing 0.1% (17 of 12 394) of all CAR T reports. These included 12 anaplastic large T-cell lymphomas (7 in tisa-cel, 3 in axi-cel, and 2 in ciltacabtagene autoleucel [cilta-cel]), 3 peripheral T-cell lymphomas (1 in tisa-cel, 1 in cilta-cel, and 1 in lisocabtagene maraleucel [liso-cel]), 1 angioimmunoblastic T-cell lymphoma (axi-cel), and 1 enteropathy-associated T-cell lymphoma (cilta-cel). (Figure 1A). Most cases were reported from the United States (n = 9). Out of the 17 cases, 8 reported death, 4 reported hypogammaglobulinemia, 3 reported cytokine release syndrome, 2 reported hemophagocytic lymphohistiocytosis, and 2 reported neurotoxicity. The enteropathy-associated T-cell lymphoma report also listed immune-mediated enterocolitis. Two cases reported out-of-specification manufacturing without additional information (Tables S13).
Analysis of SPM disproportionality in the CAR reports showed a significantly higher reporting odds ratio (ROR) for myelodysplastic syndrome in axi-cel (ROR = 3.5 [95% confidence interval {CI} 2.9-4.2]), tisa-cel (ROR = 1.3 [95% CI 1.0-1.8]), liso-cel (ROR = 4.6 [95% CI 2.4-8.5]), idecabtagene vicleucel (ide-cel) (ROR = 2.8 [95% CI 1.2-6.7]), and cilta-cel (ROR = 6.7 [95% CI 3.3-13.5]) (Figure 1C). Tisa-cel and cilta-cel were associated with higher ROR for acute myeloid leukemia (ROR = 1.5 [95% CI 1.2-2.0]; and 4.1 [95% CI 1.3-12.8], respectively). Anaplastic large T-cell lymphomas were disproportionately more reported in tisa-cel (ROR = 7.4 [95% CI 3.1-17.4]) (Figure 1C).
SPMs have been extensively documented in survivors of hematologic malignancies.2,3 However, fewer studies have reported on SPM incidence after CAR T therapies. Six of the 8 pivotal trials reported the incidence of SPMs. SPMs after Tisa-cel were reported in 3 of 137 (2.2%) in the acute lymphoblastic leukemia trials (ELIANA and ENSIGN),5 although no SPMs were reported in the large B-cell lymphoma trial (JULIET).6 The ZUMA-1 and ZUMA-7 trials reported incidence of SPMs after axi-cel of <1% and 4.7%, respectively.7,8 No SPMs were reported after the brexucabtagene autoleucel ZUMA-3 trial.9 The incidences of SPMs after liso-cel were 8.1% and 3.3% in the TRANSCEND NHL and TRANSFORM trials, respectively.10,11 Although CARTITUDE-1 reported SPMs of 25.8% after cilta-cel,12 the KarMMa-1 trial did not report on SPMs after ide-cel.13 SPM incidence of 3.6% after commercial CD19 and BCMA CAR T products was reported by Ghilardi and colleagues.14 Other reports on commercial CAR T cells indicated an SPM incidence between 3.9% and 4.5%.15,16 Although SPMs represented 4.3% of the submitted CAR T FAERS reports, this percentage only reflects the likelihood of reporting SPMs to the FDA.
ROR of myeloid neoplasms was elevated in 5 of 6 of the CAR-T products. Myeloid neoplasms after CAR T were reported in the pivotal trials with SPM data as well as other investigational studies.1,7,8,13,17 A recent study reported a shorter onset of myeloid neoplasms after CAR T compared with their development following stem cell transplantation.1,17 Additionally, cytogenetic and clonal abnormalities were frequently present in patients before receiving CAR T therapies, suggesting a clonal evolution of existing treatment-related clonal hematopoiesis.17,18
The FDA indicated that 22 cases of T-cell malignancies were reported to be associated with 5 of the 6 CAR-T products.19 Genetic sequencing was performed for 3 cases with the CAR transgene identified in the malignant clones.19 We identified 19 cases of T-cell malignancies (17 T-cell non-Hodgkin lymphomas and 2 T-cell large granular lymphocytic leukemia). Details on 2 cases associated with cilta-cel and liso-cel were previously published.20,21 CAR transgene integration into the 3′ untranslated region of the PBX2 gene was detected in the cilta-cel case. However, the evidence was inconclusive as to whether the CAR integration was a driver of the malignant transformation, given the presence of preexisting genetic mutations unrelated to CAR T infusion. Ghilardi et al identified a case of peripheral T-cell lymphoma developed 3 months after receiving axi-cel.14 The CAR transgene copies in the tumor biopsy were very low. Next-generation sequencing analysis revealed that the population giving rise to the malignant clone predated the CAR T infusion. However, CAR T manufacturing or the induced inflammation could not be excluded as contributors to the lymphoma development. Finally, additional studies have reported viral integrations into key hematopoiesis regulatory genes, such as TET2 and CBL, resulting in clonal expansion in 2 responding CAR T patients with no malignant transformation reported to date.22,23
The FAERS database remains a valuable resource for identifying AEs not captured during clinical studies; however, it has limitations such as duplicate report submissions, missing information, inability to establish causal relationships, and underreporting or overreporting based on AE severity. Additionally, the absence of a denominator reflecting the total number of prescribed products limits the ability to establish AE incidence. Finally, the quarterly release of raw data may delay independent analysis and public information dissemination.
In conclusion, SPMs after CAR T represent a small fraction of the AE reports in FAERS. The disproportionality analysis suggests an increased risk of reporting certain SPMs, notably myeloid and T-cell malignancies. The low numbers do not provide conclusive evidence of the risk of SPMs after CAR T therapy. Dedicated registries to study SPMs after CAR T therapy can offer valuable insights for patient care and future development. This becomes pertinent as CAR T therapies expand to nonmalignant conditions.24 Finally, it is imperative to recognize that the primary cause of mortality in relapses or refractory hematologic malignancies remains the primary disease.
Authorship
Contribution: M. Elsallab and M. Elithi contributed to study conception and design, data analysis and interpretation, figure creation, and draft manuscript preparation; J.M. contributed to the data compilation and curation; M.A.L., C.D., M.F., M.A.-P., and M.V.M. contributed to data interpretation and manuscript drafting and editing; all authors reviewed the results and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.A.L. reports research support by Bristol Myers Squibb and consultancy for AbbVie, AstraZeneca, Bristol Myers Squibb, Caribou, Daiichi Sankyo, Fate Therapeutics, Genentech, Genmab, Ipsen, Janssen, Kite, Loxo, Nurix, Recordati, Regeneron, SeaGen, Takeda, and ViTToria. C.D. reports being on the advisory boards of Seagen, Bristol Myers Squibb, Abbvie, and Ono Pharma, received research funding from Ono Pharma, Bristol Myers Squibb, Fate Therapeutics, Curis Inc, and BeiGene, and provided consultation to AbbVie. M.-A.P. reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma, and serves on data and safety monitoring boards for Cidara Therapeutics, Medigene, and Sellas Life Sciences and the scientific advisory board of Nexlmmune, has ownership interests in Nexlmmune, Omeros, and OrcaBio, and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. M.F. reports consultancy for Bristol Myers Squibb, Novartis, Kite, Arcellx, Iovance, and Cytoagents. M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and University of Pennsylvania (some licensed to Novartis), holds equity in 2SeventyBio, Century Therapeutics, Neximmune, Oncternal, and TCR2 and has served as a consultant for multiple companies involved in cell therapies, is on the board of directors of 2Seventy Bio, and has received grant/research support from CRISPR Therapeutics, Kite Pharma, Servier, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Marcela V. Maus, Massachusetts General Hospital Cancer Center, 149 13th St, Room 3.216, Charlestown, MA 02129; email: MVMAUS@mgh.harvard.edu.
References
Author notes
M. Elsallab, M. Ellithi, and M.V.M. contributed equally to this work.
For original data, please contact MVMAUS@mgh.harvard.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

Comments
Comment on second primary malignancies after commercial CAR T cell therapy: analysis of FDA adverse events reporting system (FAERS)
We read with interest of Magdi Elsallab et al.'s recent paper in Blood 1, which provides important insights into the risk profile of secondary primary malignancies (SPMs) following commercial CAR-T therapy. This is a crucial issue given the FDA's safety alerts regarding T-cell malignancies and package insert amendment requirements for all approved CAR-T products 2,3. The authors' analysis of the big data from the FDA Adverse Events Reporting System sheds light on the increased reporting of myeloid neoplasms, T-cell lymphomas and certain types of solid tumor after CAR-T treatment, heightening awareness of these serious risks.
We believe that further subgroup analyses could provide an even deeper understanding of the SPMs post CAR-T therapy. First, previous studies have shown significant differences in adverse event profiles between pediatric and adult patients receiving immunotherapies 4. Examining the incidence of SPMs stratified by age groups could help determine if the risk increase also applies to pediatric and adult CAR-T recipients. Second, there may be meaningful differences in time to onset for hematologic versus solid tumor SPMs following CAR-T therapy 5. Longer follow-up of CAR-T cells treated cohorts may be needed to fully characterize the kinetics and frequencies of blood cancer versus solid cancer SPMs. Finally, other interesting subgroups to explore could include the background chemotherapy regimens used bridging to CAR-T treatment, lines of therapy prior to CAR-T cells infusion, and differences in CAR target antigens or viral vectors- all of which may influence the type and frequency of SPMs developed, albeit not all data are available in the pharmacovigilance databases.
Overall, it is imperative that we comprehensively utilize global pharmacovigilance data to guide clinicians, researchers and regulators in optimizing the safety monitoring and long-term management for CAR-T recipients worldwide. Elsallab et al. have made an important contribution towards this goal.
Conflict-of-interest disclosure: The authors declare that they have no competing interests.
References
[1] Elsallab M, Ellithi M, Lunning MA, et al. Second primary malignancies after commercial CAR T cell therapy: analysis of FDA Adverse Events Reporting System (FAERS). Blood. 2024 Mar 14:blood.2024024166. doi: 10.1182/blood.2024024166.
[2] https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/2024-safety-and-availability-communications#:~:text=This%20webpage%20was%20developed%20to,and%20availability%20of%20biological%20products (last assessed on May 7, 2024).
[3] https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-boxed-warning-t-cell-malignancies-following-treatment-bcma-directed-or-cd19-directed (last assessed on May 7, 2024).
[4] Junyi Shen, Anqi Lin, Quan Cheng, et al. Exploring immune checkpoint inhibitor-related adverse events in pediatric cancer patients: a pharmacovigilance analysis of VigiBase and the FDA adverse event reporting system (FAERS) database. Cancer Res. 2024; 84 (6_Supplement): 6380. https://doi.org/10.1158/1538-7445.AM2024-6380
[5] Hsieh EM, Myers RM, Yates B, et al. Low rate of subsequent malignant neoplasms after CD19 CAR T-cell therapy. Blood Adv 2022; 6(17): 5222-5226.